Abstract
Chemical components with anti-oxidant, anti-inflammatory, and anti-cancer properties extracted from Alnus bark and leaves have been extensively studied. However, less attention has been paid to extractives from Alnus pods, which are mostly treated as waste. Here, extractives of Alnus cremastogyne pods from 12 provenances in Sichuan Province were studied for high value-added utilization of Alnus waste. The extractives were analyzed by Gas Chromatography-Mass Spectrometer (GC-MS), Ultraviolet-visible spectroscopy (UV-Vis spectra), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity. A total of 58, 49, and 51 chemical components were found when the organic solvents of ethanol, petroleum ether, and ethyl acetate were used to collect extractives, respectively. These chemical components including Phytol, CIS-5,8,11,14,17-eicosapentaenoic acid, Germacrene D, Lupeol, and β-sitosterol, etc., have wide applications in the fields of pharmacy and cosmetics. Moreover, it was also found that extractives in ethanol and ethyl acetate had impressive UV resistance, especially for UV-C and UV-B blocking. The results showed that the maximum block ratio towards UV-C and UV-B could reach 99%. In addition, the ethanol extract showed good anti-oxidant activity with a maximum free radical scavenging rate of 96.19%. This comprehensive and systematic study on extractives from Alnus cremastogyne pods promotes the development of high-value utilization of Alnus components.
1. Introduction
As a biomass material, wood plays a very important role in human life and production. Its extractives, mainly including aliphatic compounds, terpenoid, sterpenoids, and phenolic compounds, have a strong impact on the color, durability, processing, and utilization of wood [1]. This is because wood extractives can not only provide guidance on wood classification and identification but also have a wide range of applications in the fields of medicine, food, and cosmetics, etc. [2]. Alnus genus belongs to Betulaceae Gray and widely grows in Asia, Africa, Europe, and North America, with more than 40 species. Alnus cremastogyne is an endemic species to China, grown in most areas of China such as Northeast, North, East, South, Central, and Southwest China [3]. Because Alnus cremastogyne has excellent adaptability, strong wind resistance, and smoke resistance, it is an important tree species for afforestation in many places and has high ecological value. At the same time, because of its good texture and water resistance, it can be processed as plywood, paper, musical instruments, furniture, and other materials [4,5], and has a high economic value. The bark and fruit are rich in tannins and can be used as dyes and pharmaceuticals. The leaf can be used as green fodder.
Moreover, the bark, branches, and leaves of Alnus can be used as a traditional medicine to treat fever, bleeding, burns, diarrhea, and alcoholism [6,7]. Chemical components of Alnus bark, branches, and leaves have been analyzed through extraction technology, and Diarylheptanoids, polyphenols, terpenoids, and steroids have been found [8,9,10,11]. Diarylheptanoids, as the main components of Alnus hirsuta, possess excellent anti-cancer and anti-oxidant properties [12]. Diarylheptanoids extracted from the bark of Alnus glutinosa can protect healthy cells from doxorubicin injury, and thus can be used as a protective agent for non-cancerous dividing cells during chemotherapy [13]. D. Kremer studied the reducing activity, DPPH radical scavenging activity, and B-carotene-linoleic acid anti-oxidant activity of F. Rupestris bark and F. Alnus bark. The results showed that they had good anti-oxidant and antimicrobial activities, and could be used as natural anti-oxidants, antibacterial agents, functional foods, and medicines [14]. Haoxin Li and his group found that betulin and betulinic acid, which are found in Alnus Incana, have good antimycobacterial activity [15]. Sun Eun Choi believed that the leaf and bark extractives of Alnus japonica may have some efficacy in the treatment of allergic skin diseases such as atopic dermatitis [16]. This work made significant developments in the understanding and utilization of chemical components of Alnus. Alnus is widely grown in China with high seed and pod yields, and it also has a high extractives content. However, the present research mainly focuses on Alnus bark, branches, and leaves. Alnus pods with abundant resources are usually treated as waste and the study and utilization of their extractives is paid less attention. A large number of Alnus pods are post-treated by landfill, incineration, or directly discarded, resulting in a great waste of resources and environmental pollution.
Here, this work thoroughly studied the extractives of Alnus cremastogyne pods from 12 different provenances using ethanol, petroleum ether, and ethyl acetate organic solvents. Their chemical components, anti-UV properties, and anti-oxidant properties were analyzed by Gas Chromatography-Mass Spectrometer (GC-MS), Ultraviolet–visible spectroscopy (UV-Vis spectra), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity. We hoped to be able to analyze the chemical components of Alnus cremastogyne pods extractives as comprehensively as possible, and find the parts that could be used in production and utilization, so that Alnus cremastogyne could be fully applied to reduce waste. In addition, through the study of Alnus cremastogyne pod extractives in different provenances, the Alnus cremastogyne pod extractives and the provenances’ climate, soil, and other site conditions were linked to provide a theoretical basis for the directional cultivation of Alnus cremastogyne. This study provides a comprehensive and systematic understanding of the chemical components and utilization of Alnus cremastogyne pods, and is believed to promote the development of high-value and all-components utilization of Alnus.
The pods of Alnus cremastogyne in this study were collected from 12 districts and counties in Sichuan Province, as shown in Table 1 and Figure 1.

Table 1.
Information on different Alnus cremastogyne collection sites.
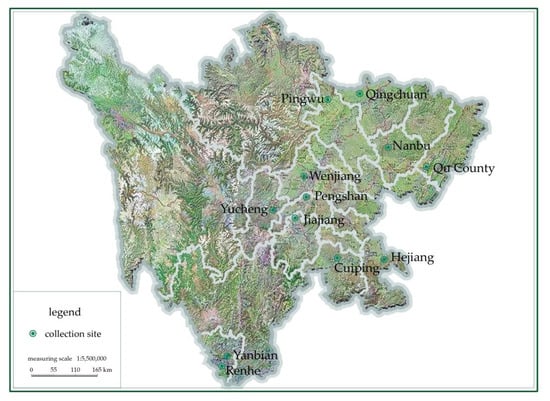
Figure 1.
The map of different Alnus cremastogyne collection sites.
2. Materials and Methods
2.1. Materials
In this study, Alnus cremastogyne pods from 12 different provenances were washed with deionized water and air-dried. The dried Alnus cremastogyne pods were broken into powder for extraction. Ethanol (≥99.9%), petroleum ether (60–90 °C), and ethyl acetate (≥99.8%) were all chromatographically pure and purchased from Knowles Company. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Duly Biotechnology Company. Vitamin C was purchased from Sinopharm Chemical Reagent Company.
2.2. Organic Solvent Extraction
Five grams (accurate to 0.001 g) of completely dried Alnus cremastogyne pod samples were accurately weighed, wrapped in filter paper, and placed in a 250 mL Soxhlet extractor. Ethanol/petroleum ether/ethyl acetate extraction solvent was added into a 250 mL round-bottomed flask for 4 h. After cooling to room temperature, the extractives were concentrated with a rotary evaporator for use.
2.3. Gas Chromatograph Mass Spectrometer (GC-MS)
GC-MS analysis was performed on an 8890A gas chromatograph (Agilent, Santa Clara, CA, USA) equipped with a 5977C and mass spectrometer (Agilent, Santa Clara, CA, USA). A fused silica capillary Agilent Technology HP-5ms (5% phenyl methyl siloxane) column (30 m × 0.25 mm i.d., film thickness 0.1 μm) was used for the separation.
Analysis conditions of ethanol and ethyl acetate extractives volatile components: the injector temperature was set at 230 °C, and the detector temperature was set at 280 °C. In the first phase, the initial temperature was kept at 50 °C for 5 min, and the temperature was gradually increased to 125 °C at a rate of 3 °C/min and was then held for 3 min at 125 °C. In the second phase, the temperature was gradually increased to 180 °C at a rate of 2 °C/min and was then held for 3 min at 180 °C. In the third phase, the temperature was gradually increased to 250 °C at a rate of 15 °C/min and was then held for 8 min at 250 °C. The linear velocity of the helium carrier gas was 1 mL/min at a split ratio of 10:1. EI was used as the ion source, which was set at 200 °C. The sector mass analyzer was set to scan from 35 to 450 amu, and the scan time was set at 1 s. Extracting solution with a volume of 1.0 μL was injected for analysis.
Analysis conditions of petroleum ether extractives volatile components: the injector temperature was set at 250 °C, and the detector temperature was set at 280 °C. The initial temperature was kept at 50 °C for 4 min, and the temperature was gradually increased to 290 °C at a rate of 4 °C/min and was then held for 20 min at 290 °C. The linear velocity of the helium carrier gas was 1 mL/min at a split ratio of 10:1. EI was used as the ion source, which was set at 200 °C. The sector mass analyzer was set to scan from 10 to 600 amu, and the scan time was set at 1 s. Extracting solution with a volume of 1.0 μL was injected for analysis.
The components were identified by matching their recorded mass spectra with the standard mass spectra from the National Institute of Standards and Technology (NIST05.LIB) libraries data provided by the software of the GC-MS system, literature data, and standards of the main components.
2.4. Ultraviolet–Visible Spectroscopy (UV-Vis Spectra)
The optical properties of extracting solutions of ethanol, petroleum ether, and ethyl acetate of Alnus cremastogyne pods were measured using a UV-Vis spectrophotometer (UNICO UV-4802H) in the wavelength range 190–800 nm.
2.5. Radical DPPH Scavenging Activity
In this study, DPPH free radical scavenging ability was used to determine anti-oxidant capacity according to the method of Brand-Williams [17] and Sultana [18], with slight modification. The extractives were dissolved in methanol, and the sample concentration was 0.005–8 mg/mL, and the DPPH concentration was 0.04 mg/mL. Methanol was used as a blank control. The following prepared solution was reacted in the shade of room temperature for 30 min, and the absorbance was measured at 517 nm. A total of 2 mL of different concentrations of solution was added to 2 mL of DPPH solution, and the absorbance was determined as Ai. A total of 2 mL of different concentrations of solution was added to 2 mL of methanol solution, and the absorbance was determined as Aj. A total of 2 mL of methanol solution was added to 2 mL of DPPH solution, and the absorbance was determined as Ac. The DPPH free radical scavenging ability SA of the sample was calculated according to the following equation:
SA(%) = [1 − (Ai − Aj)/Ac] × 100%
The IC50 value is the effective concentration that 50% of the DPPH radical scavenged by the sample. For the measurement of the IC50, analysis of regression was performed to obtain the relationship between the concentration and the DPPH scavenging rate. The regression equation was calculated and the IC50 value obtained.
3. Results and Discussion
3.1. 100-Grain Weight and Extractives Yield
Table 2 shows the 100-grain weight of Alnus cremastogyne pods from 12 different provenances. It can be found that the 100-grain weight ranged from 14.15 to 49.92 g. The sample A and sample D pods were heavy with the 100-grain weight of 49.92 g and 39.26 g, while the sample L and sample E pods were light with 100-grain weights of 14.15 g and 14.45 g. According to Table 2 combined with Figure 1, there were significant differences in the 100-grain weight of pods under different site conditions. This is because sample A and sample D pods were from Ya’an and Chengdu Plain with sufficient rainfall and good soil and climatic conditions. Sample L and sample E pods were from western and southeastern Sichuan with poor soil and water conditions. The 100-grain weight of Alnus cremastogyne pods was affected by the soil and climate conditions. The pods in Chengdu Plain and the low hilly areas nearby were larger and heavier, while the pods in the high-mountain and hilly areas in the southeast and west of Sichuan near Yunnan-Guizhou Plateau were relatively small due to the poor soil moisture and fertilizer conditions.

Table 2.
100-grain weight and extractives yield of the Alnus cremastogyne pod extractives.
The extractives yield of different samples extracted from ethanol, petroleum ether, and ethyl acetate are also presented in Table 2. The order of yield of different solvent extractives was that the yield of ethanol extractives was higher than that of ethyl acetate extractives and petroleum ether extractives, which was consistent with the order of polarity of the three solvents. The higher the polarity, the better the extraction effect. The extractives yield of ethyl acetate was from 5.20% to 17.4%, which was the highest among these three organic solvents. The extractives yield of petroleum ether and ethanol was 3.20% to 10.4%, and 5.20% to 16.8%, respectively. As for different provenances, the sample K pods had the highest extractives yield of 16.80% and 17.40% in ethanol and ethyl acetate, while the sample I pods had the highest extractives yield of 10.4% in petroleum ether. As shown in Table 2, there were also significant differences in extract yield under different site conditions. Contrary to the 100-grain weight, the extractives yield was relatively low in the regions with superior water and fertilizer conditions, because the Alnus cremastogyne pods grew faster and the chemical components accumulated less.
3.2. GC-MS Analysis
The chemical components of the Alnus cremastogyne pod extractives’ volatile components were identified by GC-MS. Figure 2, Figure 3 and Figure 4 show the total ion chromatograms of the volatile components of different extractives. In the chromatogram of ethanol extractives, an obvious Eicosane characteristic peak appeared at the retention time of 74.3 min (Figure 2), and in the chromatogram of petroleum ether extractives, the characteristic peak of Lup-20(29)-en-3-one appeared at the retention time of 67.2 min (Figure 3). Around 29.9 min, the characteristic peak of Isoledene appeared in the ethyl acetate extractives (Figure 4). The above characteristic peaks can be used as a reference for Alnus cremastogyne identification.
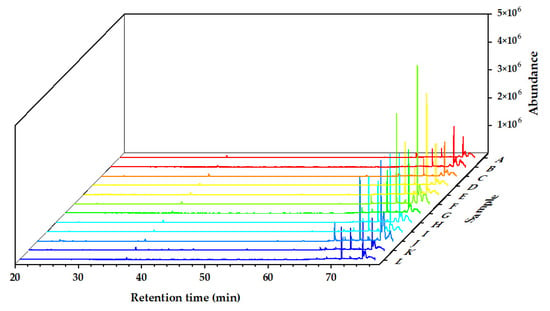
Figure 2.
GC-MS chromatograms of the volatile components of the ethanol extractives of Alnus cremastogyne pods.
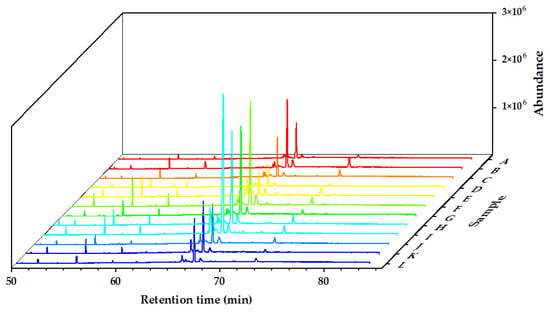
Figure 3.
GC-MS chromatograms of the volatile components of the petroleum ether extractives of Alnus cremastogyne pods.
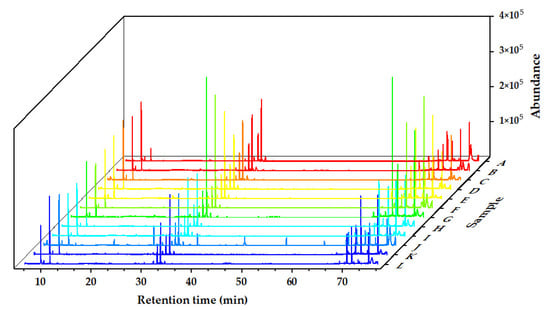
Figure 4.
GC-MS chromatograms of the volatile components of the ethyl acetate extractives of Alnus cremastogyne pods.
The identified chemical components are shown in Table 3, Table 4 and Table 5. A total of 59, 49, and 51 chemical components were identified around when ethanol, petroleum ether, and ethyl acetate were used as the organic solvent, respectively. Table 3, Table 4 and Table 5 show that the main chemical components of the three solvent extractives of Alnus cremastogyne pods were alkenes, ketones, carboxylates, ethers, benzenes, esters, and a small number of halides and complex compounds. Table 3 shows that the ethanol extractives were mainly chemical components of Phenol, 3,5-bis(1,1-dimethylethyl)-, 1R,4S,7S,11R-2,2,4,8-Tetramethyltricyclo[5.3.1.0(4,11)] undec-8-ene, Nonadecane, 6β-Bicyclo[4.3.0] nonane, 5β-iodomethyl-1β-isopropenyl-4α.,5α.-dimethyl-, Octadecane, and Eicosane. As shown in Table 4, for petroleum ether extractives, the main chemical components were Phenol, 2,2’-methylenebis[6-(1,1-dimethylethyl)-4-methyl-, Undecane, 3,8-dimethyl-, D-Friedoolean-14-en-3-one, A’-Neogammacer-22(29)-en-3-one, 13,27-Cycloursan-3-one, γ-Sitosterol, Lup-20(29)-en-3-one, Lupeol, and Eicosane, 1-iodo-. As for ethyl acetate extractives (Table 5), the main constituents were Bicyclo[3.1.0]hex-2-ene, 4-methyl-1-(1-methylethyl)-, Camphene, Ethanol, 1,1’-oxybis-, diacetate, α-Cubebene, 1,3-Cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]-, (1R,5R)-2-Methyl-5-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hex-2-ene, β-Gurjunene, isoledene, (E)-β-Famesene, Phytol, Eicosane, 1-iodo-, Phenol, 2,2’-methylenebis[6-(1,1-dimethylethyl)-4-methyl-, Bicyclo[3.1.1]heptane, 6,6-dimethyl-2-methylene-, (1S)-, cis-Muurola-4(15),5-diene, cis-muurola-3,5-diene, Di-n-decylsulfone, Eicosane, 7-hexyl-, Undecane, 3,8-dimethyl-, and A’-Neogammacer-22(29)-en-3-one. Many of these chemical components have been studied and utilized in the pharmaceutical, cosmetic, and food industries.

Table 3.
Chemical compositions of the ethanol extractives of Alnus cremastogyne pods.

Table 4.
Chemical compositions of the petroleum ether extractives of Alnus cremastogyne pods.

Table 5.
Chemical compositions of the petroleum ether extractives of Alnus cremastogyne pods.
In the ethanol extractives, Phytol has anti-injury, anti-oxidant, anti-inflammatory and anti-allergic effects. It also has antibacterial activity against Mycobacterium tuberculosis and Staphylococcus aureus [19,20]. Cis-5,8,11,14,17-eicosapentaenoic acid can reduce blood viscosity, reduce thrombosis, improve serum, and reduce blood sugar, and also has cerebrovascular protection, anti-allergy, and anti-cancer effects [21]. Picrotoxinin is a potent convulsant, and a noncompetitive GABAA receptor antagonist that negatively modulates the effects of GABA on GABAA receptors [22]. Germacrene D as a precursor plays an important role in sesquiterpene biosynthesis [23]. Caryophyllene oxide in clinical trials shows good central and peripheral analgesic and anti-inflammatory activity, and it can be used for the treatment of onychomycosis. It also has antifungal properties and is widely used as a preservative in cosmetics and daily necessities [24,25]. Other chemical components such as Butanoic acid, 2-methyl-, Decanoic acid, ethyl ester, Citronellyl isobutyrate, Nonanoic acid, 5-methyl-, ethyl ester, 2H-Pyran-2-One, Tetrahydro-6-nonyl-, and so on, can be used as raw materials as a configuration of a variety of fruit-based flavors, added to a variety of common food and drinks.
In petroleum ether extractives, existing studies have found that Squalene is effective on hypoxia resistance and thus can be widely used in the domestic market. Because Squalene possesses a stronger anti-oxidant capacity compared with other lipid molecules in the skin, it can help the skin resist damage caused by UV irradiation and other oxidation reactions. It has also been reported that Squalene has moisturizing properties, and it is widely used in creams, lotions, hair creams, lipsticks, and other cosmetics [26,27,28]. Lupeol has anti-oxidant, anti-inflammatory, and skin-healing properties. It also has an inhibitory effect on breast cancer, prostate cancer, and melanoma [29,30]. β-sitosterol can reduce cholesterol, act as an anti-diabetic cough expectorant, inhibit tumors, and repair tissue [31,32]. Octacosanal has obvious biological activities on both humans and animals. It has physiological functions such as fatigue, metabolism promotion, anti-atherosclerosis, and prevention and treatment of Parkinson’s disease and Alzheimer’s disease. It is widely used in medicine, food, cosmetics, feed, and other fields [33,34,35]. Betulinaldehyde and Betulin also have anti-tumor, anti-inflammatory, and anti-allergic effects, which can be used in content determination, identification, and pharmacological experiments [36,37]. In addition to the pharmaceutical effects of the above-mentioned ingredients, 1H-tetrazol-5-amine, Nonadecane, Pentacosane, Hexadecane, and 3, 5-dimethoxycinnamic acid can be used as raw materials for chemical analysis and pharmaceutical synthesis.
In the ethyl acetate extractives, divided by the mentioned Germacrene D, Phytol, and other chemical components, Camphene with good anti-oxidant and cholesterol-reducing properties, can also be used as a raw material for the synthesis of camphor, and spices [38,39]. 1-Adamantanemethylamine, α-methyl- has a good therapeutic and inhibitory effect on influenza A, and can be used in the manufacture of various kinds of levofloxacin capsules, tablets, and other anti-microbial preparations [40,41,42]. γ-sitosterol has anti-hyperglycemia and lipid-lowering effects and can be used as an effective lipid-lowering agent for the treatment of hyperglycemia [43,44]. Cyclobarbital is a type of barbiturate derivative that has sedative and hypnotic effects and can be used as a surgical anesthetic [45].
3.3. UV Absorbability
Table 3, Table 4 and Table 5 show that the main chemical components of the three solvent extractives of Alnus cremastogyne pods are olefin, ketones, carboxylates, ethers, benzenes, esters, and a small number of halides and complex compounds. Most of these substances have good UV absorption capacity, especially olefin, ketones, and benzene compounds. As shown in Figure 5, Figure 6 and Figure 7, in the visible spectrum, the 12 samples in near 665 nm wavelength visible light peak, it may be because the extraction process also extracts the chlorophyll A components from the Alnus cremastogyne pods. The peak values of the ethyl acetate and ethanol extracting solutions were lower than those of the petroleum ether extracting solution, which may be due to their greater polarity and better extraction effect. In the UV light band, the absorbability of the three solvent control samples in the UV-A (315–400 nm) and UV-B (280–315 nm) bands was lower than 20% and increased in the UV-C (230–280 nm) and below bands. In general, the three solvents had poor UV absorbability, but three kinds of solvent extracting solution of 12 samples showed good UV absorbability, especially the UV-C (230–280 nm) and UV-B (280–315 nm) bands. Three kinds of solvent extracting solution ultraviolet transmittance were all less than 50%, the ethanol extracting solution in the UV-B (280–315 nm) band absorbability was greater than 96%, and the ethyl acetate extracting solution absorbability was more than 94%. The absorbability of ethanol and ethyl acetate was more than 99% in the UV-C (230–280 nm) band. In the UV-A (315–400 nm) band, the ethanol extracting solution has the best absorbability, which absorbed almost more than 50%–85% of the UV-A (315–400 nm) band, followed by the ethyl acetate extracting solution, which absorbed almost more than 45%–80% of the UV-A (315–400 nm) band. The petroleum ether extracting solution had poor absorbability in the UV-A (315–400 nm) band. It only absorbed 10%–30% around the wavelength of 385 nm, which may have been related to the composition and content of the extractives that absorb UV. For example, the benzene substances in the absorbability of ethanol and ethyl acetate were significantly more than those of petroleum ether, while the ethanol extractives were more than that of ethyl acetate extractives. In addition, the low UV absorbability in the UV-A (315–400 nm) band may have been caused by the presence of conjugated bonds in the chemical components, which made the UV absorption peak of -S=O, -S-O, C6H6, COO-, -O-, C=O, -Coor and other chromogenic functional groups in the compound red-shift, thus reducing the UV absorbability of the solution in this band. These studies showed that the ethanol and ethyl acetate extractives of Alnus cremastogyne pods had good UV absorbability, which is valuable for research, and they could be used in strong UV protection products after separation and purification.
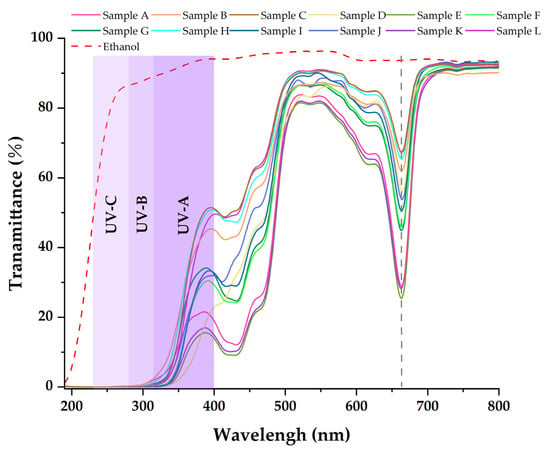
Figure 5.
Transmittance spectra of the ethanol extractives from Alnus cremastogyne pods.
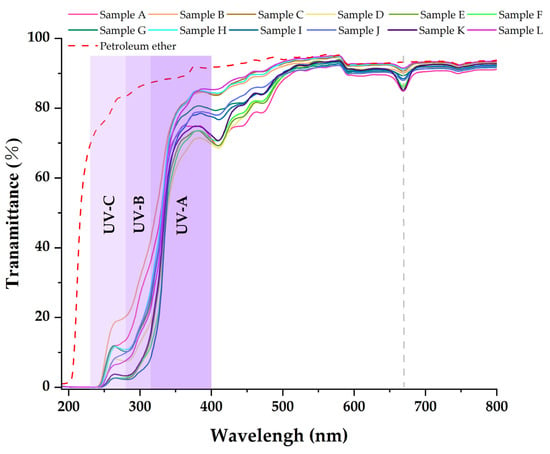
Figure 6.
Transmittance spectra of the petroleum ether extractives from Alnus cremastogyne pods.
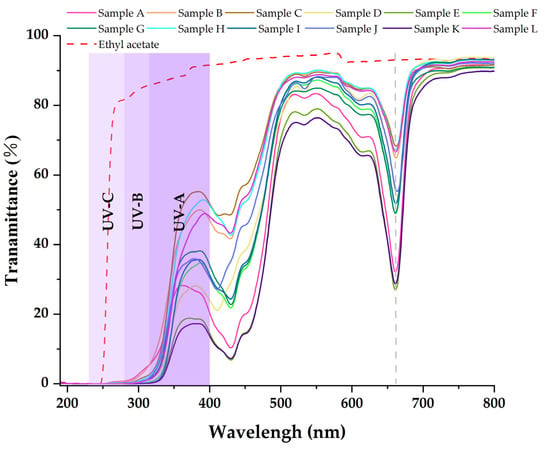
Figure 7.
Transmittance spectra of the ethyl acetate extractives from Alnus cremastogyne pods.
3.4. Anti-Oxidant Activity
The anti-oxidant activity was evaluated by measuring the scavenging ability of the Alnus cremastogyne pod extractives against DPPH free radicals. As Table 6 shows, the scavenging ability of the extracting solution against DPPH radicals increased with the increase of the concentration of the extracting solution. By calculating the concentration of extracting solution when the DPPH free radical scavenging rate of extractives was 50% (IC50), the anti-oxidant activity of Alnus cremastogyne pods extractives from different provenances of three solvents was compared. As Table 6 shows, when the DPPH free radical scavenging rate of ethanol extracting was 50%, the concentration of the extracting solution was the lowest (0.076 mg/mL–1.992 mg/mL), and the anti-oxidant activity was the best. When the concentration of petroleum ether extracting solution was the highest (1.518 mg/mL–5.265 mg/mL), the anti-oxidant activity was the worst. The IC50 value of ethyl acetate was 0.349 mg/mL to 3.283 mg/mL. This is related to the amount of -OH ions in the compound.

Table 6.
Radical scavenging capacity of DPPH of ethanol, petroleum ether, and ethyl acetate extractives from Alnus cremastogyne pods.
In extractives of Alnus cremastogyne pods from 12 different provenances. The IC50 values of ethanol, petroleum ether, and ethyl acetate extractives of sample D and sample J were lower than those of the other 10 provenances, indicating that they had better anti-oxidant activity. The IC50 values of the ethanolic extractives of sample D and sample J were only 0.076 mg/mL and 0.184 mg/mL, and the DPPH free radical scavenging rates of 0.5mg/mL ethanolic extractives reached 95.79% and 90.35%; DPPH free radical scavenging rate can be up to 95.91% and 96.19%. It has a high anti-oxidant activity that is higher than tea extractives [46], Essential Oil [47], Oilseeds [48], and other plants and foods. The IC50 value of sample D is more than 30 times higher than that of Vitamin C, but it may have a high anti-oxidant potential for further isolation and purification in later studies. These studies indicate that the anti-oxidant activity of Alnus cremastogyne pod extractives is valuable to be studied and utilized. Alnus cremastogyne pods can also be used as a raw material, along with Alnus cremastogyne bark, branches, and leaves, to extract the active ingredients of anti-inflammatory and anti-cancer drugs.
4. Conclusions
The results show that the volume and weight of Alnus cremastogyne pods are closely related to the site conditions. The pods are larger and heavier in areas with superior climate and soil conditions, and smaller in areas near the plateau due to poor soil and water conditions. The same solvent extractives’ yield rules are different, which may be the pods’ growth speed and less chemical accumulation. This study on Alnus cremastogyne pod extractives can be used to guide the directed cultivation of Alnus cremastogyne.
A total of 59, 49, 51 chemical components were found when the organic solvents of ethanol, petroleum ether, and ethyl acetate were used to collect extractives. Many chemical components have anti-oxidant, anti-inflammatory, and other effects that can be used in pharmaceuticals, cosmetics, and other fields. The ethanol extractives of Alnus cremastogyne pods show good performance in UV absorbability and anti-oxidant activity analysis and can be further separated and purified as natural raw materials for anti-UV and anti-oxidant products. The ethanol extractives have higher relative content, the most types of chemical components, the best ability of UV absorption and anti-oxidant activity, and have the value of development and utilization. In the future, through the directional cultivation of Alnus cremastogyne, its pod extractives have great application potential in the fields of biomedicine, fine chemical intermediates, light industry and food, health care products, and cosmetics.
Author Contributions
Conceptualization, Y.J., J.Q. and S.J.; Data curation, Y.G. and X.Q.; Formal analysis, F.P., Y.G. and Y.J.; Funding acquisition, S.J.; Investigation, G.C.; Methodology, Y.J., J.X. and S.J.; Project administration, S.J.; Resources, J.X. and S.J.; Software, H.L. and J.Q.; Supervision, Y.J. and S.J.; Validation, G.C.; Visualization, F.P. and H.L.; Writing—original draft, G.C., F.P. and Y.J.; Writing—review & editing, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Provincial Tianfu Emei Young Talents Project (031/2212199007), China Postdoctoral Science Foundation (2022M712287), Natural Science Foundation of Sichuan Province (2022NSFSC1034).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Financial support from Sichuan Provincial Tianfu Emei Young Talents Project, China Postdoctoral Science Foundation and Natural Science Foundation of Sichuan Province is gratefully acknowledged. Also, sincere thanks to the equipment of Forestry College of Sichuan Agricultural University.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Kirker, G.T.; Blodgett, A.B.; Arango, R.A.; Lebow, P.K.; Clausen, C.A. The role of extractives in naturally durable wood species. Int. Biodeteri. Biodegr. 2013, 82, 53–58. [Google Scholar] [CrossRef]
- Schultz, T.P.; Nicholas, D.D. Naturally durable heartwood: Evidence for a proposed dual defensive function of the extractives. Phytochemistry 2000, 54, 47–52. [Google Scholar] [CrossRef]
- Flora Reipublicae Popularis Sinicae. Available online: http://www.iplant.cn/info/Alnus (accessed on 10 November 2022).
- Bühlmann, T.; Caprez, R.; Hiltbrunner, E.; Körner, C.; Niklaus, P.A. Nitrogen fixation by Alnus species boosts soil nitrous oxide emissions. Eur. J. Soil Sci. 2017, 68, 740–748. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.; Zhang, Y.; Mou, Q.; Gou, Y.; Liu, K.; Huang, N.; Ouyang, C.; Hu, J.; Du, B. Simulation of potential suitable distribution of Alnus cremastogyne Burk. In China under climate change scenarios. Ecol. Indic. 2021, 133, 108396. [Google Scholar] [CrossRef]
- Sati, S.C.; Sati, N.; Sati, O.P. Bioactive constituents and medicinal importance of genus Alnus. Pharm. Rev. 2011, 5, 174–183. [Google Scholar] [CrossRef]
- Park, D.; Kim, H.J.; Jung, S.Y.; Yook, C.S.; Jin, C.; Lee, Y.S. A new diarylheptanoid glycoside from the stem bark of Alnus hirsuta and protective effects of diarylheptanoid derivatives in human HepG2 cells. Chem. Pharm. Bull. 2010, 58, 238–241. [Google Scholar] [CrossRef]
- Ren, X.; He, T.; Chang, Y.; Zhao, Y.; Chen, X.; Bai, S.; Wang, L.; Shen, M.; She, G. The genus Alnus, a comprehensive outline of Its chemical constituents and biological activities. Molecules 2017, 22, 1383. [Google Scholar] [CrossRef]
- Lee, M.; Tanaka, T.; Nonaka, G.; Nishioka, I. Dimeric ellagitannins from Alnus japonica. Phytochemistry 1992, 31, 2835–2839. [Google Scholar] [CrossRef]
- Wollenweber, E. Flavonoids from Alnus crispa, A. Japonica, A. Koehnei and A. Sinuata. Phytochemistry 1974, 13, 2318–2319. [Google Scholar] [CrossRef]
- Phan, M.G.; Truong, T.T.C.; Phan, T.S. Katsuyoshi Matsunami, Hideaki Otsuka, Mangiferonic acid, 22-hydroxyhopan-3-one, and physcion as specific chemical markers for Alnus nepalensis. Biochem. Syst. Ecol. 2010, 38, 1065–1068. [Google Scholar] [CrossRef]
- Hu, W.; Wang, M. Antioxidative activity and anti-inflammatory effects of diarylheptanoids isolated from Alnus hirsute. J. Wood Sci. 2011, 57, 323–330. [Google Scholar] [CrossRef]
- Dinić, J.; Ranđelović, T.; Stanković, T.; Dragoj, M.; Isaković, A.; Novaković, M.; Pešić, M. Chemo-protective and regenerative effects of diarylheptanoids from the bark of black alder (Alnus glutinosa) in human normal keratinocytes. Fitoterapia 2015, 105, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Kosalec, I.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Končića, M.Z. Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. Bark. Food Chem. 2012, 131, 1174–1180. [Google Scholar] [CrossRef]
- Li, H.; Webster, D.; Johnson, J.A.; Gray, C.A. Anti-mycobacterial triterpenes from the Canadian medicinal plant Alnus incana. J. Ethnopharmacol. 2015, 165, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Park, K.H.; Jeong, M.S.; Kim, H.H.; Lee, D.I.; Joo, S.S.; Lee, C.S.; Bang, H.; Choi, Y.W.; Lee, M.K.; et al. Effect of Alnus japonica extract on a model of atopic dermatitis in NC/Nga mice. J. Ethnopharmacol. 2011, 136, 406–413. [Google Scholar] [CrossRef]
- Williams, W.B.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Usman, M.A.; Usman, F.I.; Abubakar, M.S.; Salman, A.A.; Adamu, A.; Ibrahima, M.A. Phytol suppresses parasitemia and ameliorates anaemia and oxidative brain damage in mice infected with Plasmodium berghei. Exp. Parasitol. 2021, 224, 108097. [Google Scholar] [CrossRef]
- Shariare, M.H.; Noor, H.B.; Khan, J.H.; Uddin, J.; Ahamad, S.R.; Altamimi, M.A.; Alanazi, F.K.; Kazi, M. Liposomal drug delivery of Corchorus olitorius leaf extract containing phytol using design of experiment (DoE): In-vitro anticancer and in-vivo anti-inflammatory studies. Colloid. Surfaces B 2021, 199, 111543. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Salmon, J.A.; Ubatuba, F.B.; Weatherly, B.C.; Moncada, S.; Vane, J.R. Effects of all CIS-5,8,11,14,17 eicosapentaenoic acid and PGH3 on platelet aggregation. Prostaglandins 1979, 18, 453–478. [Google Scholar] [CrossRef]
- Smart, T.G.; Constanti, A. Studies on the mechanism of action of picrotoxinin and other convulsants at the crustacean muscle GABA receptor. P. Roy. Soc. B-Biol. Sci. 1986, 227, 91–216. [Google Scholar] [CrossRef]
- Bülow, N.; König, W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2000, 55, 141–168. [Google Scholar] [CrossRef]
- Yang, D.; Michel, L.; Chaumont, J.P.; Clerc, J.M. Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathologia 2001, 148, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.H.S.; Anandan, R.; Kumar, S.H.S.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar] [CrossRef]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Phenolic compounds and squalene in olive oils: The concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignansand squalene. Food Chem. Toxicol. 2000, 38, 647–659. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, Y.; Fang, J. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef]
- Geetha, T.; Varalakshmi, P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J. Ethnopharmacol. 2001, 76, 77–80. [Google Scholar] [CrossRef]
- Grundy, S.M.; Jr, E.H.A.; Salen, G. Dietary β-sitosterol as an internal standard to correct for cholesterol losses in sterol balance studies. J. Lipid Res. 1968, 9, 374–387. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cureton, T.K. The Physical Effect of Wheat Germ Oil on Human in Exercise, Springfield Illinois; Thomas Publishes: New York, NY, USA, 1976. [Google Scholar]
- Kato, S.; Karino, K.; Hasegawa, S.; Nagasawa, J.; Nagasaki, A.; Eguchi, M.; Ichinose, T.; Tago, K.; Okumori, H.; Hamatani, K. Octacosanol affects lipid metabolism in rats fed on a high-fat diet. Br. J. Nutr. 1995, 73, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Lin, Q.; Li, X.; Nie, Y.; Wang, L.; Shi, L.; Xu, W.; Hu, T.; Guo, T.; Luo, F. Octacosanol attenuates inflammation in both RAW264.7 macrophages and a mouse model of colitis. J. Agric. Food Chem. 2017, 65, 3647–3658. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, R.; Ni, D.; Luo, X.; Chen, S.; Luo, C.; Xiao, W. Discovery of betulinaldehyde as a natural RORγt agonist. Fitoterapia 2019, 137, 104200. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.C.; Wang, H.K.; Kashiwada, Y.; Shen, J.K.; Cosentino, L.M.; Chen, C.H.; Yang, L.M.; Lee, K.H. Anti-AIDS Agents. 34. Synthesis and Structure−Activity Relationships of Betulin Derivatives as Anti-HIV Agents. J. Med. Chem. 1998, 41, 4648–4657. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants—Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. In Vitro 2009, 23, 295–301. [Google Scholar] [CrossRef]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Cladaras, M.H. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS ONE 2011, 6, 20516. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Sperber, S.J.; Belshe, R.B.; Clover, R.D.; Hay, A.J.; Pyke, S. Recovery of drug-resistant influenza A virus during therapeutic use of rimantadine. Antimicrob. Agents Ch. 1991, 35, 1741–1747. [Google Scholar] [CrossRef]
- Hayden, F.G.; Hay, A.J. Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr. Top. Microbiol. Immunol. 1992, 176, 119–130. [Google Scholar] [CrossRef]
- Dolin, R.; Reichman, R.C.; Madore, H.P.; Maynard, R.; Linton, P.N.; Webber-Jones, J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza a infection. N. Engl. J. Med. 1982, 307, 580–584. [Google Scholar] [CrossRef]
- Balamurugan, R.; Duraipandiyan, V.; Ignacimuthu, S. Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2011, 667, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Stalin, A.; Aravinthan, A.; Kim, J. γ-sitosterol a potent hypolipidemic agent: In silico docking analysis. Med. Chem. Res. 2015, 24, 124–130. [Google Scholar] [CrossRef]
- Breyer-Pfaff, U.; Jerg, H.; Petruch, F. Cyclobarbital as a test substance for oxidative drug metabolism in man. Findings in neuropsychiatric patients. Eur. J. Clin. Pharmacol. 1979, 15, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.; Chen, H. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Matthäus, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).