Abstract
New salts of photochromic indoline spiropyrans capable of reversibly responding to UV radiation were synthesized to develop light-controlled materials. Photoinduced reactions of the synthesized compounds were studied using absorption and luminescence spectroscopies, and the quantum yields of photoisomerization and other spectral and kinetic characteristics were measured. It was shown that the light sensitivity and photostability of the synthesized compounds are considerably influenced by the length of the spacer between the indole and ammonium nitrogen atoms.
1. Introduction
Spiropyrans form one of the most interesting classes of organic photochromic compounds capable of reversible isomerization under the action of external stimuli. The optical and other physicochemical properties of spiropyran isomers considerably differ; therefore, these photochromes can be used as sensors [1,2,3,4,5,6], optoelectronic and holographic devices [7,8], memory cells [9,10,11], etc. Moreover, an obvious advantage of spiropyrans over other classes of photochromic compounds is the relative ease of their synthesis and structural modification. Finally, modification of the spiropyran structure by introduction of functional groups offers broad opportunities for the targeted synthesis of new photochromes with a wide variation of the spectral and kinetic properties [12,13,14].
In addition, it is known that ionic liquids are capable of triggering apoptosis in cells via the mitochondrial pathway. Such molecules can be incorporated into membranes of eukaryotic cells and disrupt their integrity to cause their death due to long side alkyl chains in their structure [15,16,17].
Merocyanines obtained via photochemical isomerization are such ionic compounds. At the same time, the luminescent properties of the open forms of these molecules will not only allow the visualization of the pathway itself and the accumulation of these compounds in specific organelles of target cells, but they will also allow one to study the mechanism of cytotoxic activity in different cell lines. This would open up prospects of obtaining anticancer drugs based on them for the treatment of human oncological diseases due to the solubility of these compounds in water. The only work which describes photoswitchable cytotoxicity against Hek293 cancer cells using spiropyran as an example serves as the feasibility of this approach [18].
2. Results and Discussion
2.1. Chemistry
Here we continued these studies by synthesizing new indoline spiropyrans with the aim of expanding the scope of applicability of spirophotochromic compounds, obtaining new light-controlled materials with a variety of properties, and studying the effect of the length of the spacer between the indole and ammonium nitrogen atoms on the spectral and photochromic properties. The presence of the formyl group in the molecule gives hope that the obtained compounds would exhibit bright luminescence [19].
Spiropyrans were synthesized using methods reported in the literature [20,21] according to Scheme 1:
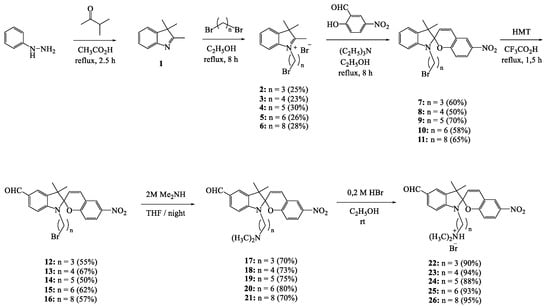
Scheme 1.
Synthesis of compounds 22–26.
The structures of compounds 22–26 were determined using 1H and 13C NMR spectroscopy and high-resolution mass spectrometry (HRMS, see Supplementary Materials).
In the 1H NMR spectrum of compound 22 in CDCl3, the signal positions and integrated intensities, as well as the spin–spin coupling constants, are in line with the presented structure. For example, two singlets at 1.18 and 1.30 ppm correspond to methyl protons at the indole nitrogen atom. The singlet with a chemical shift at 2.52 ppm corresponds to protons of two methyl groups at the ammonium nitrogen. In the case of compound 17, the singlet is shifted up-field and is observed at 2.21 ppm. The signal indicating the presence of a spirocyclic structure is a doublet in the aromatic region at 5.93 ppm with a spin–spin coupling constant of 10.3 Hz, which corresponds to the CH group at the spiro atom in the pyran moiety. The formyl proton signal is manifested at 9.77 ppm (see Supplementary Materials).
In the 13C NMR spectrum of 22 in CDCl3, the number of signals is equal to the number of carbon atoms in the molecule. The characteristic signal of the spirocyclic carbon atom is manifested at 106.19 ppm and is correlated with the proton signal of the gem–methyl groups and the C3′ and C4′ proton signals in the 1H–13C HMBC spectrum. The formyl carbon signal is detected at 190.50 ppm (see Supplementary Materials).
2.2. UV–Vis and Luminescent Studies
Considering the sensitivity of spiropyrans to a broad range of external stimuli [5,18,19,20,21,22,23,24,25,26,27,28,29], the photoinduced reactions of the synthesized salts 22–26 in tetrahydrofuran (THF) were studied using absorption spectroscopy. THF was chosen as the solvent due to its moderate polarity, high dissolving capacity, and inertness to the spiropyran salts.
Figure 1 shows the absorption spectra typical of spiropyrans 22–26 without and under UV irradiation, taking compound 22 as an example. The measured spectra exhibit four most characteristic bands, three being in the UV range with peaks at 266–267, 324–327, and 374–377 nm, and one being in the visible range with a maximum at 599–604 nm (Figure 1 and see Supplementary Materials). The two first-mentioned bands are known to correspond to spiropyran molecules in the closed-ring form (Figure 1, curve 1), while the longer wavelength bands are attributable to absorption of molecules in the open merocyanine form (Figure 1, curve 2). The strong absorption of photochromic compounds 22–26 in the orange spectral range (599–604 nm) is responsible for the deep blue color of their solutions (Figure 2).
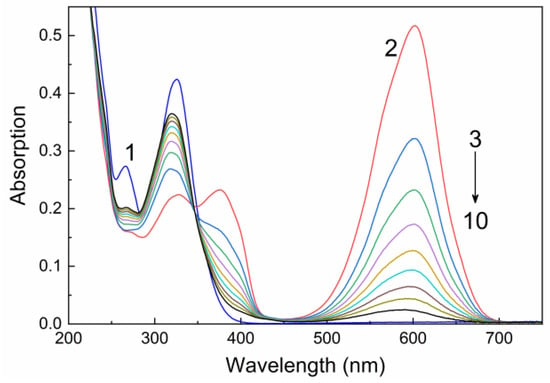
Figure 1.
Absorption spectra of 22 in THF in spiropyran (1) and merocyanine forms (2–10) measured before (1) and upon UV irradiation (2) through a UFS-1 light filter and during bleaching in the dark (3–10). C = 10−4 M, L = 0.1 cm.

Figure 2.
Photo of solutions of compound 22 in THF before (left) and after irradiation (right).
Upon UV irradiation, the intensities of bands at 266–267 and 324–327 nm somewhat decrease, while the intensities of the 374–377 and 599–604 nm bands considerably increase (Figure 1, spectra 2–10). These changes unambiguously indicate that UV irradiation induces photochromic transformations of spiropyrans in the adsorption layer to give the open merocyanine form of the molecules. The observed changes are reversible: molecules 22–26 cyclize again in the dark (dark bleaching) or under the action of visible light (photobleaching). This switching cycle can be repeated more than 10 times. The complete photoinduced transition of spiropyrans 22–26 (10−4 M in THF) from closed to open form takes 11–18 s. The reverse transition is characterized by a longer duration: 180–190 s with dark discoloration, and 100–170 s with visible light irradiation.
Analysis of the absorption spectra of compound 22 showed that the absorption bands with maxima at 374–377 and 599–604 nm are irregularly broadened and, as the period of spontaneous dark bleaching increases, the long wavelength absorption bands not only decrease in intensity, but also shift to shorter wavelengths. The resolution of experimental spectra into Gaussian bands (see Supplementary Materials Figures) demonstrates that the absorption spectra of merocyanine forms are complex. This attests to the presence of cis- and trans-isomers of open-ring spiropyrans (Figure 3), which are characterized by different stabilities and different lifetimes of the metastable state.
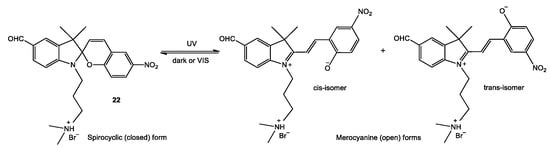
Figure 3.
Spiropyran (SP) closed-ring structure and its stimuli-induced open-ring isomers (MC).
It can be seen from the data of Table 1 that the positions of absorption bands of compounds 22–26 remain almost invariable in both spirocyclic and merocyanine forms, i.e., they do not depend on the number of methylene groups in the spacer chain. This attests to the absence of electronic interactions between the N–amine moiety and the π-conjugated system in the electronic ground state of the molecule.

Table 1.
Spectral and kinetic characteristics of the studied photochromic compounds in spiropyran (SP) and merocyanine (MC) forms.
Study of the relaxation kinetics of compounds 22–26 without irradiation or on exposure to visible light showed that the kinetic curves are exponential (Figure 4A); their linearization in the ln(I)–t(s) coordinates gives linear plots for the initial stage (Figure 4B). This fact indicates that the reverse transition is a first-order reaction.
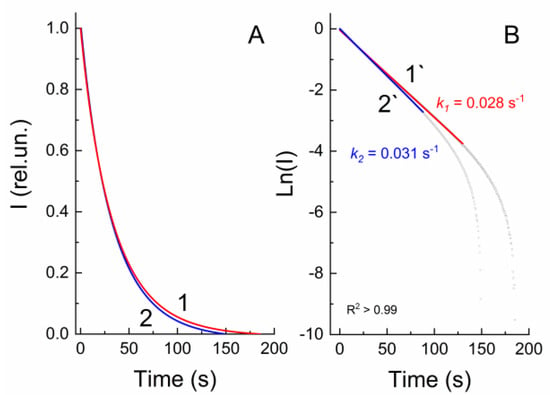
Figure 4.
Normalized kinetic curves (A) of the transition process of the merocyanine form of 22 to the spiropyran form measured at 605 nm without (1) (dark bleaching) and upon irradiation (2) through a SZS–9 light filter (photobleaching). Linear anamorphoses (B) of kinetic curves 1 and 2 in the Ln(I)–time(s) coordinates.
A comparison of the rate constants for dark bleaching (k1) and photobleaching (k2) for 22–26 derived from the slopes of the linearized plots indicates that k2 are slightly greater than k1, and generally the constants are of the same order of magnitude. Thus, the rate of relaxation of compounds 22–26 from the open-ring to the closed-ring form also does not depend on the number of the methylene groups in the spacer.
In addition, considerable variation was found for other important characteristics of photochromic compounds, such as photodegradation efficiency and light sensitivity defined as the ratio ∆Dmax/Dmax. Ongoing from 22 to 24, the light sensitivity and the photostability of compounds increase. Further increase in the number of methylene groups in the chains from 24 to 26 leads to a decrease in both the light sensitivity and the stability to photodegradation (Figure 5).
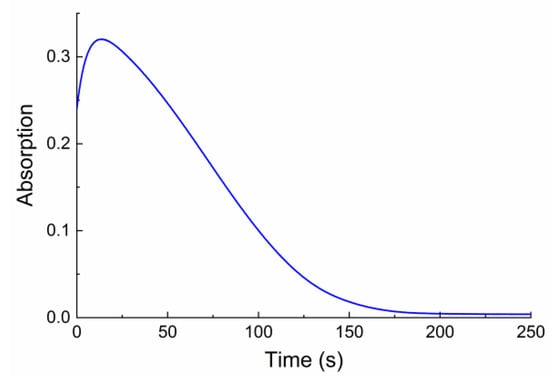
Figure 5.
Kinetics of photodegradation of 22 upon UV irradiation through a UFS-1 light filter, recorded at 605 nm.
It is known that the control spectral and luminescent properties of photochromic compounds is a promising trend in the field of photochromic technologies, owing to the exceptionally high sensitivity and luminescence response time. Meanwhile, it is known from the literature that the ammonium salt of spiropyran may act as an antitumor agent [18]. The detected luminescence behavior of the synthesized compound would enable visualization of the route and cellular location of a test compound, which is a promising trend in the design of anticancer drugs for humans. Therefore, we studied the spectral and luminescent properties of the photochromic spiropyran 22 in a THF solution at room temperature.
According to the experimental results, spirophotochrome 22 does not possess photoluminescence (PL) when it is in the closed spirocyclic form. However, UV irradiation of its THF solution at room temperature gives rise to PL in the red region of the visible spectrum with a peak at 645 nm (Figure 6). The measured PL quantum yield of 22 in merocyanine form was found to be low (ϕPL = 3.7 × 10−4).
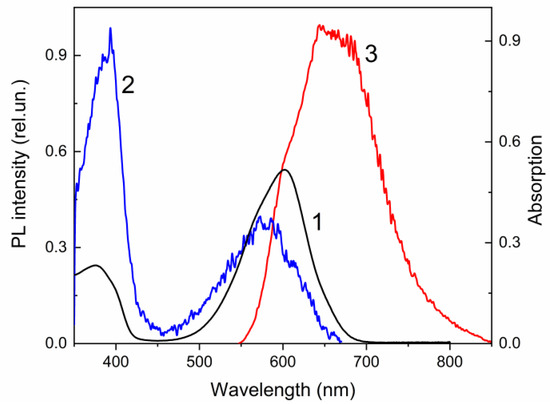
Figure 6.
The absorption (1), PL excitation (2) and PL (3) spectra of compound 22 in THF solution. λem = 680 nm (2), λexc = 530 nm (3). T = 298 K.
3. Materials and Methods
The detailed procedure of the synthesis and characterization of the products are given in Supplementary Materials.
4. Conclusions
Thus, we synthesized new indoline-derived spiropyran salts containing functional substituents with different lengths of spacer between the indole and ammonium nitrogen atoms in the molecules, and studied their photochromic and luminescent properties. The results are that all synthesized compounds exhibit positive photochromism at room temperature. Study of the effect of structural factors on the spectral and kinetic characteristics of photochromic transformations of the synthesized spiropyrans demonstrated that the positions of the absorption bands of the closed and merocyanine forms of spiropyrans, and the rate constants of spiropyran relaxation from the open to closed form virtually do not depend on the length of the spacer between the indole and ammonium nitrogen atoms. Meanwhile, the light sensitivity and photostability of the compounds depends considerably on the number of methylene units. An increase in the number of methylene units induces first an increase and then a decrease in the light sensitivity and stability to photodegradation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238492/s1, they are as: NMR spectra of compound 1 (Figures S1, S2), compounds 2–6 (Figures S3–S12), compounds 7–11 (Figures S13–S22), compounds 12–16 (Figures S23–S32), compound 17–21 (Figures S33–S42), compound 22 (Figures S43, S44, S46), compound 23 (Figures S47, S48), compound 24 (Figures S53, S54), compound 25 (Figures S59, S60), compound 26 (Figures S65, S66), ESI-HRMS spectra of compound 22 (Figure S45), compound 23 (Figure S49), compound 24 (Figure S55), compound 25 (Figure S61), compound 26 (Figure S67), absorption spectrum of compound’s 23–26 (Figures S50, S56, S62, S68, respectively), kinetic curves of the transition process of the merocyanine forms of compounds 23–26 to the spiropyran forms (Figures S51, S57, S63, S69, respectively), kinetics of photodegradation of compounds 23–26 (Figures S52, S58, S64, S70, respectively).
Author Contributions
Conceptualization, A.A.K.; methodology, A.A.K., D.I.G. and L.L.K.; validation, A.A.K. and D.I.G.; formal analysis, L.L.K. and A.R.T.; investigation, A.A.K. and L.L.K.; writing—original draft preparation, A.A.K. and D.I.G.; writing—review and editing, A.A.K.; data analysis and visualization, A.R.T. and D.I.G.; funding acquisition, A.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Russian Science Foundation (grant № 21-73-10112).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was financially supported by the Russian Science Foundation (grant № 21-73-10112). The structural studies were performed using the equipment of the Collective Usage Centre ‘Agidel’ at the Institute of Petrochemistry and Catalysis of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Phillips, J.P.; Mueller, A.; Przystal, F. Photochromic Chelating Agents. J. Am. Chem. Soc. 1965, 87, 4020. [Google Scholar] [CrossRef]
- Kimura, K. Photocontrol of ionic conduction by photochromic crown ethers. Coord. Chem. Rev. 1996, 148, 41–61. [Google Scholar] [CrossRef]
- Willner, I. Photoswitchable Biomaterials: En Route to Optobioelectronic Systems. Acc. Chem. Res. 1997, 30, 347–356. [Google Scholar] [CrossRef]
- Evans, L.; Collins, G.E.; Shaffer, R.E.; Mechelet, V.; Winkler, J.D. Selective Metals Determination with a Photoreversible Spirobenzopyran. Anal. Chem. 1999, 71, 5322. [Google Scholar] [CrossRef] [PubMed]
- Minkin, V.I. Photo-, Thermo-, Solvato-, and Electrochromic Spiroheterocyclic Compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef]
- Krayushkin, M.M.; Bogacheva, A.M.; Levchenko, K.S.; Kobeleva, O.I.; Valova, T.M.; Barachevskii, V.A.; Pozzo, J.-L.; Struchkova, M.I.; Shmelin, P.S.; Kalik, M.A.; et al. Synthesis of photochromic 6-aryl-substituted bis(benzothiophenyl)- perfluorocyclopentenes by the Suzuki–Miyaura cross-coupling. Mendeleev Commun. 2013, 23, 78–80. [Google Scholar] [CrossRef]
- Tomasulo, M.; Yildiz, I.; Raymo, F.M. Nanoparticle-induced transition from positive to negative photochromism. Inorg. Chim. Acta 2007, 360, 938–944. [Google Scholar] [CrossRef]
- Ramos-Garcia, R.; Delgado-Macuil, R.; Iturbe-Castillo, D.; de los Santos, E.G.; Corral, F.S. Polarization dependence on the holographic recording in spiropyran-doped polymers. Opt. Quantum Electron. 2003, 35, 641–650. [Google Scholar] [CrossRef]
- Bouas-Laurent, H.; Dürr, H. Organic photochromism (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 639–665. [Google Scholar] [CrossRef]
- Orgiu, E.; Samori, P. 25th Anniversary Article: Organic Electronics Marries Photochromism: Generation of Multifunctional Interfaces, Materials, and Devices. Adv. Mater. 2014, 26, 1827–1845. [Google Scholar] [CrossRef]
- Barachevsky, V.A.; Lashkov, G.I.; Tsekhomsky, V.A. Photochromism and Its Uses; Chemistry: Moscow, Russia, 1977; p. 280. [Google Scholar]
- Traven, V.F.; Miroshnikov, V.S.; Chibisova, T.A.; Barachevsky, V.A.; Venediktova, O.V.; Strokach, Y.P. Synthesis and structure of indoline spiropyrans of the coumarin series. Rus. Chem. Bull. 2005, 54, 2417–2424. [Google Scholar] [CrossRef]
- Bertelson, R.C. Photochromic processes involving heterocyclic cleavage. In Photochromism; Wiley-Interscience: New York, NY, USA, 1971; Chapter 3; p. 45. [Google Scholar]
- Dolotov, S.M.; Miroshnikov, V.S.; Chibisova, T.A.; Su-Lan, S.; Venidiktova, O.V.; Valova, T.M.; Dunaev, A.A.; Strokach, Y.P.; Barachevsky, V.A.; Traven, V.F. Photochromism of indoline spiropyrans of the coumarin series in polymeric matrices. Rus. Chem. Bull. 2007, 56, 904–909. [Google Scholar] [CrossRef]
- Benedetto, A.; Ballone, P. Room-Temperature Ionic Liquids and Biomembranes: Setting the Stage for Applications in Pharmacology, Biomedicine, and Bionanotechnology. Langmuir 2018, 34, 9579–9597. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Pillai, V.V.S.; Benedetto, A. Mechanisms of action of ionic liquids on living cells: The state of the art. Biophys. Rev. 2020, 12, 1187–1215. [Google Scholar] [CrossRef] [PubMed]
- Dzhemileva, L.U.; D’yakonov, V.A.; Seitkalieva, M.M.; Kulikovskaya, N.S.; Egorova, K.S.; Ananikov, V.P. A large-scale study of ionic liquids employed in chemistry and energy research to reveal cytotoxicity mechanisms and to develop a safe design guide. Green Chem. 2021, 23, 6414–6430. [Google Scholar] [CrossRef]
- Nilsson, J.R.; Li, S.; Önfelt, B.; Andréasson, J. Light-induced cytotoxicity of a photochromic spiropyran. Chem. Commun. 2011, 47, 11020–11022. [Google Scholar] [CrossRef]
- Galimov, D.I.; Tuktarov, A.R.; Sabirov, D.S.; Khuzin, A.A.; Dzhemilev, U.M. Reversible luminescence switching of a photochromic fullerene[60]-containing spiropyran. J. Photochem. Photobiol. A Chem. 2019, 375, 64–70. [Google Scholar] [CrossRef]
- Hammarson, M.; Andersson, J.; Li, S.; Lincoln, P.; Andréasson, J. Molecular AND-logic for dually controlled activation of a DNA-binding spiropyran. Chem. Commun. 2010, 46, 7130–7132. [Google Scholar] [CrossRef]
- Feeney, M.J.; Thomas, S.W. Tuning the Negative Photochromism of Water-Soluble Spiropyran Polymers. Macromolecules 2018, 51, 8027–8037. [Google Scholar] [CrossRef]
- Bahr, J.L.; Kodis, G.; de la Garza, L.; Lin, S.; Moore, A.L.; Moore, T.A.; Gust, D. Photoswitched Singlet Energy Transfer in a Porphyrin-Spiropyran Dyad. J. Am. Chem. Soc. 2001, 123, 7124–7133. [Google Scholar] [CrossRef]
- Song, X.; Zhou, J.; Li, Y.; Tang, Y. Correlations between solvatochromism, Lewis acid-base equilibrium and photochromism of an indoline spiropyran. J. Photochem. Photobiol. A Chem. 1995, 92, 99–103. [Google Scholar] [CrossRef]
- Yagi, S.; Nakamura, S.; Watanabe, D.; Nakazumi, H. Colorimetric sensing of metal ions by bis(spiropyran) podands: Towards naked-eye detection of alkaline earth metal ions. Dyes Pigment. 2009, 80, 98–105. [Google Scholar] [CrossRef]
- Darwish, T.A.; Evans, R.A.; James, M.; Malic, N.; Triani, G.; Hanley, T.L. CO2 Triggering and Controlling Orthogonally Multiresponsive Photochromic Systems. J. Am. Chem. Soc. 2010, 132, 10748–10755. [Google Scholar] [CrossRef] [PubMed]
- Darwish, T.A.; Evans, R.A.; James, M.; Hanley, T.L. Spiropyran–Amidine: A Molecular Canary for Visual Detection of Carbon Dioxide Gas. Chem. Eur. J. 2011, 17, 11399–11404. [Google Scholar] [CrossRef]
- Wojtyk, J.T.C.; Wasey, A.; Xiao, N.-N.; Kazmaier, P.M.; Hoz, S.; Yu, C.; Lemieux, R.P.; Buncel, E. Elucidating the Mechanisms of Acidochromic Spiropyran-Merocyanine Interconversion. J. Phys. Chem. A 2007, 111, 2511–2516. [Google Scholar] [CrossRef]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; Van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martinez, T.J.; White, S.R.; et al. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Itoh, M.; Hirai, T. Thermal isomerization of spiropyran to merocyanine in aqueous media and its application to colorimetric temperature indication. Phys. Chem. Chem. Phys. 2010, 12, 13737–13745. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).