Valorization of Pimenta racemosa Essential Oils and Extracts: GC-MS and LC-MS Phytochemical Profiling and Evaluation of Helicobacter pylori Inhibitory Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. GC/MS Analysis of the Essential Oils
2.2. HPLC-PDA-ESI-MS/MS Analysis
2.2.1. Proanthocyanidins
2.2.2. Organic Acids, Phenolic Acids and Their Derivatives
2.2.3. Flavonoids
| Compound | Rt (min) | UV λ (nm) | [M−H]− | (PRL-ME) | (PRS-ME) | Fragment Ions (MS/MS) | Class | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | B-type proanthocyanidin pentamer | 1.37 | 279 | 1425 | - | + | 1257, 1187, 1155 | Proanthocyanidin | [23] |
| 2. | Caffeoylglucaric acid | 1.42 | 236, 270 | 371 | + | - | 325, 191 | Phenolic acid | |
| 3. | Quinic acid | 1.47 | 236, 265 | 191 | + | - | 173 | Organic acid | [20] |
| 4. | B-type proanthocyanidin trimer (EC→EG→EG) | 1.52 | 267 | 897 | + | - | 879, 711, 693, 543, 407, 289 | Proanthocyanidin | |
| 5. | B-type proanthocyanidin dimer (EC→EG) | 1.61 | 275 | 593 | + | - | 575, 467, 441, 305, 289 | Proanthocyanidin | |
| 6. | Galloylated prodelphinidin dimer (EG→EG)2 g | 4.17 | 273 | 914 | + | - | 727, 559, 423, 305 | Proanthocyanidin | [23] |
| 7. | (Epi)gallocatechin | 4.28 | 273 | 305 | + | - | 287, 261, 221, 219, 179, 165, 125 | Flavonoid | [23,24] |
| 8. | Gallic acid | 4.68 | 270 | 169 | + | - | 125 | Phenolic acid | [25] |
| 9. | (Epi)gallocatechin | 5.46 | 273 | 305 | + | - | 287, 261, 221, 219, 179, 165, 125 | Flavonoid | |
| 10. | B-type Procyanidin dimer (EC→EC) | 5.71 | 274 | 577 | + | + | 559, 451, 425, 407, 299, 289, 287 | Proanthocyanidin | [23,26] |
| 11. | B-type Prodelphinidin dimer (EG→EG) | 5.96 | 274 | 609 | + | - | 591, 483, 441, 423, 305 | Proanthocyanidin | [23] |
| 12. | Galloylated procyanidin dimer (EC→EC)2 g | 6.22 | 276 | 881 | + | - | 729, 711 | Proanthocyanidin | [27] |
| 13. | B-type proanthocyanidin trimer EG→EG→EC | 6.63 | 277 | 897 | + | + | 879, 771, 729, 711, 593, 407, 289 | Proanthocyanidin | [27,28] |
| 14. | B-type procyanidin trimer (EC→EC→EC) | 6.83 | 278 | 865 | + | + | 847, 695, 577, 449, 407, 287 | Proanthocyanidin | [29] |
| 15. | Prodelphinidin trimer (EG→EG→EG) | 7.04 | 277 | 913 | + | - | 895, 787, 745, 727, 609, 559, 483, 305 | Proanthocyanidin | [23] |
| 16. | B-type procyanidin trimer (EC→EC→EC) | 7.09 | 277 | 865 | + | - | 847, 695, 577, 407, 287 | Proanthocyanidin | |
| 17. | A-type procyanidin trimer EC→EC→EC | 7.11 | 277 | 863 | - | + | 737, 711, 693, 591, 575, 289 | Proanthocyanidin | [29] |
| 18. | B-type Procyanidin dimer (EC→EC) | 7.14 | 277 | 577 | + | - | 559, 451, 425, 407, 299, 289, 287 | Proanthocyanidin | |
| 19. | Proanthocyanidin dimer EC→EG | 7.76 | 278 | 593 | + | + | 575, 467, 425, 407, 305, 289, 245 | Proanthocyanidin | [27] |
| 20. | (Epi)gallocatechin | 8.2 | 278 | 305 | + | - | 287, 261, 221, 219, 179 | Flavonoid | [23,24] |
| 21. | B-type proanthocyanidin trimer EG→EG→EC | 8.3 | 277 | 897 | + | - | 879, 771, 729, 711, 593, 577, 305, 289 | Proanthocyanidin | [27] |
| 22. | B-type Procyanidin dimer (EC→EC) | 8.55 | 277 | 577 | + | - | 559, 451, 425, 407, 299, 289, 287 | Proanthocyanidin | |
| 23. | B-type proanthocyanidin dimer (EC→EG) | 8.79 | 278 | 593 | 575, 467, 441, 407, 305, 289 | Proanthocyanidin | |||
| 24. | B-type proanthocyanidin trimer EC→EC→EG | 9.54 | 278 | 881 | + | - | 755, 729, 711, 695, 593, 425, 407, 289 | Proanthocyanidin | [23] |
| 25. | (Epi)catechin | 9.73 | 278 | 289 | + | + | 245, 205, 179 | Flavonoid | [24,29] |
| 26. | B-type procyanidin tetramer EC→EC→EC→EC | 9.81 | 278 | 577 | - | + | 559, 451, 425, 407, 299, 289, 287 | Proanthocyanidin | [23] |
| 27. | B-type Procyanidin dimer (EC→EC) | 9.9 | 278 | 577 | + | - | 559, 451, 425, 407, 299, 289, 287 | Proanthocyanidin | [23] |
| 28. | B-type procyanidin tetramer EC→EC→EC→EC | 10.21 | 278 | 1153 | + | + | 983, 863,695, 575 | Proanthocyanidin | [23] |
| 29. | B-type proanthocyanidin dimer (EC→EG) | 10.26 | 278 | 593 | + | + | 575, 467, 441, 407, 305, 289 | Proanthocyanidin | [27] |
| 30. | (Epi)catechin | 10.82 | 278 | 289 | + | + | 245, 205, 179, 151 | Flavonoid | [24,30] |
| 31. | B-type procyanidin trimer EC→EC→EC | 10.86 | 278 | 865 | + | + | 847, 695, 577, 575, 407, 289 | Proanthocyanidin | [23] |
| 32. | Galloylated procyanidin dimer (EC→EC)g | 11.00 | 278 | 729 | + | + | 711, 603, 577, 559, 425, 407, 289 | Proanthocyanidin | [26] |
| 33. | Tri-O-galloyl-hexoside | 11.1 | 278 | 635 | + | - | 483, 465 | Gallotannin | [20] |
| 34. | Galloylated procyanidin trimer (EC→EC→EC)→2 g | 11.15 | 278 | 1169 | + | - | 1042,890, 864, 703, 633, 443, 424 | Proanthocyanidin | [23] |
| 35. | B-type proanthocyanidin dimer EA→EC | 11.31 | 278 | 559 | + | - | 541, 453, 407, 321, 289 | Proanthocyanidin | |
| 36. | B-type procyanidin pentamer EC→EC→EC→EC→EC | 11.49 | 278 | 1441 | - | + | 1421, 1315, 1271, 1153, 1151, 1027, 865, 863,739, 575 | Proanthocyanidin | [23] |
| 37. | Galloylated procyanidin trimer (EC→EC→EC)g | 11.75 | 277 | 1017 | + | - | 999, 891, 865, 739, 729, 575, 425, 407 | Proanthocyanidin | [23] |
| 38. | Tri-O-galloyl-hexoside isomer | 12.38 | 278 | 635 | + | - | 483, 465 | Gallotannin | |
| 39. | B-type Procyanidin dimer (EC→EC) | 12.43 | 278 | 577 | + | - | 559, 451, 425, 407, 299, 289, 287 | Proanthocyanidin | [23] |
| 40. | Galloylated procyanidin dimer (EC→EC)g | 12.66 | 277 | 729 | + | + | 711, 603, 577, 559, 425, 407, 289 | Proanthocyanidin | [23] |
| 41. | B-type Procyanidin dimer (EC→EC) | 12.79 | 279 | 577 | - | + | 559, 451, 425, 407, 299, 289, 287 | Proanthocyanidin | [23] |
| 42. | Tetra-O-galloyl hexoside | 13.12 | 277 | 787 | + | - | 635, 617, 465, 331, 313 | Gallotannin | [20] |
| 43. | Galloylated procyanidin trimer (EC→EC→EC)g | 13.82 | 278 | 1017 | + | + | 999, 891, 865, 847, 739, 729, 695, 677, 575 | Proanthocyanidin | [23] |
| 44. | Tetra-O-galloyl hexoside isomer | 14.57 | 274 | 787 | + | - | 635, 617, 465, 331, 313 | Gallotannin | |
| 45. | Quercetin-O-hexoside | 14.59 | 274, 349 | 463 | + | + | 301, 179, 151 | Flavonoid | [20] |
| 46. | Penta-O-galloyl hexoside | 14.80 | 274 | 939 | + | - | 921, 787, 769, 635, 617, 555, 465, 447, 313, 295 | Gallotannin | [20] |
| 47. | Quercetin-O-galloyl hexoside | 15.15 | 272, 351 | 615 | + | - | 463, 301, 300, 179 | Flavonoid | [31] |
| 48. | Pentahydroxyflavone-C-hexoside | 15.70 | 266, 353 | 463 | + | - | 445, 373, 343, 301, 179, 151, 133 | Flavonoid | |
| 49. | Pentahydroxyflavone-C-pentoside | 15.83 | 271, 352 | 433 | + | - | 415, 373, 343, 301, 300, 287, 251, 193, 179, 151, 125 | Flavonoid | |
| 50. | Penta-O-galloyl hexoside | 15.96 | 268, 353 | 939 | + | - | 921, 787, 769, 635, 617, 555, 465, 447, 313 | Gallotannin | [20] |
| 51. | Gallic acid dihexoside | 16.11 | 266 | 493 | + | + | 341, 313, 179, 169 | Phenolic acid | [20] |
| 52. | Quercetin-O-deoxyhexoside | 16.55 | 255, 353 | 447 | + | + | 301, 255, 179, 151 | Flavonoid | [10,20] |
| 53. | Ellagic acid-O-pentoside | 16.88 | 266, 351 | 433 | + | - | 301, 191, 169 | Phenolic acid | |
| 54. | Quercetin-O-pentoside | 16.92 | 268, 349 | 433 | + | + | 415, 301, 300, 179, 151 | Flavonoid | |
| 55. | Quercetin-O-deoxyhexoside | 17.05 | 264, 348 | 447 | + | - | 301, 255, 179, 151 | Flavonoid | |
| 56. | Gallic acid dihexoside isomer | 17.21 | 273 | 493 | 341, 313, 179, 169, 151 | Phenolic acid | [20] | ||
| 57. | Quercetin-di-O-hexoside | 18.25 | 271, 350 | 625 | + | - | 463, 301, 179 | Flavonoid | [22] |
| 58. | Gallic acid derivative | 19.23 | 268, 348 | 477 | + | - | 313, 301, 223, 169 | Phenolic acid | [31,32,33] |
| 59. | Gallic acid derivative | 19.38 | 268, 336 | 447 | + | - | 313, 301, 269, 169, 125 | Phenolic acid | |
| 60. | Quercetin O-acetyl-deoxyhexoside | 20.25 | 275, 350 | 489 | + | - | 471, 447, 301, 300, 179, 151 | Flavonoid | |
| 61. | Unidentified | 27.03 | 275 | 313 | + | - | 313, 298, 283, 269, 257, 243, 227, 163, 135, 113 | ||
| 62. | Unidentified | 33.32 | 291, 311 | 289 | + | - | 245, 163, 119 | ||
| 63. | Unidentified | 36.67 | 279 | 325 | + | + | 325, 310, 307, 295, 281, 252, 191 | ||
| 64. | Hydroxypalmitic acid | 48.09 | 271 | + | - | 271, 253, 225 | Fatty acid | ||
| 65. | Unidentified | 55.83 | 817 | - | + | 796, 711 |
2.3. Evaluation of Anti-H. pylori Activity
2.4. In Silico Evaluation of Anti-H. pylori Activity
3. Materials and Methods
3.1. Plant Material
3.2. Essential Oils Isolation
3.3. Preparation of Plant Extracts
3.4. GC/MS Analysis of Essential Oils
3.5. Identification of Essential Oil Components
3.6. HPLC-PDA-ESI-MS/MS Analysis
3.7. Evaluation of Anti-H. pylori Activity
3.8. In Silico Molecular Docking Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Youssef, F.S.; Labib, R.M.; Gad, H.A.; Eid, S.; Ashour, M.L.; Eid, H.H. Pimenta dioica and Pimenta racemosa: GC-based metabolomics for the assessment of seasonal and organ variation in their volatile components, in silico and in vitro cytotoxic activity estimation. Food Funct. 2021, 12, 5247–5259. [Google Scholar] [CrossRef]
- Bailey, L.H. Manual of Cultivated Plants; The MacMillan Company: New York, NY, USA, 1951. [Google Scholar]
- Garcıa, M.; Fernandez, M.; Alvarez, A.; Saenz, M. Antinociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. ozua (Myrtaceae). J. Ethnopharmacol. 2004, 91, 69–73. [Google Scholar] [CrossRef]
- DeFilipps, R.A.; Maina, S.L.; Crepin, J. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana); Department of Botany, National Museum of Natural History, Smithsonian: Washington, DC, USA, 2004. [Google Scholar]
- Al-Gendy, A.; Moharram, F.; Zarka, M. Chemical composition, antioxidant, cytotoxic and antimicrobial activities of Pimenta racemosa (Mill.) JW Moore flower essential oil. J. Pharmacogn. Phytochem. 2017, 6, 312–319. [Google Scholar]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Bamawa, C.; Ndjele, L.; Foma, F. Characterization of leaf phenolic compounds of Sabicea johnstonii by HPLC-MSn. J. Nat. Prod. Resour. 2016, 2, 86–89. [Google Scholar]
- Gad, H.; Al-Sayed, E.; Ayoub, I. Phytochemical discrimination of Pinus species based on GC-MS and ATR-IR analyses and their impact on Helicobacter pylori. Phytochem. Anal. 2021, 32, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Shams-Ardakani, M.; Foroumadi, A. Medicinal plants in the treatment of Helicobacter pylori infections. Pharm. Biol. 2015, 53, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Moharram, F.A.; Al-Gendy, A.A.; El-Shenawy, S.M.; Ibrahim, B.M.; Zarka, M.A. Phenolic profile, anti-inflammatory, antinociceptive, anti-ulcerogenic and hepatoprotective activities of Pimenta racemosa leaves. BMC Complement. Altern. Med. 2018, 18, 208. [Google Scholar] [CrossRef]
- Yousif, F.; Hifnawy, M.S.; Soliman, G.; Boulos, L.; Labib, T.; Mahmoud, S.; Ramzy, F.; Yousif, M.; Hassan, I.; Mahmoud, K. Large-scale in vitro screening of Egyptian native and cultivated plants for schistosomicidal activity. Pharm. Biol. 2007, 45, 501–510. [Google Scholar] [CrossRef]
- Alitonou, G.A.; Noudogbessi, J.-P.; Sessou, P.; Tonouhewa, A.; Avlessi, F.; Menut, C.; Sohounhloue, D. Chemical composition and biological activities of essential oils of Pimenta racemosa (Mill.) JW Moore. from Benin. Int. J. Biosci. 2012, 2, 1–12. [Google Scholar]
- Pragadheesh, V.; Yadav, A.; Singh, S.; Gupta, N.; Chanotiya, C. Leaf essential oil of cultivated Pimenta racemosa (Mill.) JW Moore from North India: Distribution of phenylpropanoids and chiral terpenoids. Med. Aromat. Plants 2013, 2. [Google Scholar] [CrossRef]
- Contreras-Moreno, B.Z.; Velasco, J.J.; Rojas, J.d.C.; Méndez, L.d.C.; Celis, M.T. Antimicrobial activity of essential oil of Pimenta racemosa var. racemosa (Myrtaceae) leaves. J. Pharm. Pharmacogn. Res. 2016, 4, 224–230. [Google Scholar]
- Ayoub, I.M.; Korinek, M.; El-Shazly, M.; Wetterauer, B.; El-Beshbishy, H.A.; Hwang, T.-L.; Chen, B.-H.; Chang, F.-R.; Wink, M.; Singab, A.N.B.; et al. Anti-Allergic, anti-inflammatory, and anti-hyperglycemic activity of Chasmanthe aethiopica leaf extract and its profiling using LC/MS and GLC/MS. Plants 2021, 10, 1118. [Google Scholar] [CrossRef]
- Faheem, S.A.; Saeed, N.M.; El-Naga, R.N.; Ayoub, I.M.; Azab, S.S. Hepatoprotective effect of cranberry nutraceutical extract in non-alcoholic fatty liver model in rats: Impact on insulin resistance and Nrf-2 expression. Front Pharm. 2020, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, I.M.; George, M.Y.; Menze, E.T.; Mahmoud, M.; Botros, M.; Essam, M.; Ashmawy, I.; Shendi, P.; Hany, A.; Galal, M.; et al. Insights into the neuroprotective effects of Salvia officinalis L. and Salvia microphylla Kunth in the memory impairment rat model. Food Funct. 2022, 13, 2253–2268. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Farrag, A.R.H.; Ayoub, I.M.; Mahdy, K.A.; Taher, R.F.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Al-Rejaie, S.S.; EI-Amier, Y.A.; Abd-EIGawad, A.M.; et al. UPLC-qTOF-MS phytochemical profile and antiulcer potential of Cyperus conglomeratus Rottb. Alcoholic Extract. Molecules 2020, 25, 4234. [Google Scholar] [CrossRef]
- Saeed Kotb, S.; Ayoub, I.M.; El-Moghazy, S.A.; Singab, A.N.B. Phytochemical analysis of Pithecellobium dulce (Roxb) Benth bark via UPLC-ESI-MS/MS and evaluation of its biological activity. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L.(Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Elkady, W.M.; Ayoub, I.M.; Abdel-Mottaleb, Y.; ElShafie, M.F.; Wink, M. Euryops pectinatus L. flower extract inhibits P-glycoprotein and reverses multi-drug resistance in cancer cells: A mechanistic study. Molecules 2020, 25, 647. [Google Scholar] [CrossRef]
- Ablajan, K.; Abliz, Z.; Shang, X.Y.; He, J.M.; Zhang, R.P.; Shi, J.G. Structural characterization of flavonol 3, 7di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMS n profiling method to identify and quantify oligomeric proanthocyanidins in plant products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, J.; Miao, A.; Xie, Z.; Yang, D. HPLC-DAD-ESI-MS/MS analysis of polyphenols and purine alkaloids in leaves of 22 tea cultivars in China. J. Food Compos. Anal. 2008, 21, 361–369. [Google Scholar] [CrossRef]
- Teixeira, N.; Nabais, P.; de Freitas, V.; Lopes, J.A.; Melo, M.J. In-depth phenolic characterization of iron gall inks by deconstructing representative Iberian recipes. Sci. Rep. 2021, 11, 8811. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- Teixeira, N.; Azevedo, J.; Mateus, N.; de Freitas, V. Proanthocyanidin screening by LC–ESI-MS of Portuguese red wines made with teinturier grapes. Food Chem. 2016, 190, 300–307. [Google Scholar] [CrossRef]
- Dvorakova, M.; Moreira, M.M.; Dostalek, P.; Skulilova, Z.; Guido, L.F.; Barros, A.A. Characterization of monomeric and oligomeric flavan-3-ols from barley and malt by liquid chromatography–ultraviolet detection–electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1189, 398–405. [Google Scholar] [CrossRef]
- Lv, Q.; Luo, F.; Zhao, X.; Liu, Y.; Hu, G.; Sun, C.; Li, X.; Chen, K. Identification of proanthocyanidins from litchi (Litchi chinensis Sonn.) pulp by LC-ESI-Q-TOF-MS and their antioxidant activity. PLoS ONE 2015, 10, e0120480. [Google Scholar] [CrossRef]
- Liu, P.; Shi, Y.; Zhu, L. Genetic variation in resistance to valsa canker is related to arbutin and gallic acid content in Pyrus bretschneideri. Hortic. Plant J. 2018, 4, 233–238. [Google Scholar] [CrossRef]
- Sobeh, M.; Rezq, S.; Sabry, O.M.; Abdelfattah, M.A.O.; El Raey, M.A.; El-Kashak, W.A.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. Albizia anthelmintica: HPLC-MS/MS profiling and in vivo anti-inflammatory, pain killing and antipyretic activities of its leaf extract. Biomed. Pharmacother. 2019, 115, 108882. [Google Scholar] [CrossRef]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of nonvolatile compounds in clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017, 1862, 030079. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Fan, M.-X.; Wu, X.; Wang, H.-J.; Yang, J.; Si, N.; Bian, B.-L. Chemical profiling of the Chinese herb formula Xiao-Cheng-Qi decoction using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. Sci. 2012, 51, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Périco, L.L.; Emílio-Silva, M.T.; Ohara, R.; Rodrigues, V.P.; Bueno, G.; Barbosa-Filho, J.M.; Rocha, L.R.M.d.; Batista, L.M.; Hiruma-Lima, C.A. Systematic analysis of monoterpenes: Advances and challenges in the treatment of peptic ulcer diseases. Biomolecules 2020, 10, 265. [Google Scholar] [CrossRef]
- Youssef, F.S.; Sobeh, M.; Dmirieh, M.; Bogari, H.A.; Koshak, A.E.; Wink, M.; Ashour, M.L.; Elhady, S.S. Metabolomics-based profiling of Clerodendrum speciosum (Lamiaceae) leaves using LC/ESI/MS-MS and in vivo evaluation of its antioxidant activity using Caenorhabditis elegans model. Antioxidants 2022, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A Polyphenol- rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front. Pharmacol. 2018, 9, 566. [Google Scholar] [CrossRef]
- Wink, M.; Schimmer, O. Molecular modes of action of defensive secondary metabolites. Annu. Plant Rev. 2010, 39, 21–161. [Google Scholar]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/ Mass Spectrometry, 4th ed.; Allured Publisher: Carol Stream, IL, USA, 2007. [Google Scholar]
- Elkady, W.M.; Ayoub, I.M. Chemical profiling and antiproliferative effect of essential oils of two Araucaria species cultivated in Egypt. Ind. Crops Prod. 2018, 118, 188–195. [Google Scholar] [CrossRef]
- Ashmawy, A.M.; Ayoub, I.M.; Eldahshan, O.A. Chemical composition, cytotoxicity and molecular profiling of Cordia africana Lam. on human breast cancer cell line. Nat. Prod. Res. 2021, 35, 4133–4138. [Google Scholar] [CrossRef]
- Korany, D.A.; Ayoub, I.M.; Labib, R.M.; El-Ahmady, S.H.; Singab, A.N.B. The impact of seasonal variation on the volatile profile of leaves and stems of Brownea grandiceps (Jacq.) with evaluation of their anti-mycobacterial and anti-inflammatory activities. S. Afr. J. Bot. 2021, 142, 88–95. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS profiling, anti-collagenase, anti-elastase, anti-tyrosinase and anti-hyaluronidase activities of a Stenocarpus sinuatus leaves extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- Thabet, A.A.; Ayoub, I.M.; Youssef, F.S.; Al Sayed, E.; Singab, A.N.B. Essential oils from the leaves and flowers of Leucophyllum frutescens (Scrophulariaceae): Phytochemical analysis and inhibitory effects against elastase and collagenase in vitro. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset, S.; El-Kersh, D.M.; Ayoub, I.M.; Eldahshan, O.A. GC-MS profiling of Vitex pinnata bark lipophilic extract and screening of its anti-TB and cytotoxic activities. Nat. Prod. Res. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saeed Kotb, S.; Ayoub, I.M.; El-Moghazy, S.A.; Singab, A.N.B. Profiling the lipophilic fractions of Pithecellobium dulce bark and leaves using GC/MS and evaluation of their antioxidant, antimicrobial and cytotoxic activities. Chem. Biodivers. 2020, 17, e2000048. [Google Scholar] [CrossRef] [PubMed]
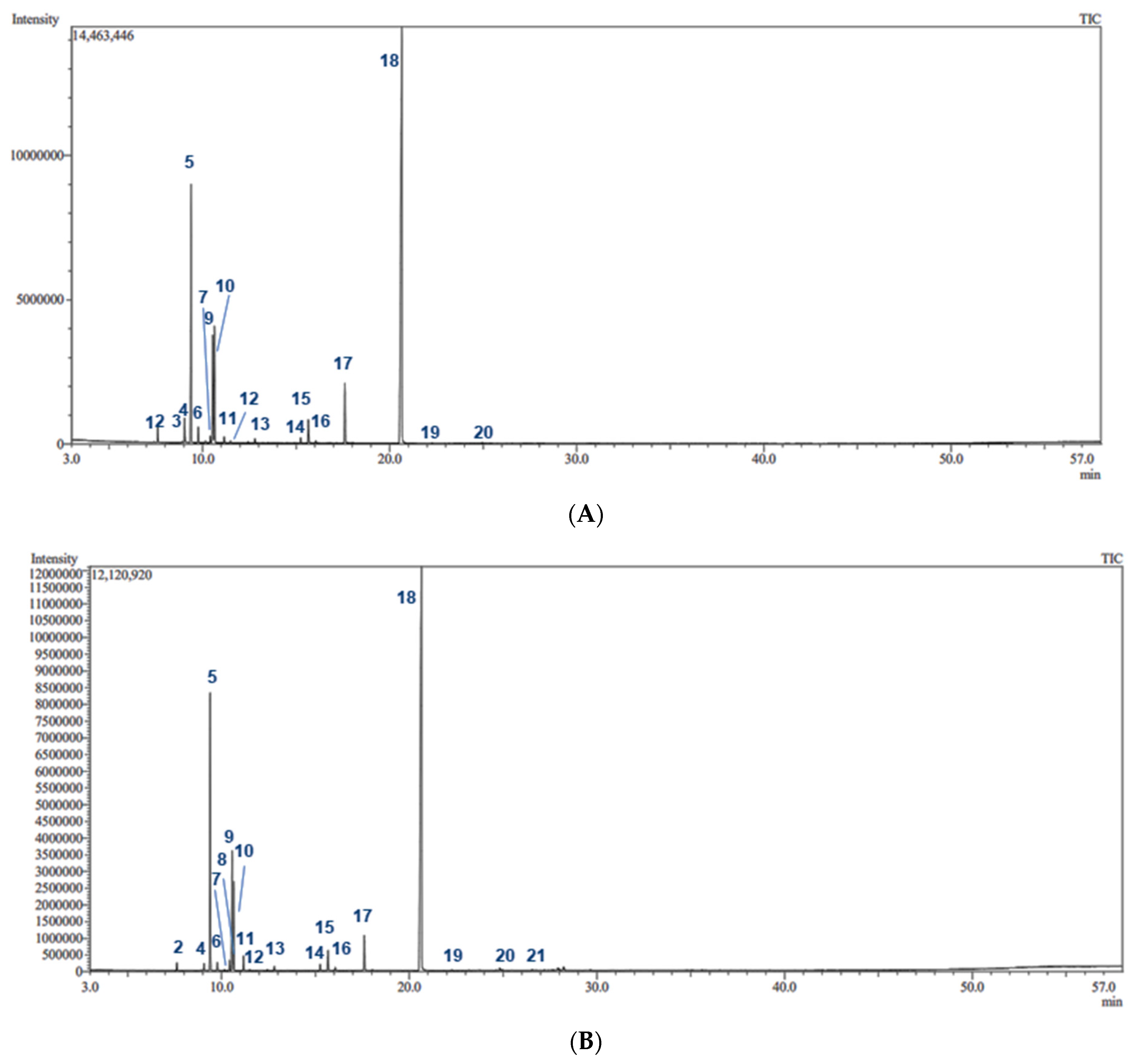





| No. | Rt (min) | Compounds a | Molecular Formula | RIexp b | RIlit c | Content % | |
|---|---|---|---|---|---|---|---|
| PRL-EO | PRS-EO | ||||||
| 1 | 7.42 | α-Thujene | C10H16 | 917 | 917 | 0.04 | - |
| 2 | 7.61 | α-Pinene | C10H16 | 938 | 938 | 0.89 | 0.48 |
| 3 | 8.92 | β-Pinene | C10H16 | 973 | 973 | 0.06 | - |
| 4 | 9.05 | 1-Octen-3-ol (Amyl vinyl carbinol) | C8H16O | 976 | 976 | 1.51 | 0.45 |
| 5 | 9.39 | β-Myrcene | C10H16 | 990 | 990 | 16.30 | 17.43 |
| 6 | 9.77 | α-Phellandrene | C10H16 | 1004 | 1004 | 1.02 | 0.58 |
| 7 | 10.16 | 2-Carene | C10H16 | 1015 | 1014 | 0.11 | 0.07 |
| 8 | 10.42 | p-Cymene | C10H14 | 1023 | 1023 | 0.43 | 0.71 |
| 9 | 10.55 | Limonene | C10H16 | 1029 | 1029 | 7.20 | 7.70 |
| 10 | 10.63 | Eucalyptol | C10H18O | 1033 | 1033 | 7.31 | 5.45 |
| 11 | 11.16 | β-Ocimene | C10H16 | 1037 | 1037 | 0.36 | 0.92 |
| 12 | 11.50 | γ-Terpinene | C10H16 | 1060 | 1060 | 0.12 | 0.08 |
| 13 | 12.43 | Terpinolene | C10H16 | 1090 | 1090 | 0.07 | 0.07 |
| 14 | 15.26 | Terpinen-4-ol | C10H18O | 1179 | 1179 | 0.34 | 0.43 |
| 15 | 15.66 | α-Terpineol | C10H18O | 1193 | 1193 | 1.68 | 1.49 |
| 16 | 16.05 | n-Decanal(Capraldehyde) | C10H20O | 1204 | 1204 | 0.13 | 0.24 |
| 17 | 17.61 | Chavicol(p-Allylphenol) | C9H10O | 1259 | 1259 | 4.18 | 2.60 |
| 18 | 20.65 | Eugenol | C10H12O2 | 1359 | 1359 | 57.84 | 59.76 |
| 19 | 22.27 | Caryophyllene | C15H24 | 1424 | 1424 | 0.05 | 0.16 |
| 20 | 24.96 | δ-Cadinene | C15H24 | 1529 | 1529 | 0.05 | 0.10 |
| 21 | 26.56 | Viridiflorol | C15H24 | 1591 | 1591 | - | 0.10 |
| Monoterpene hydrocarbons | 26.48 | 28.04 | |||||
| Oxygenated monoterpenes | 9.33 | 7.37 | |||||
| Sesquiterpene hydrocarbons | 0.1 | 0.36 | |||||
| Phenyl propanoids | 62.02 | 62.36 | |||||
| Others | 1.64 | 0.69 | |||||
| Total identified % | 99.57 | 98.82 | |||||
| Inhibition % | |||||
|---|---|---|---|---|---|
| Sample Conc. (µg/mL) | PRL-EO | PRS-EO | PRL-ME | PRS-ME | Clarithromycin |
| 125 | 100 ± 0 | 100 ± 0 | 46.52 ± 0.58 | 100 ± 0 | 100 ± 0 |
| 62.5 | 100 ± 0 | 100 ± 0 | 19.85 ± 1.8 | 100 ± 0 | 100 ± 0 |
| 31.25 | 100 ± 0 | 100 ± 0 | 5.74 ± 2.3 | 100 ± 0 | 100 ± 0 |
| 15.63 | 83.25 ± 3.1 | 100 ± 0 | 0 | 100 ± 0 | 100 ± 0 |
| 7.81 | 64.85 ± 1.2 | 100 ± 0 | 0 | 86.32 ±1.5 | 100 ± 0 |
| 3.9 | 39.17 ± 2.5 | 100 ± 0 | 0 | 55.34 ± 2.4 | 100 ± 0 |
| 1.95 | 23.14 ± 1.3 | 92.14 ± 0.95 | 0 | 34.38 ± 1.3 | 100 ± 0 |
| 0.98 | 9.32 ± 1.2 | 78.95 ± 1.3 | 0 | 26.34 ± 0.69 | 92.45 ± 1.2 |
| 0.48 | 0 | 56.38 ± 1.6 | 0 | 19.3 ± 0.95 | 87.65 ± 0.58 |
| 0.24 | 0 | 37.28 ± 2.4 | 0 | 7.2 ± 0.83 | 81.35 ± 1.5 |
| 0 | 0 | 0 | 0 | 0 | 0 |
| MIC (µg/mL) | 31.25 | 3.9 | >125 | 15.63 | 1.95 |
| Compound | Free Binding Energy (∆G) (Kcal/mol) | |
|---|---|---|
| pH Based | Rule-Based | |
| Co-crystalized ligand (HAE) | −22.51 | −22.51 |
| Decanal | −29.76 | −29.76 |
| Eugenol | −29.44 | −29.44 |
| α-Terpineol | −23.09 | −23.09 |
| δ-Cadinene | −22.68 | −22.68 |
| Amyl vinyl | −22.63 | −26.57 |
| Chavicol | −21.97 | −21.97 |
| Ocimene | −21.91 | −21.91 |
| Myrcene | −21.63 | −21.63 |
| Terpinolene | −20.24 | −20.24 |
| Terpinene | −19.76 | −19.76 |
| Phellandrene | −19.71 | −19.71 |
| Caryophyllene | −18.83 | −18.83 |
| Limonene | −18.71 | −18.71 |
| Cymene | −18.51 | −18.51 |
| Pinene | −15.48 | −15.48 |
| Eucalyptol | −13.31 | −13.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, I.M.; Abdel-Aziz, M.M.; Elhady, S.S.; Bagalagel, A.A.; Malatani, R.T.; Elkady, W.M. Valorization of Pimenta racemosa Essential Oils and Extracts: GC-MS and LC-MS Phytochemical Profiling and Evaluation of Helicobacter pylori Inhibitory Activity. Molecules 2022, 27, 7965. https://doi.org/10.3390/molecules27227965
Ayoub IM, Abdel-Aziz MM, Elhady SS, Bagalagel AA, Malatani RT, Elkady WM. Valorization of Pimenta racemosa Essential Oils and Extracts: GC-MS and LC-MS Phytochemical Profiling and Evaluation of Helicobacter pylori Inhibitory Activity. Molecules. 2022; 27(22):7965. https://doi.org/10.3390/molecules27227965
Chicago/Turabian StyleAyoub, Iriny M., Marwa M. Abdel-Aziz, Sameh S. Elhady, Alaa A. Bagalagel, Rania T. Malatani, and Wafaa M. Elkady. 2022. "Valorization of Pimenta racemosa Essential Oils and Extracts: GC-MS and LC-MS Phytochemical Profiling and Evaluation of Helicobacter pylori Inhibitory Activity" Molecules 27, no. 22: 7965. https://doi.org/10.3390/molecules27227965
APA StyleAyoub, I. M., Abdel-Aziz, M. M., Elhady, S. S., Bagalagel, A. A., Malatani, R. T., & Elkady, W. M. (2022). Valorization of Pimenta racemosa Essential Oils and Extracts: GC-MS and LC-MS Phytochemical Profiling and Evaluation of Helicobacter pylori Inhibitory Activity. Molecules, 27(22), 7965. https://doi.org/10.3390/molecules27227965







