Impact of Sample Pretreatment and Extraction Methods on the Bioactive Compounds of Sugar Beet (Beta vulgaris L.) Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition and Water Activity of SBLs
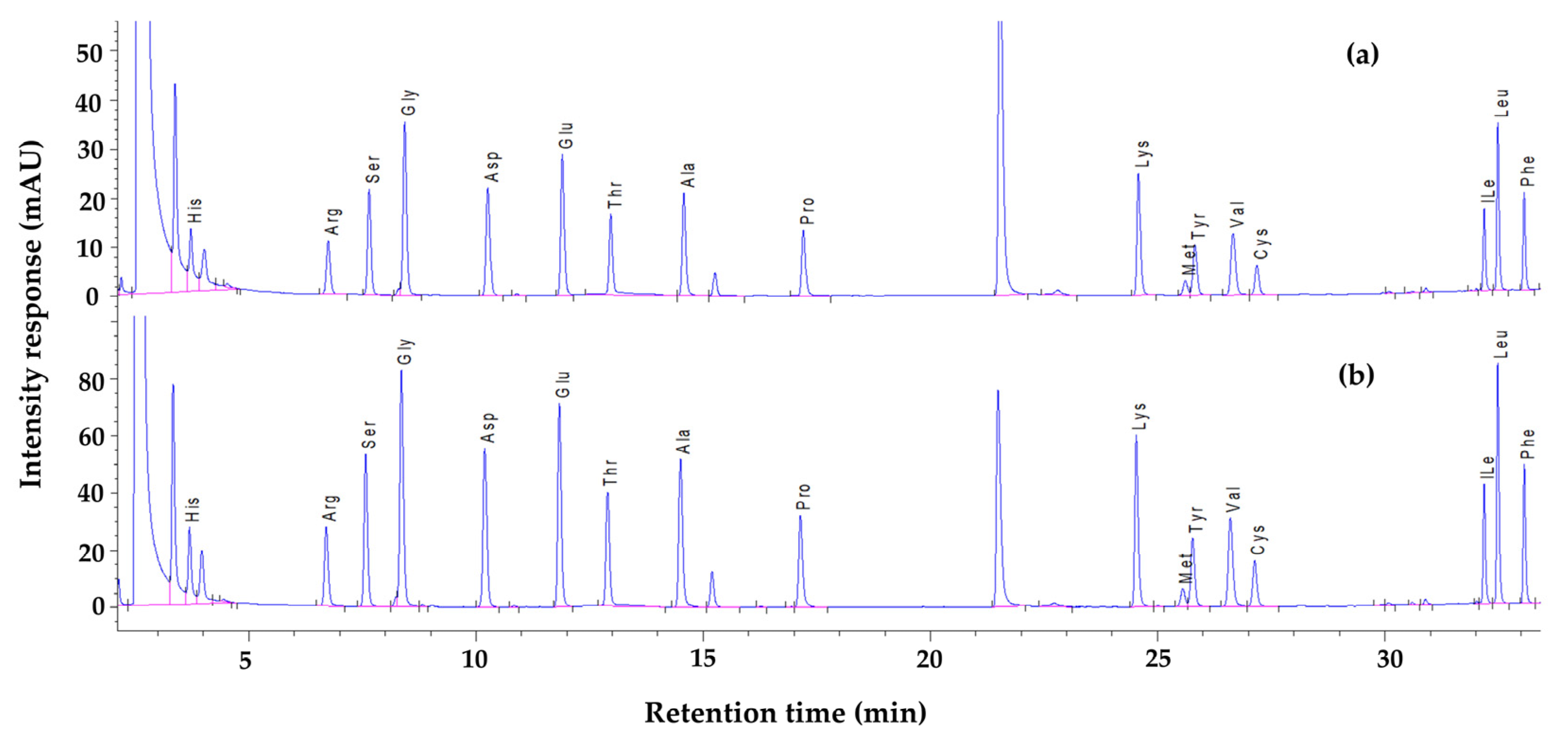
2.2. Amino Acid Profile of Fresh and Lyophilized Leaves
2.3. Fatty Acid Profile of SBLs
2.4. Polyphenol Oxidase Activity of SBLs
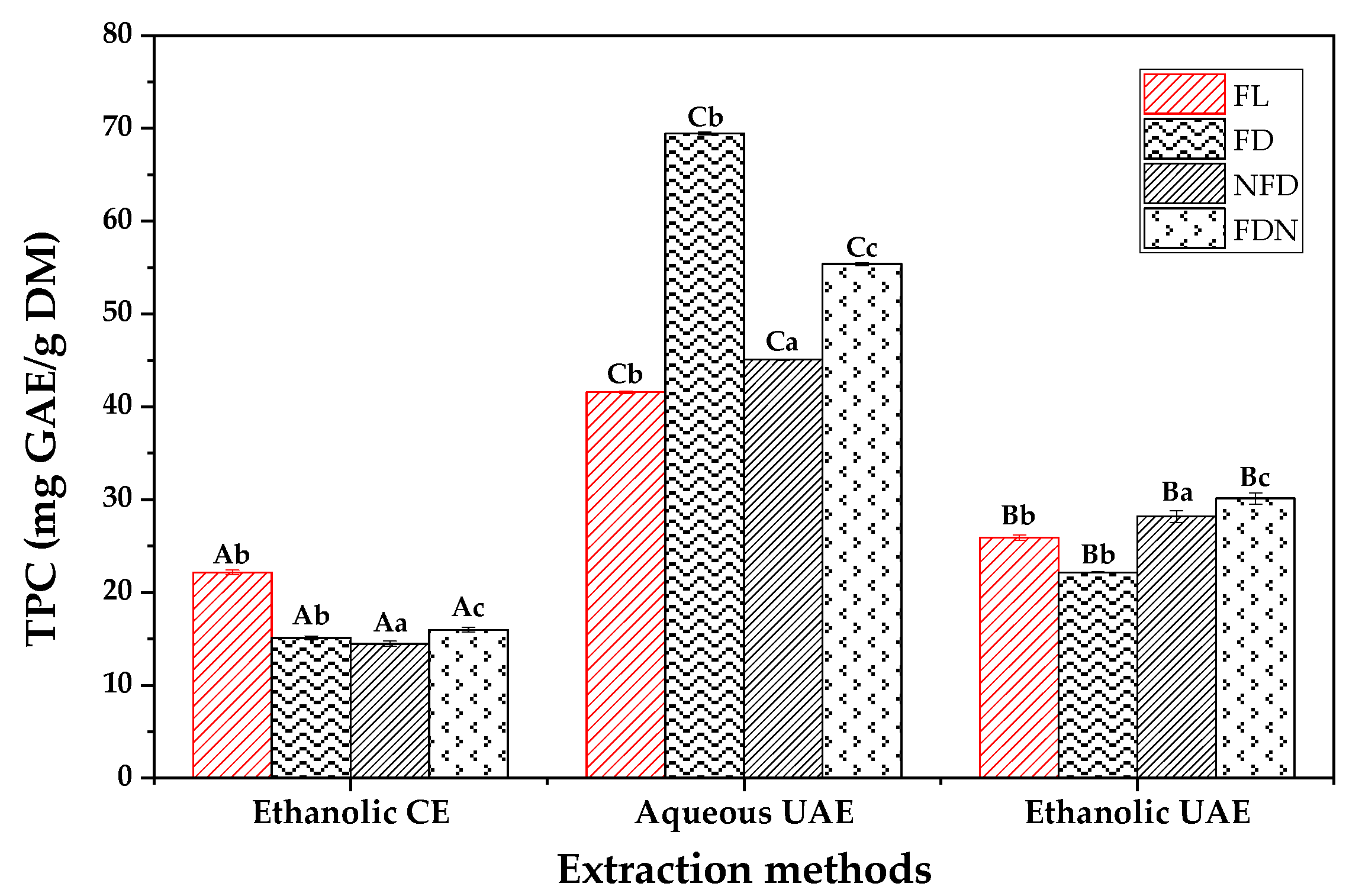
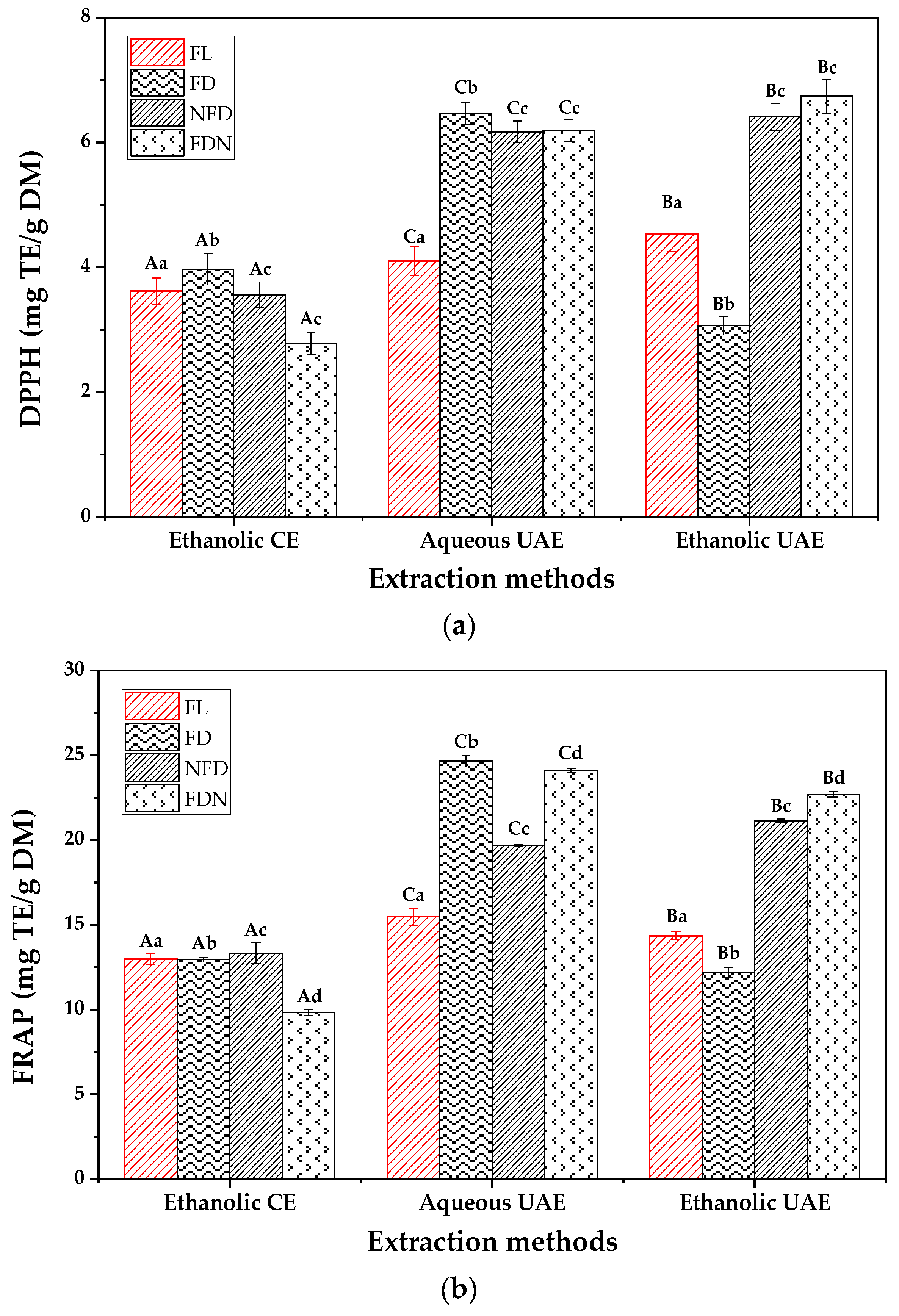
2.5. Total Phenolic Content (TPC) and Antioxidant Activity of Extracts
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Sample Preparation
3.3. Extraction of PPO from SBLs
3.4. UAE and CE of Polyphenols from SBLs
3.5. Analysis of Chemical Properties and PPO Activity of the Leaves
3.5.1. Proximate Composition and Water Activity of the Leaves
3.5.2. Determination of AA Profile
3.5.3. Determination of FA Profile
3.5.4. Determination of Polyphenol Oxidase Activity
3.6. Analysis of Phenolic Extracts
3.6.1. Determination of TPC
3.6.2. Determination of Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
- Combining aqueous UAE and freeze-drying techniques could be a potential tool for extracting polyphenols more efficiently, which may result from the inactivation of PPO by freeze-drying and the breakage of the cell membrane by UAE.
- The freeze-drying method decreases the lipid content and increases the fiber content, essential fatty acids, and some essential amino acids and phenolic content of SBLs, making this by-product a potential dietary food supplement and a suitable substrate for fermentation.
- The combined effect of aqueous UAE and freeze-drying techniques could be a circular economy approach enhancing the by-products’ valorization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, J.P.B.; Liberal, Â.; Petropoulos, S.A.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Fernandes, Â.; Barros, L. Agri-Food Surplus, Waste and Loss as Sustainable Biobased Ingredients: A Review. Molecules 2022, 27, 5200. [Google Scholar] [CrossRef] [PubMed]
- Molotoks, A.; Smith, P.; Dawson, T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2021, 10, e261. [Google Scholar] [CrossRef]
- Yeasmen, N.; Orsat, V. Green extraction and characterization of leaves phenolic compounds: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.B.N.; Ferreira, M.S.L.; Fai, A.E.C. Utilization of Agricultural By-products: Bioactive Properties and Technological Applications. Food Rev. Int. 2022, 38, 1305–1329. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Polyphenols: A Comprehensive Review of their Nutritional Properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Modelska, M.; Berlowska, J.; Kregiel, D.; Cieciura, W.; Antolak, H.; Tomaszewska, J.; Binczarski, M.; Szubiakiewicz, E.; Witonska, I.A. Concept for recycling waste biomass from the sugar industry for chemical and biotechnological purposes. Molecules 2017, 22, 1544. [Google Scholar] [CrossRef]
- Dukić, J.; Hunić, M.; Nutrizio, M.; Režek Jambrak, A. Influence of High-Power Ultrasound on Yield of Proteins and Specialized Plant Metabolites from Sugar Beet Leaves (Beta vulgaris subsp. vulgaris var. altissima). Appl. Sci. 2022, 12, 8949. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Biondo, P.B.F.; Boeing, J.S.; Barizão, É.O.; de Souza, N.E.; Matsushita, M.; de Oliveira, C.C.; Boroski, M.; Visentainer, J.V. Evaluation of beetroot (Beta vulgaris L.) leaves during its developmental stages: A chemical composition study. Food Sci. Technol. 2014, 34, 94–101. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, T.; Wang, X.; Lü, X. Apple pomace as a potential valuable resource for full-components utilization: A review. J. Clean. Prod. 2021, 329, 129676. [Google Scholar] [CrossRef]
- Sehsah, M.D.; El-Kot, G.A.; El-Nogoumy, B.A.; Alorabi, M.; El-Shehawi, A.M.; Salama, N.H.; El-Tahan, A.M. Efficacy of Bacillus subtilis, Moringa oleifera seeds extract and potassium bicarbonate on Cercospora leaf spot on sugar beet. Saudi J. Biol. Sci. 2022, 29, 2219–2229. [Google Scholar] [CrossRef]
- Esteban-Lustres, R.; Sanz, V.; Domínguez, H.; Torres, M.D. Ultrasound-Assisted Extraction of High-Value Fractions from Fruit Industrial Processing Waste. Foods 2022, 11, 2089. [Google Scholar] [CrossRef]
- Garcìa, L.M.; Ceccanti, C.; Negro, C.; De Bellis, L.; Incrocci, L.; Pardossi, A.; Guidi, L. Effect of drying methods on phenolic compounds and antioxidant activity of Urtica dioica L. leaves. Horticulturae 2021, 7, 10. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of Drying Processes and Pretreatments on Nutritional and Bioactive Characteristics of Dried Vegetables: A Review. Food Eng. Rev. 2016, 8, 134–163. [Google Scholar] [CrossRef]
- Dal-Bó, V.; Freire, J.T. Effects of lyophilization on colorimetric indices, phenolics content, and antioxidant activity of avocado (Persea americana) pulp. Food Control 2022, 132, 108526. [Google Scholar] [CrossRef]
- Dziki, D. Recent Trends in Pretreatment of Food before Freeze-Drying. Processes 2020, 8, 1661. [Google Scholar] [CrossRef]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Dek, M.S.P.; Hairuddin, M.R. Effect of freeze-drying on the antioxidant compounds and antioxidant activity of selected tropical fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef]
- Romani, A.; Mulas, S.; Heimler, D. Polyphenols and secoiridoids in raw material (Olea europaea L. leaves) and commercial food supplements. Eur. Food Res. Technol. 2017, 243, 429–435. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, R.; Correa, V.G.; de Morais, G.R.; de Souza, C.G.M.; Bracht, A.; Peralta, R.A.; Peralta-Muniz Moreira, R.F.; Peralta, R.M. Liquid nitrogen pretreatment of eucalyptus sawdust and rice hull for enhanced enzymatic saccharification. Bioresour. Technol. 2017, 224, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Žauhar, G.; Žuvić, M. Optimization of ultrasonic-assisted extraction of major phenolic compounds from olive leaves (Olea europaea L.) using response surface methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, A.; Ersus, S. Optimization of enzyme assisted extraction of protein from the sugar beet (Beta vulgaris L.) leaves for alternative plant protein concentrate production. Food Chem. 2021, 335, 127673. [Google Scholar] [CrossRef] [PubMed]
- Kiskini, A.; Vissers, A.; Vincken, J.-P.; Gruppen, H.; Wierenga, P.A. Effect of Plant Age on the Quantity and Quality of Proteins Extracted from Sugar Beet (Beta vulgaris L.) Leaves. J. Agric. Food Chem. 2016, 64, 8305–8314. [Google Scholar] [CrossRef] [PubMed]
- Starke, P.; Hoffmann, C. Dry matter and sugar content as parameters to assess the quality of sugar beet varieties for anaerobic digestion. Sugar Ind. 2014, 139, 232–240. [Google Scholar] [CrossRef]
- Oliveira, A.M.B.; Viganó, J.; Sanches, V.L.; Rostagno, M.A.; Martínez, J. Extraction of potential bioactive compounds from industrial Tahiti lime (Citrus latifólia Tan.) by-product using pressurized liquids and ultrasound-assisted extraction. Food Res. Int. 2022, 157, 111381. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, W.; Sun, D.W. Effects of liquid nitrogen quick freezing on polyphenol oxidase and peroxide activities, cell water states and epidermal microstructure of wolfberry. LWT 2020, 120, 108923. [Google Scholar] [CrossRef]
- Alfaro, L.; Siramard, S.; Chouljenko, A.; Sathivel, S. Effects of liquid nitrogen pretreatment on the osmotic dehydration and quality of cryogenically frozen blueberries (Vaccinium angustifolium Ait.). Food Biosci. 2018, 22, 165–169. [Google Scholar] [CrossRef]
- Harguindeguy, M.; Fissore, D. On the effects of freeze-drying processes on the nutritional properties of foodstuff: A review. Dry. Technol. 2020, 38, 846–868. [Google Scholar] [CrossRef]
- Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Arámbula-Villa, G.; Flores-Casamayor, V.; Álvarez-Fitz, P.; Ramírez-Ruano, M.; Salazar, R. Effect of water activity on extractable polyphenols and some physical properties of Hibiscus sabdariffa L. calyces. J. Food Meas. Charact. 2019, 13, 687–696. [Google Scholar] [CrossRef]
- Mutukuri, T.T.; Wilson, N.E.; Taylor, L.S.; Topp, E.M.; Zhou, Q.T. Effects of drying method and excipient on the structure and physical stability of protein solids: Freeze drying vs. spray freeze drying. Int. J. Pharm. 2021, 594, 120169. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Evaluation of the chemical composition and nutritional potential of brown macroalgae commercialised in China. Algal Res. 2022, 64, 102683. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Schreuders, F.K.G.; Zisopoulos, F.K.; Boom, R.M.; van der Goot, A.J. Processing concepts for the use of green leaves as raw materials for the food industry. J. Clean. Prod. 2017, 164, 736–748. [Google Scholar] [CrossRef]
- Di Stefano, E.; Agyei, D.; Njoku, E.N.; Udenigwe, C.C. Plant RuBisCo: An Underutilized Protein for Food Applications. J. Am. Oil Chem. Soc. 2018, 95, 1063–1074. [Google Scholar] [CrossRef]
- Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef]
- Ağar, B.; Gençcelep, H.; Saricaoğlu, F.T.; Turhan, S. Effect of sugar beet fiber concentrations on rheological properties of meat emulsions and their correlation with texture profile analysis. Food Bioprod. Process. 2016, 100, 118–131. [Google Scholar] [CrossRef]
- Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Evaluation and Characterization of Nutritional, Microbiological and Sensory Properties of Beet Greens. Acta Sci. Nutr. Health 2017, 1, 37–45. [Google Scholar]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Becerril-Campos, A.A.; Ocampo-Anguiano, P.V.; Mondragón-Jacobo, C.; Escobar-García, K.; Camacho-Barrón, M.; Anaya-Loyola, M.A.; Feregrino-Perez, A.A.; García-Gasca, T.; Ahumada-Solórzano, S.M. Phaseolus vulgaris L. Leaves Increase Short-Chain Fatty Acid (SCFA) Production, Ameliorating Early Metabolic Alterations. Plant Foods Hum. Nutr. 2022, 1, 3. [Google Scholar] [CrossRef]
- Asadi, S.Z.; Khan, M.A. The Effect of Beetroot (Beta vulgaris L.) Leaves Powder on Nutritional, Textural, Sensorial and Antioxidant Properties of Cookies. J. Culin. Sci. Technol. 2021, 19, 424–438. [Google Scholar] [CrossRef]
- Abdo, E.; El-Sohaimy, S.; Shaltout, O.; Abdalla, A.; Zeitoun, A. Nutritional Evaluation of Beetroots (Beta vulgaris L.) and Its Potential Application in a Functional Beverage. Plants 2020, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Hegde, S.; Kumar, A.; Chaudhary, G.; Tewari, S.K.; Upreti, D.K.; Pal, M. Fatty acid composition and antibacterial potential of Cassia tora (leaves and stem) collected from different geographic areas of India. J. Food Drug Anal. 2018, 26, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.A.; Desouza, C. Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, Y.; Meghwal, M. Encapsulated oil powder: Processing, properties, and applications. J. Food Process. Eng. 2022, 45, e14047. [Google Scholar] [CrossRef]
- Ennigrou, A.; Casabianca, H.; Vulliet, E.; Hanchi, B.; Hosni, K. Assessing the fatty acid, essential oil composition, their radical scavenging and antibacterial activities of Schinus terebinthifolius Raddi leaves and twigs. J. Food Sci. Technol. 2018, 55, 1582–1590. [Google Scholar] [CrossRef]
- Da Silva, L.G.S.; Morelli, A.P.; Pavan, I.C.B.; Tavares, M.R.; Pestana, N.F.; Rostagno, M.A.; Simabuco, F.M.; Bezerra, R.M.N. Protective effects of beet (Beta vulgaris) leaves extract against oxidative stress in endothelial cells in vitro. Phyther. Res. 2020, 34, 1385–1396. [Google Scholar] [CrossRef]
- Radzimierska-Kaźmierczak, M.; Śmigielski, K.; Sikora, M.; Nowak, A.; Plucińska, A.; Kunicka-Styczyńska, A.; Czarnecka-Chrebelska, K.H. Olive Oil with Ozone-Modified Properties and Its Application. Molecules 2021, 26, 3074. [Google Scholar] [CrossRef]
- Moretto, Â.; Byruchko, R.T.; Modesto, E.C.; da Motta, A.S.; Friedrich, M.T.; Rezzadori, K. Effect of olive oil replacement on physicochemical, technological, and microbiological properties of buffalo burger modification. J. Food Process. Preserv. 2020, 44, e14624. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83. [Google Scholar] [CrossRef]
- Öztürk, C.; Aksoy, M.; Küfrevioğlu, Ö.İ. Purification of tea leaf (Camellia sinensis) polyphenol oxidase by using affinity chromatography and investigation of its kinetic properties. J. Food Meas. Charact. 2020, 14, 31–38. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Sanz, M.T.; Blanco, B.; Beltrán, S.; Niknam, S.M. Freeze dried extract from olive leaves: Valorisation, extraction kinetics and extract characterization. Food Bioprod. Process. 2020, 124, 196–207. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of bioactive compounds from beet leaves through simultaneous extraction: Modelling and process optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Goyeneche, R.; Di Scala, K.; Ramirez, C.L.; Fanovich, M.A. Recovery of bioactive compounds from beetroot leaves by supercritical CO2 extraction as a promising bioresource. J. Supercrit. Fluids 2020, 155, 104658. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A.; Mihaylova, D. Comparison of Green Technologies for Valorizing Sugar Beet (Beta vulgaris L.) Leaves. Food Sci. Appl. Biotechnol. 2022, 5, 119–130. [Google Scholar] [CrossRef]
- De Castro, A.P.R.B.; da Cunha, D.T.; Antunes, A.E.C.; Corona, L.P.; Bezerra, R.M.N. Effect of Freeze-Dried Red Beet (Beta vulgaris L.) Leaf Supplementation on Biochemical and Anthropometrical Parameters in Overweight and Obese Individuals: A Pilot Study. Plant Foods Hum. Nutr. 2019, 74, 232–234. [Google Scholar] [CrossRef]
- Lorizola, I.M.; Furlan, C.P.B.; Portovedo, M.; Milanski, M.; Botelho, P.B.; Bezerra, R.M.N.; Sumere, B.R.; Rostagno, M.A.; Capitani, C.D. Beet stalks and leaves (Beta vulgaris L.) protect against high-fat diet-induced oxidative damage in the liver in mice. Nutrients 2018, 10, 872. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Determination of Anti-Alzheimer’s Disease Activity of Selected Plant Ingredients. Molecules 2022, 27, 3222. [Google Scholar] [CrossRef]
- Thuy, N.M.; Tuyen, N.T.M.; Thanh, N.V.; Tai, N.V. Evaluation of freeze-drying conditions on the process kinetics and physicochemical properties of purple shallot. Food Res. 2020, 4, 1630–1636. [Google Scholar] [CrossRef]
- Tan, S.; Tang, J.; Shi, W.; Wang, Z.; Xiang, Y.; Deng, T.; Gao, X.; Li, W.; Shi, S. Effects of three drying methods on polyphenol composition and antioxidant activities of Litchi chinensis Sonn. Food Sci. Biotechnol. 2020, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous Determination of All Polyphenols in Vegetables, Fruits, and Teas. J. Agric. Food Chem. 2003, 51, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhou, Q.; Sun, D.W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Arjeh, E.; Mahsa, S.; Mohsen, K.; Sajad, B.; Karimi, I.; Shiva, S.; Farzad, R. Phenolic compounds of sugar beet (Beta vulgaris L.): Separation method, chemical characterization, and biological properties. Food Sci. Nutr. 2022, 00, 1–9. [Google Scholar] [CrossRef]
- Silva Júnior, M.E.; Araújo, M.V.R.L.; Santana, A.A.; Silva, F.L.H.; Maciel, M.I.S. Ultrasound-assisted extraction of bioactive compounds from ciriguela (Spondias purpurea L.) peel: Optimization and comparison with conventional extraction and microwave. Arab. J. Chem. 2021, 14, 103260. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Hu, W.; Ahmad, I.; Ahmed, A.; Xu, X. Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioprod. Process. 2019, 117, 170–182. [Google Scholar] [CrossRef]
- Hazra, S.; Gallou, F.; Handa, S. Water: An Underestimated Solvent for Amide Bond-Forming Reactions. ACS Sustain. Chem. Eng. 2022, 10, 5299–5306. [Google Scholar] [CrossRef]
- Alves, T.P.; Triques, C.C.; Palsikowski, P.A.; da Silva, C.; Fiorese, M.L.; da Silva, E.A.; Fagundes-Klen, M.R. Improved extraction of bioactive compounds from Monteverdia aquifolia leaves by pressurized-liquid and ultrasound-assisted extraction: Yield and chemical composition. J. Supercrit. Fluids 2022, 181, 105468. [Google Scholar] [CrossRef]
- Mihaylova, D.; Lante, A. Water an Eco-Friendly Crossroad in Green Extraction: An Overview. Open Biotechnol. J. 2019, 13, 155–162. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Nagbhushan, P.; Giridhar, P.; Shetty, N.P.; Yannam, S.K.; Mahadevappa, P. Evaluation of Various Drying Methods on Bioactives, Ascorbic Acid and Antioxidant Potentials of Talinum triangulare L., foliage. Plant Foods Hum. Nutr. 2020, 75, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Tinello, F.; Mihaylova, D.; Lante, A. Effect of Dipping Pre-treatment with Unripe Grape Juice on Dried “Golden Delicious” Apple Slices. Food Bioprocess Technol. 2018, 11, 2275–2285. [Google Scholar] [CrossRef]
- Bosch, L.; Alegría, A.; Farré, R. Application of the 6-aminoquinolyl-N-hydroxysccinimidyl carbamate (AQC) reagent to the RP-HPLC determination of amino acids in infant foods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 831, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Tuan, Y.H.; Phillips, R.D. Optimized Determination of Cystine/Cysteine and Acid-Stable Amino Acids from a Single Hydrolysate of Casein- and Sorghum-Based Diet and Digesta Samples. J. Agric. Food Chem. 1997, 45, 3535–3540. [Google Scholar] [CrossRef]
- European Union Commission Directive 2000/45/EC of 6 July 2000 establishing Community methods of analysis for the determination of vitamin A, vitamin E and tryptophan in feeding stuffs. Off. J. Eur. Union 2000, L174, 32–50.
- Ebrahimi, P.; Lante, A.; Scapin, R.M.; Zannoni, S.; Contiero, B.; Catellani, P.; Giaccone, V. Evaluation of quality and safety of beef hamburgers fortified with Ozonated Extra Virgin Olive Oil. LWT 2022, 170, 114100. [Google Scholar] [CrossRef]
- Zocca, F.; Lomolino, G.; Lante, A. Dog rose and pomegranate extracts as agents to control enzymatic browning. Food Res. Int. 2011, 44, 957–963. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Azuma, K.; Ippoushi, K.; Ito, H.; Higashio, H.; Terao, J. Evaluation of antioxidative activity of vegetable extracts in linoleic acid emulsion and phospholipid bilayers. J. Sci. Food Agric. 1999, 79, 2010–2016. [Google Scholar] [CrossRef]
- Massini, L.; Rico, D.; Martin-Diana, A.B.; Barry-Ryan, C. Apple peel flavonoids as natural antioxidants for vegetable juice applications. Eur. Food Res. Technol. 2016, 242, 1459–1469. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of Total Content of Phenolic Compounds and Their Antioxidant Activity in Vegetables Evaluation of Spectrophotometric Methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
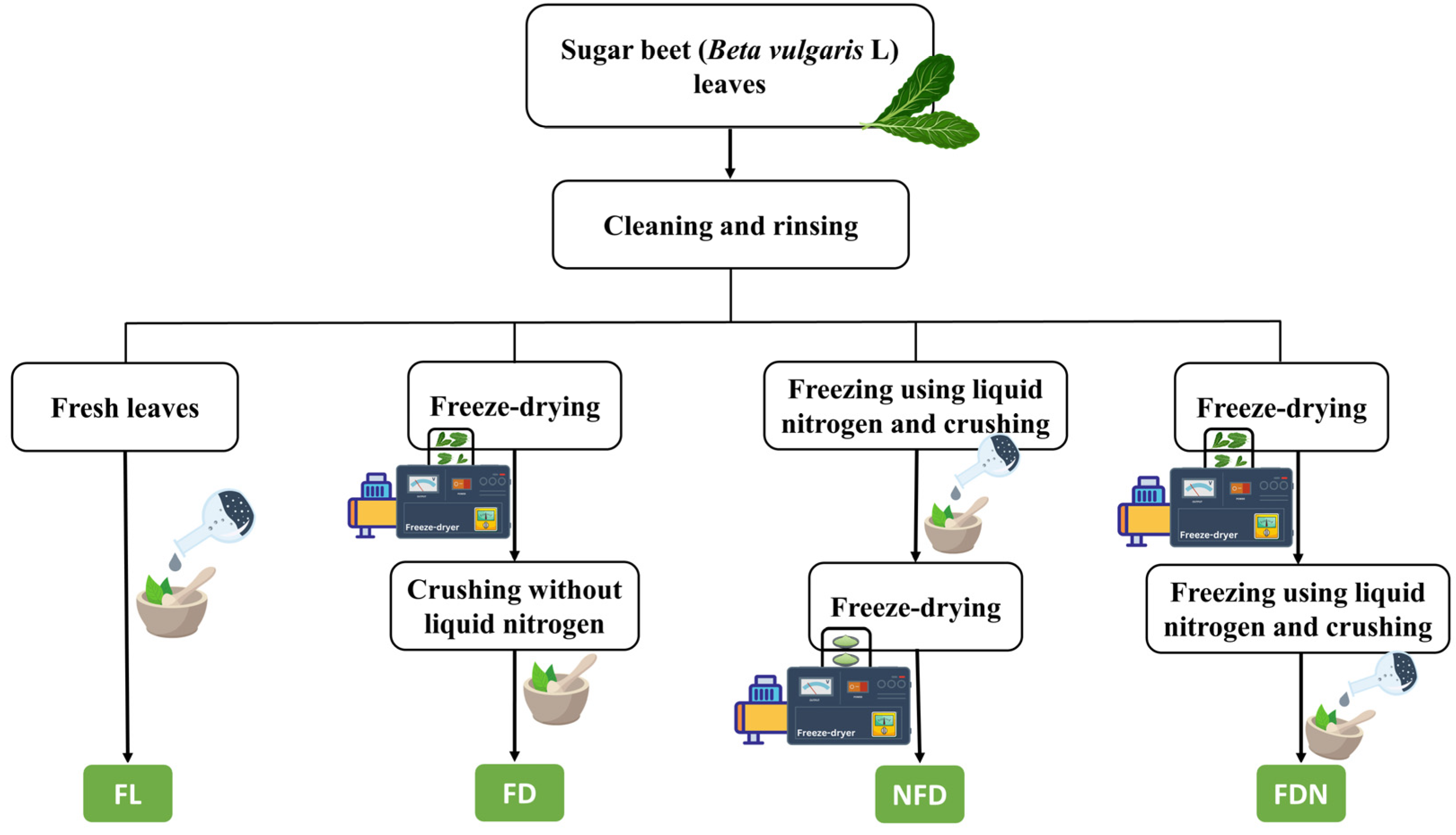


| Component | Type of Leaves | |||
|---|---|---|---|---|
| FL 1 | FD 3 | NFD 2 | FDN 4 | |
| Water activity | 0.98 ± 0.00 c | 0.10 ± 0.00 b | 0.08 ± 0.00 a | 0.08 ± 0.00 a |
| Moisture (%) | 82.42 ± 0.08 b | 9.26 ± 0.33 a | 8.63 ± 0.18 a | 8.95 ± 0.60 a |
| Dry matter (%) | 17.58 ± 0.08 a | 90.74 ± 0.33 b | 91.37 ± 0.18 b | 91.05 ± 0.64 b |
| Ash (g/100 g DM 5) | 10.23 ± 0.13 a | 10.49 ± 0.18 a | 10.08 ± 0.08 a | 10.23 ± 0.25 a |
| Crude protein (g/100 g DM) | 31.31 ± 0.05 d | 27.76 ± 0.07 a | 28.29 ± 0.08 b | 28.75 ± 0.05 c |
| Crude fiber (g/100 g DM) | 2.13 ± 0.03 a | 4.04 ± 0.04 c | 3.76 ± 0.04 b | 3.77 ± 0.08 b |
| Crude lipid (g/100 g DM) | 1.16 ± 0.05 bc | 1.08 ± 0.08 b | 1.30 ± 0.09 c | 0.46 ± 0.10 a |
| Amino Acids (% Total AAs) | Type of Leaves | |||
|---|---|---|---|---|
| FL | FD | NFD | FDN | |
| Histidine | 3.81 ± 0.16 a | 3.59 ± 0.00 a | 3.67 ± 0.01 a | 3.59 ± 0.08 a |
| Arginine | 5.45 ± 0.06 a | 5.54 ± 0.00 a | 5.43 ± 0.10 a | 5.44 ± 0.05 a |
| Serine | 5.63 ± 0.14 a | 5.62 ± 0.01 a | 5.80 ± 0.02 a | 5.71 ± 0.08 a |
| Glycine | 7.34 ± 0.37 a | 6.88 ± 0.01 ab | 6.71 ± 0.03 a | 6.73 ± 0.03 a |
| Aspartic acid | 9.89 ± 0.15 a | 10.24 ± 0.01 b | 10.86 ± 0.00 c | 10.79 ± 0.08 c |
| Glutamic acid | 14.32 ± 0.21 a | 14.49 ± 0.02 a | 15.92 ± 0.02 a | 14.50 ± 1.44 a |
| Threonine | 5.81 ± 0.54 b | 5.13 ± 0.01 ab | 4.98 ± 0.02 a | 5.23 ± 0.27 ab |
| Alanine | 5.57 ± 0.27 a | 5.82 ± 0.03 a | 5.73 ± 0.01 a | 5.86 ± 0.12 a |
| Proline | 4.85 ± 0.16 a | 4.77 ± 0.01 a | 4.66 ± 0.02 a | 4.70 ± 0.05 a |
| Lysine | 7.06 ± 0.46 a | 8.14 ± 0.01 b | 7.95 ± 0.01 b | 8.32 ± 0.38 b |
| Methionine | 1.29 ± 0.14 a | 1.38 ± 0.01 a | 1.28 ± 0.02 a | 1.23 ± 0.04 a |
| Tyrosine | 3.91 ± 0.19 b | 3.62 ± 0.01 a | 3.64 ± 0.01 a | 3.62 ± 0.01 a |
| Valine | 4.93 ± 0.19 ab | 5.12 ± 0.01 b | 4.64 ± 0.01 a | 4.91 ± 0.26 ab |
| Cysteine + Cystine | 1.54 ± 0.087 a | 1.44 ± 0.01 a | 1.39 ± 0.01 a | 1.46 ± 0.09 a |
| Isoleucine | 3.44 ± 0.29 ab | 3.78 ± 0.01 b | 3.26 ± 0.01 a | 3.54 ± 0.28 ab |
| Leucine | 7.88 ± 0.11 ab | 7.97 ± 0.00 b | 7.54 ± 0.01 a | 7.81 ± 0.27 ab |
| Phenylalanine | 5.09 ± 0.12 b | 4.91 ± 0.01 ab | 4.73 ± 0.01 a | 4.85 ± 0.13 a |
| Tryptophan | 2.19 ± 0.14 c | 1.54 ± 0.01 a | 1.82 ± 0.01 b | 1.70 ± 0.11 ab |
| Fatty Acids (% of Total FA Content) | FL | FD | NFD | FDN | |
|---|---|---|---|---|---|
| SFAs 2 | Caproic acid (6:0) | 0.37 ± 0.06 bc | ND 1 | 0.50 ± 0.10 c | 0.24 ± 0.03 b |
| Enanthic acid (7:0) | 0.18 ± 0.03 b | ND | ND | 0.01 ± 0.01 a | |
| Caprylic acid (8:0) | 0.09 ± 0.02 a | 0.34 ± 0.06 b | 2.72 ± 0.02 c | 0.32 ± 0.03 b | |
| Pelargonic acid (9:0) | 0.08 ± 0.02 b | ND | ND | 0.01 ± 0.01 a | |
| Capric acid (10:0) | 0.07 ± 0.04 a | 0.25 ± 0.04 b | 1.88 ± 0.04 c | 0.23 ± 0.02 b | |
| Lauric acid (12:0) | 0.11 ± 0.03 a | 0.29 ± 0.03 b | 1.41 ± 0.02 c | 0.38 ± 0.11 b | |
| Myristic acid (14:0) | 0.68 ± 0.03 b | 0.53 ± 0.05 a | 0.74 ± 0.06 b | 0.54 ± 0.04 a | |
| Pentadecanoic acid (15:0) | 0.34 ± 0.05 a | 0.24 ± 0.03 a | 0.27 ± 0.02 a | 0.27 ± 0.06 a | |
| Palmitic acid (16:0) | 27.87 ± 0.33 c | 15.97 ± 0.35 a | 15.83 ± 0.14 a | 18.40 ± 0.50 b | |
| 14-Methylhexadecanoic acid (17:0 anteiso) | 0.03 ± 0.01 a | 0.03 ± 0.02 a | ND | 0.01 ± 0.01 a | |
| Margaric acid (17:0) | 1.60 ± 0.07 c | 1.45 ± 0.04 b | 1.41 ± 0.02 b | 1.17 ± 0.05 a | |
| 16-Methylheptadecanoic acid (18:0 iso) | 0.02 ± 0.01 a | 0.04 ± 0.02 a | 0.02 ± 0.01 a | 0.03 ± 0.02 a | |
| Stearic acid (18:0) | 3.05 ± 0.09 c | 1.14 ± 0.09 b | 0.91 ± 0.04 a | 1.20 ± 0.02 b | |
| Arachidic acid (20:0) | 0.24 ± 0.03 a | 0.31 ± 0.16 a | 0.23 ± 0.03 a | 0.27 ± 0.09 a | |
| Heneicosylic acid (21:0) | 0.14 ± 0.02 b | 0.12 ± 0.03 b | 0.04 ± 0.01 a | 0.12 ± 0.03 b | |
| Behenic acid (22:0) | 0.69 ± 0.02 a | 0.61 ± 0.04 a | 0.57 ± 0.02 a | 0.66 ± 0.10 a | |
| Tricosanoic acid (23:0) | 0.34 ± 0.03 a | 0.23 ± 0.03 a | 0.23 ± 0.03 a | 0.30 ± 0.07 a | |
| Lignoceric acid (24:0) | 1.10 ± 0.10 a | 1.06 ± 0.02 a | 1.05 ± 0.01 a | 1.28 ± 0.27 a | |
| MUSFAs 3 | Myristoleic acid (14:1 cis-9) | 0.04 ± 0.01 b | ND | ND | 0.01 ± 0.01 a |
| trans-10-Pentadecenoic acid (15:1 trans-10) | 0.00 ± 0.01 a | ND | ND | 0.02 ± 0.03 a | |
| trans-6-Hexadecenoic acid (16:1 trans-6) | 0.10 ± 0.03 b | 0.02 ± 0.00 a | ND | 0.05 ± 0.04 ab | |
| cis-7-Hexadecenoic acid (16:1 cis-7) | 1.58 ± 0.08 b | 0.86 ± 0.04 a | 0.91 ± 0.02 a | 0.68 ± 0.16 a | |
| Palmitoleic acid (16:1 cis-9) | 3.47 ± 0.22 d | 1.09 ± 0.04 b | 0.53 ± 0.03 a | 2.35 ± 0.20 c | |
| cis-11-Hexadecenoic acid (16:1 cis-11) | 0.01 ± 0.02 a | ND | ND | ND | |
| cis-9-Heptadecenoic acid (17:1 cis-9) | 1.29 ± 0.03 b | 0.85 ± 0.02 a | 0.94 ± 0.04 a | 0.85 ± 0.15 a | |
| Elaidic acid (18:1 trans-9) | 0.10 ± 0.02 b | ND | ND | 0.01 ± 0.02 a | |
| Oleic acid (18:1 cis-9) | 25.34 ± 0.39 c | 8.77 ± 0.23 a | 6.60 ± 0.28 a | 14.60 ± 2.96 b | |
| Vaccenic acid (18:1 cis-11) | 1.69 ± 0.05 c | 0.67 ± 0.06 b | 0.38 ± 0.08 a | 0.69 ± 0.04 b | |
| cis-10-Nonadecenoic acid (19:1 cis-10) | 0.17 ± 0.03 a | 0.12 ± 0.01 a | 0.14 ± 0.01 a | 0.14 ± 0.03 a | |
| Gondoic acid (20:1 cis-11) | 0.28 ± 0.03 b | 0.19 ± 0.01 a | 0.20 ± 0.02 a | 0.28 ± 0.02 b | |
| Gerucic acid (22:1 cis-13) | 0.27 ± 0.02 b | 0.03 ± 0.02 a | ND | 0.06 ± 0.04 a | |
| Nervonic acid (24:1 cis-15) | 0.00 ± 0.01 a | ND | ND | 0.02 ± 0.03 a | |
| PUSFAs 4 | cis, cis-6,9-Hexadecadienoic acid (16:2 cis, cis-6,9) | 0.02 ± 0.01 a | 0.10 ± 0.02 bc | 0.12 ± 0.02 c | 0.06 ± 0.03 ab |
| All cis-7,10,13-Hexadecatrienoic acid (16:3 all cis-7,10,13) | 0.32 ± 0.04 a | 1.31 ± 0.02 b | 1.23 ± 0.05 b | 0.79 ± 0.55 ab | |
| trans, cis-8,13-Octadecadienoic acid (18:2 trans, cis-8,13) | 0.07 ± 0.03 b | 0.02 ± 0.02 a | ND | 0.02 ± 0.01 a | |
| Linoleic acid (18:2 cis, cis-9,12) | 15.15 ± 0.42 a | 19.30 ± 0.06 b | 22.86 ± 0.04 c | 19.82 ± 1.43 b | |
| γ-Linolenic (18:3 all cis-6,9,12) | 0.10 ± 0.02 a | 0.19 ± 0.04 b | 0.13 ± 0.02 ab | 0.11 ± 0.04 b | |
| Dihomo-γ-Linolenic acid (DGLA) (18:3 all cis-8,11,14) | 0.01 ± 0.01 a | 0.06 ± 0.02 b | 0.04 ± 0.01 ab | 0.02 ± 0.02 a | |
| α-Linolenic acid (18:3 all cis-9,12,15) | 12.70 ± 0.20 a | 43.65 ± 0.15 d | 37.97 ± 0.04 c | 33.70 ± 1.90 b | |
| Stearidonic acid (18:4 all cis-6,9,12,15) | 0.03 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | |
| cis, cis-11,14-Eicosadienoic acid (20:2 cis, cis-11,14) | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.04 ± 0.00 a | 0.10 ± 0.08 a | |
| All cis-8,11,14-Eicosatrienoic acid (20:3 all cis-8,11,14) | 0.03 ± 0.01 b | ND | ND | ND | |
| All cis-11,14,17-eicosatrienoic acid (20:3 all cis-11,14,17) | 0.02 ± 0.01 a | 0.04 ± 0.02 ab | 0.04 ± 0.01 ab | 0.05 ± 0.00 b | |
| Arachidonic acid (20:4 all cis 5,8,11,14) | 0.13 ± 0.02 b | 0.04 ± 0.00 a | 0.03 ± 0.01 a | 0.06 ± 0.02 a | |
| Cetoleic acid (22:2 cis-11) | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.02 ± 0.00 a | 0.03 ± 0.02 a | |
| Osbond acid (22:5 all cis-4,7,10,13,16) | ND | 0.01 ± 0.00 b | ND | 0.02 ± 0.00 c | |
| Cervonic acid (22:6 all cis-4,7,10,13,16,19) | 0.02 ± 0.01 b | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, P.; Mihaylova, D.; Marangon, C.M.; Grigoletto, L.; Lante, A. Impact of Sample Pretreatment and Extraction Methods on the Bioactive Compounds of Sugar Beet (Beta vulgaris L.) Leaves. Molecules 2022, 27, 8110. https://doi.org/10.3390/molecules27228110
Ebrahimi P, Mihaylova D, Marangon CM, Grigoletto L, Lante A. Impact of Sample Pretreatment and Extraction Methods on the Bioactive Compounds of Sugar Beet (Beta vulgaris L.) Leaves. Molecules. 2022; 27(22):8110. https://doi.org/10.3390/molecules27228110
Chicago/Turabian StyleEbrahimi, Peyman, Dasha Mihaylova, Christine Mayr Marangon, Luca Grigoletto, and Anna Lante. 2022. "Impact of Sample Pretreatment and Extraction Methods on the Bioactive Compounds of Sugar Beet (Beta vulgaris L.) Leaves" Molecules 27, no. 22: 8110. https://doi.org/10.3390/molecules27228110
APA StyleEbrahimi, P., Mihaylova, D., Marangon, C. M., Grigoletto, L., & Lante, A. (2022). Impact of Sample Pretreatment and Extraction Methods on the Bioactive Compounds of Sugar Beet (Beta vulgaris L.) Leaves. Molecules, 27(22), 8110. https://doi.org/10.3390/molecules27228110








