Trimetallic Chalcogenide Species: Synthesis, Structures, and Bonding
Abstract
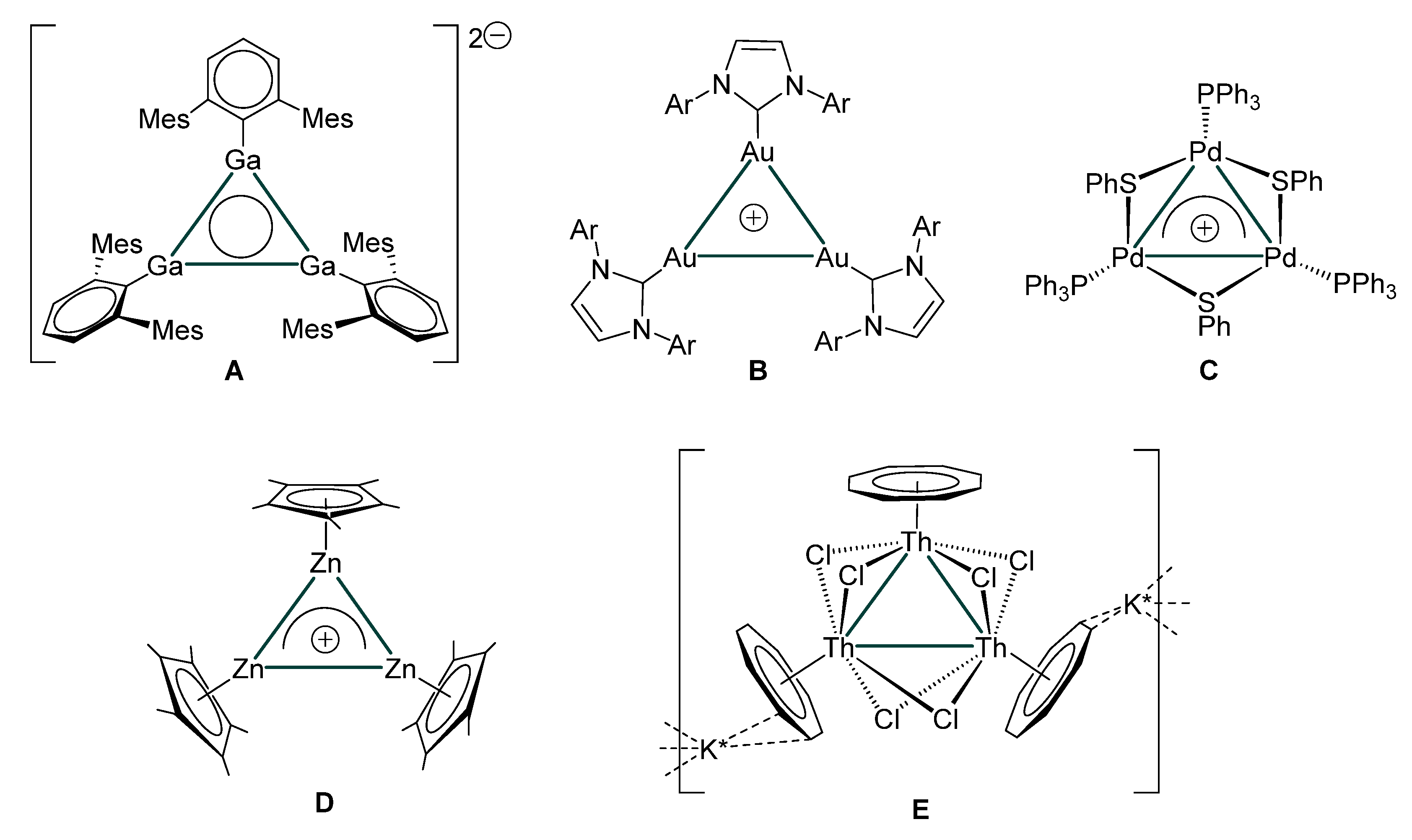
1. Introduction
2. Results and Discussion
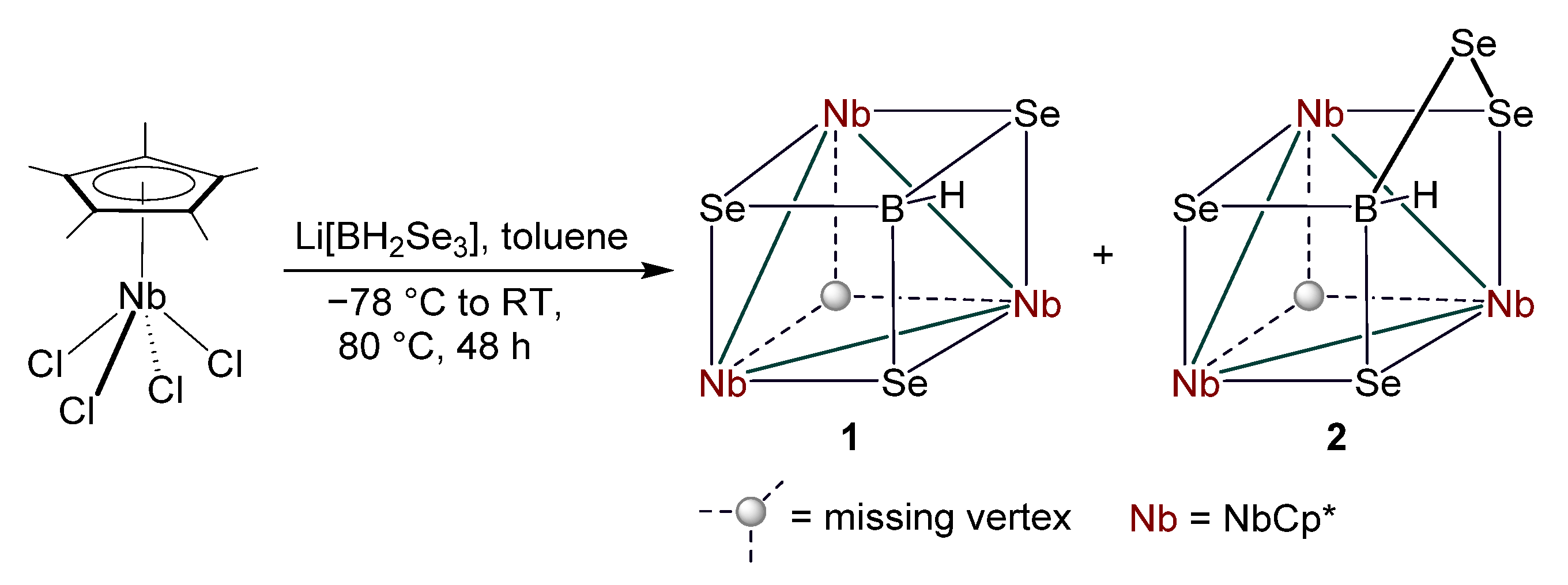
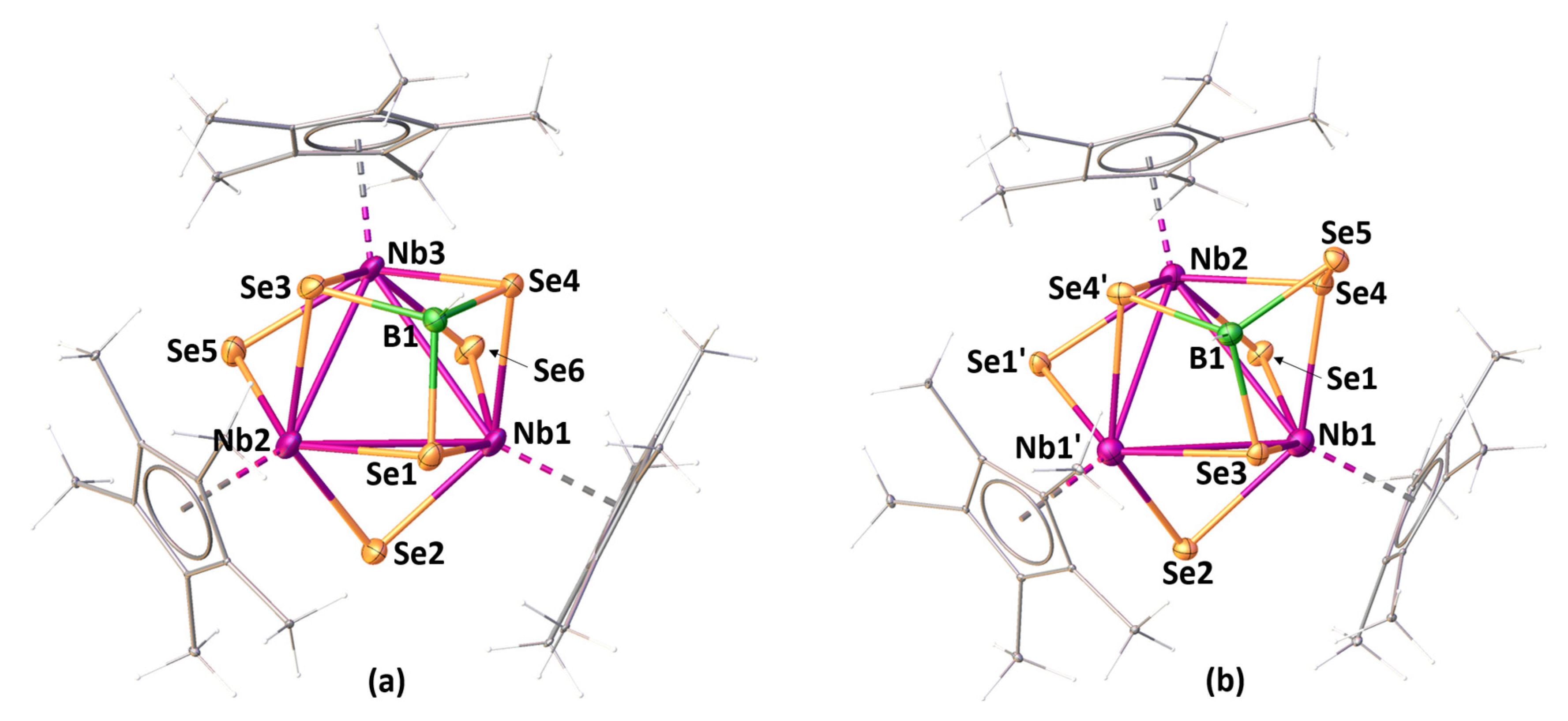
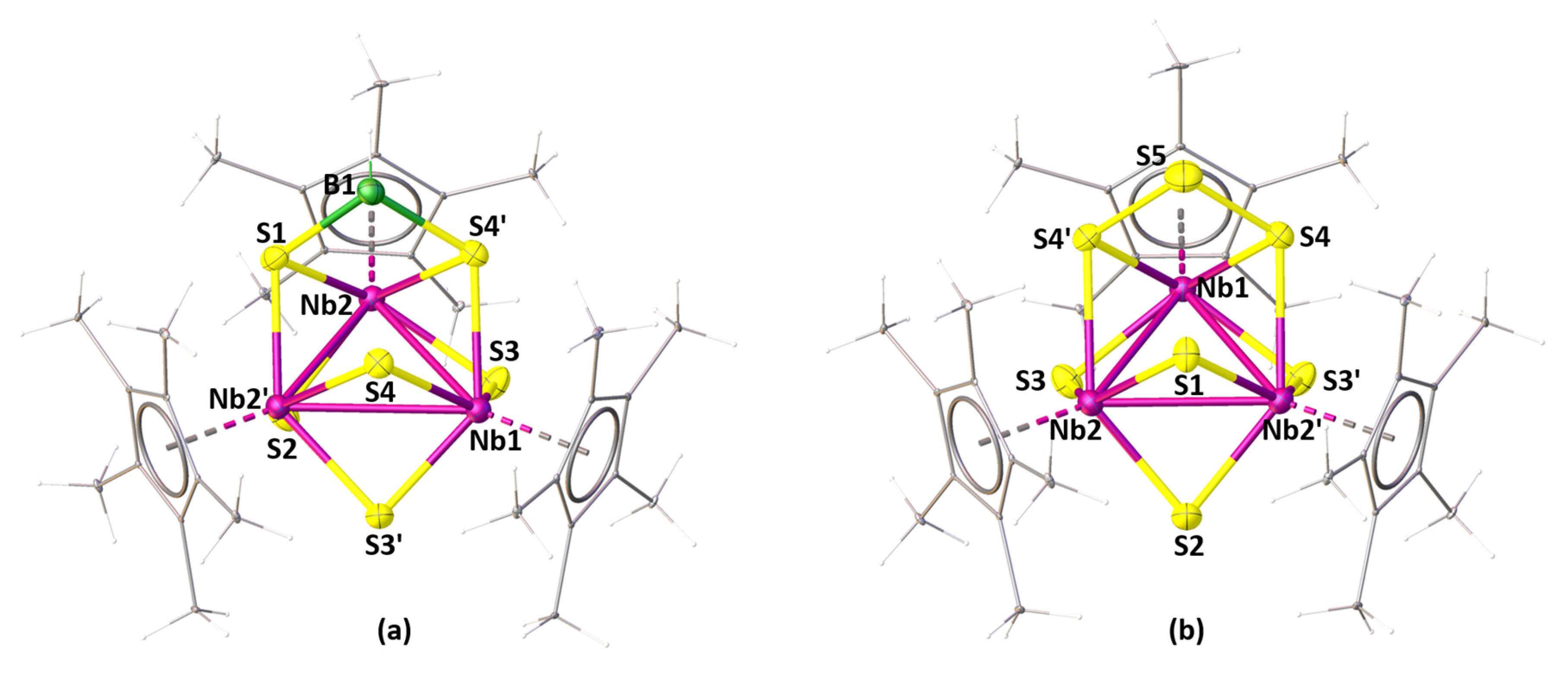
2.1. Reactivity of [Cp*NbCl4] with Li[BH2Se3]
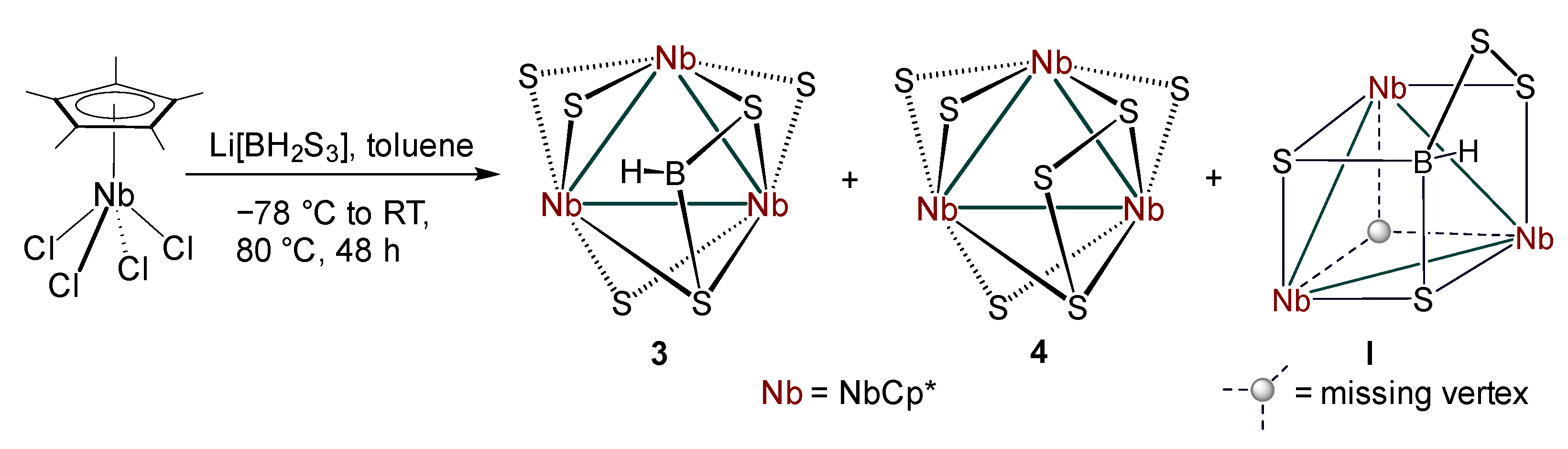
2.2. Reactivity of [Cp*NbCl4] with Li[BH2S3]
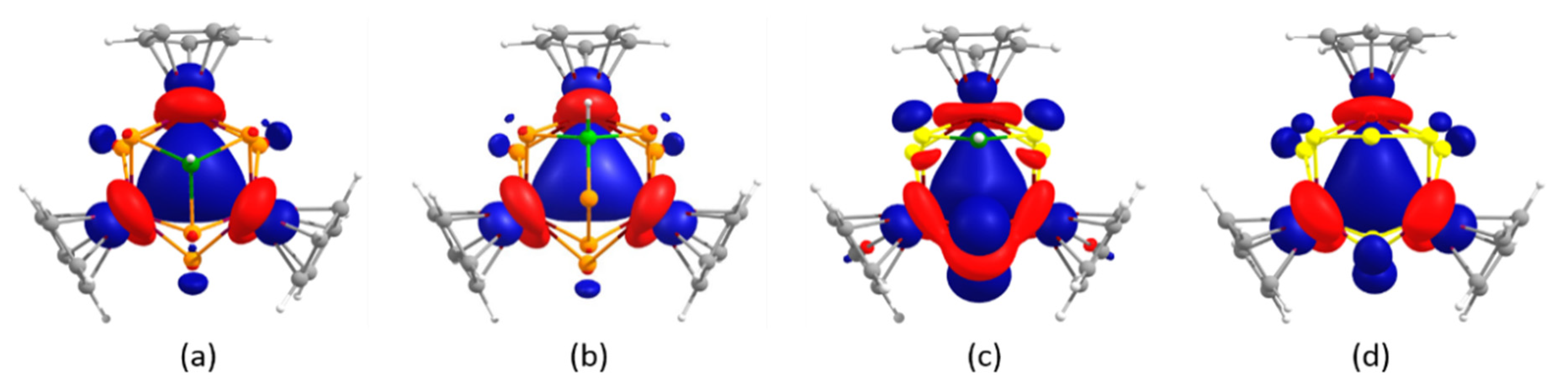
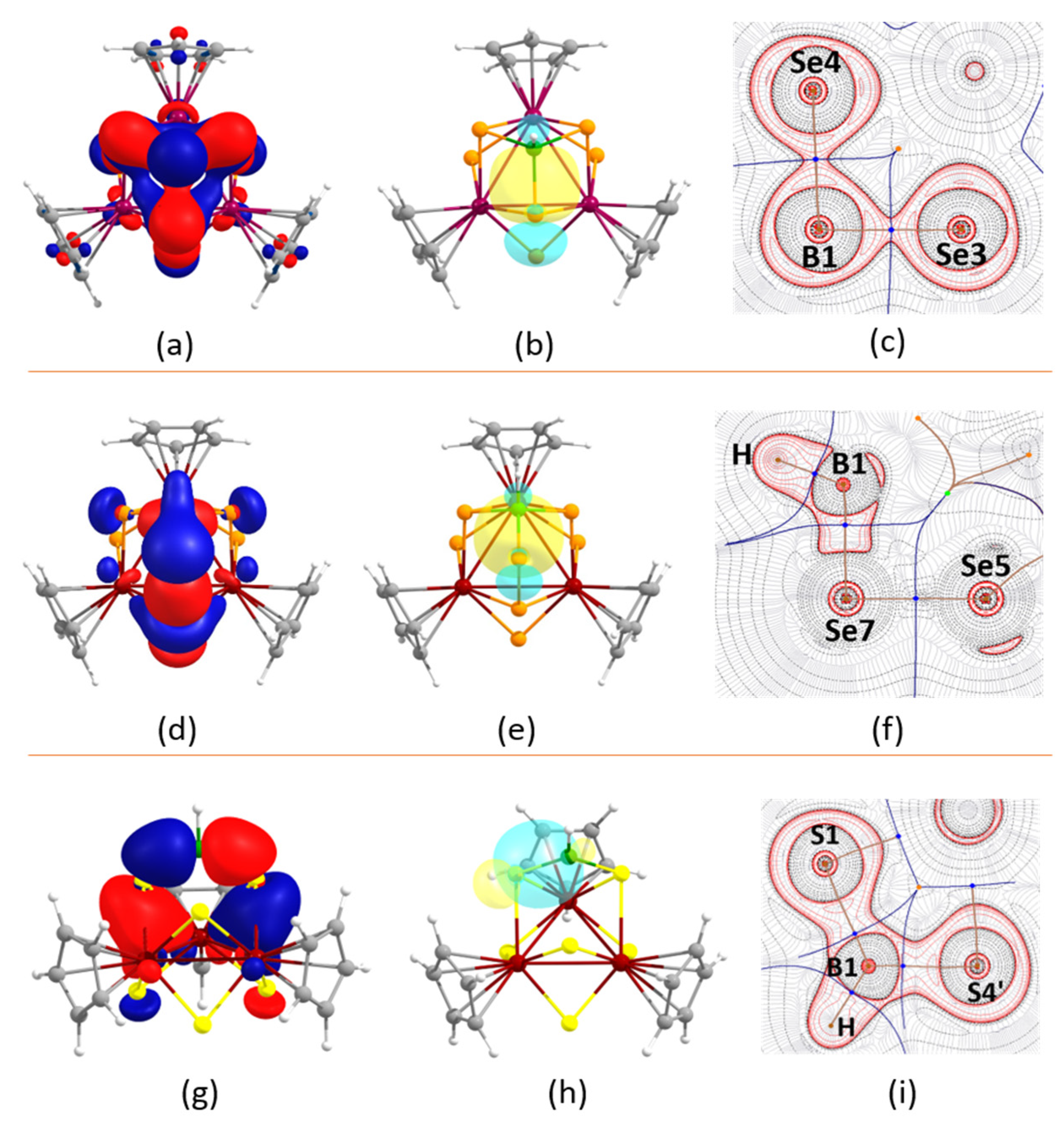
2.3. Electronic Structural and Bonding Analysis of 1–4
3. Materials and Methods
3.1. Synthesis of 1 and 2
3.2. Synthesis of 3 and 4
3.3. X-ray Structure Determination
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References and Note
- Liddle, S.T. Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties; Wiley-VCH: London, UK, 2015. [Google Scholar] [CrossRef]
- Wang, X.-B.; Wang, L.-S. Probing the Electronic Structure and Metal−Metal Bond of Re2Cl82− in the Gas Phase. J. Am. Chem. Soc. 2000, 122, 2096–2100. [Google Scholar] [CrossRef]
- Roy, D.K.; Ghosh, S. Group 5 Metal–Metal Bonds. In Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties; Liddle, S., Ed.; Wiley-VCH: London, UK, 2015; Chapter 5; pp. 91–138. [Google Scholar]
- Le Guennic, B.; Jiao, H.; Kahlal, S.; Saillard, J.-Y.; Halet, J.-F.; Ghosh, S.; Beatty, A.M.; Rheingold, A.L.; Fehlner, T.P. Synthesis and Characterization of Hypoelectronic Rhenaboranes. Analysis of the Geometric and Electronic Structures of Species Following Neither Borane nor Metal Cluster Electron Counting Paradigms. J. Am. Chem. Soc. 2004, 126, 3203–3217. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, D.Y.; Averkiev, B.B.; Zhai, H.-J.; Wang, L.-S.; Boldyrev, A.I. Aromaticity and antiaromaticity in transition-metal systems. Phys. Chem. Chem. Phys. 2008, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Pradhan, A.N.; Ghosh, S. Polyhedral Metallaboranes and Metallacarboranes. In Comprehensive Organometallic Chemistry IV, 4th ed.; Parkin, G., Meyer, K., O’hare, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 9, pp. 263–369. [Google Scholar] [CrossRef]
- Kar, S.; Pradhan, A.N.; Ghosh, S. Metal-rich Metallaboranes: Clusters Containing Triply and Tetra Bridging Borylene and Boride Units. Coord. Chem. Rev. 2021, 436, 213796. [Google Scholar] [CrossRef]
- Kar, S.; Bairagi, S.; Haridas, A.; Joshi, G.; Jemmis, E.D.; Ghosh, S. Hexagonal Planar [B6H6] within a [B6H12] Borate complex; Structure and Bonding of [(Cp*Ti)2(μ–η6:η6-B6H6)(μ-H)6]. Angew. Chem. Int. Ed. 2022, 61, e202208293. [Google Scholar] [CrossRef]
- Cotton, F.A.; Walton, R.A. Metal-metal multiple bonds in dinuclear clusters. In Clusters. Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1985; Volume 62, pp. 1–49. [Google Scholar] [CrossRef]
- Vivó, D.G.; Ramos, A.; Ruiz, M.A. Cyclopentadienyl and related complexes of the group 6 elements having metal–metal triple bonds: Synthesis, structure, bonding and reactivity. Coord. Chem. Rev. 2013, 257, 2143–2191. [Google Scholar] [CrossRef]
- Chisholm, M.H. Mixed valency and metal–metal quadruple bonds. Coord. Chem. Rev. 2013, 257, 1576–1583. [Google Scholar] [CrossRef]
- Nguyen, T.; Sutton, A.D.; Brynda, M.; Fettinger, J.C.; Long, G.J.; Power, P.P. Synthesis of a Stable Compound with Fivefold Bonding Between Two Chromium(I) Centers. Science 2005, 310, 844–847. [Google Scholar] [CrossRef]
- Doz, U.R.P.; Breher, F. To Boldly Pass the Metal–Metal Quadruple Bond. Angew. Chem. Int. Ed. 2006, 45, 3006–3010. [Google Scholar] [CrossRef]
- Fehlner, T.P.; Halet, J.-F.; Saillard, J.-Y. Molecular Clusters. A Bridge to Solid-State Chemistry; Cambridge University Press: Cambridge, UK, 2007; pp. 85–138. [Google Scholar] [CrossRef]
- Anju, R.S.; Saha, K.; Mondal, B.; Dorcet, V.; Roisnel, T.; Halet, J.-F.; Ghosh, S. Chemistry of Diruthenium Analogue of Pentaborane(9) with Heterocumulenes: Towards Novel Trimetallic Cubane-type Clusters. Inorg. Chem. 2014, 53, 10527–10535. [Google Scholar] [CrossRef]
- Okamura, R.; Tada, K.; Matsubara, K.; Oshima, M.; Suzuki, H. Novel Trinuclear Trihydride Complexes of Ruthenium Having a Triply Bridging Borylene Ligand, {(η5-C5Me5)Ru}3(μ-H)3(μ3-BX) (X = H, CN, OMe, OEt). Synthesis, Structure Determination, and Reaction with Benzothiophene. Organometallics 2001, 20, 4772–4774. [Google Scholar] [CrossRef]
- Takao, T.; Suwa, H.; Okamura, R.; Suzuki, H. Formation of a Boraruthenacyclopentenyl Skeleton via B–C Bond Formation across a Triruthenium Plane. Organometallics 2012, 31, 1825–1831. [Google Scholar] [CrossRef]
- Arnold, N.; Braunschweig, H.; Dewhurst, R.D.; Ewing, W.C. Unprecedented Borane, Diborane(3), Diborene, and Borylene Ligands via Pt-Mediated Borane Dehydrogenation. J. Am. Chem. Soc. 2016, 138, 76–79. [Google Scholar] [CrossRef]
- Mednikov, E.G.; Dahl, L.F. Syntheses, structures and properties of primarily nanosized homo/heterometallic palladium CO/PR3-ligated clusters. Philos. Trans. R. Soc. A 2010, 368, 1301–1332. [Google Scholar] [CrossRef]
- Bag, R.; Kar, S.; Saha, S.; Gomosta, S.; Raghavendra, B.; Roisnel, T.; Ghosh, S. Heterometallic Triply-Bridging Bis-Borylene Complexes. Chem. Asian J. 2020, 15, 780–786. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.v.R. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef]
- Tsipis, C.A. Aromaticity/antiaromaticity in “bare” and “ligand-stabilized” rings of metal atoms. In Metal-Metal Bonding. Structure and Bonding; Parkin, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 136, pp. 217–274. [Google Scholar] [CrossRef]
- King, R.B. Three-Dimensional Aromaticity in Polyhedral Boranes and Related Molecules. Chem. Rev. 2001, 101, 1119–1152. [Google Scholar] [CrossRef]
- Wang, Y.; Deyris, P.-A.; Caneque, T.; Blanchard, F.; Li, Y.; Bigi, F.; Maggi, R.; Blanchard, S.; Maestri, G.; Malacria, M. A Simple Synthesis of Triangular All-Metal Aromatics Allowing Access to Isolobal All-Metal Heteroaromatics. Chem. Eur. J. 2015, 21, 12271–12274. [Google Scholar] [CrossRef]
- Boldyrev, A.I. All-Metal Aromaticity and Antiaromaticity. Chem. Rev. 2005, 105, 3716–3757. [Google Scholar] [CrossRef]
- Popov, I.A.; Pan, F.-X.; You, X.R.; Li, L.J.; Matito, E.; Liu, C.; Zhai, H.-J.; Sun, Z.-M.; Boldyrev, A.I. Peculiar All-Metal s-Aromaticity of the [Au2Sb16]4− Anion in the Solid State. Angew. Chem. Int. Ed. 2016, 55, 15344–15346. [Google Scholar] [CrossRef]
- Boldyrev, A.I.; Wang, L.S. Beyond organic chemistry: Aromaticity in atomic clusters. Phys. Chem. Chem. Phys. 2016, 18, 11589–11605. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhai, H.J.; Kiran, B.; Wang, L.S. Observation of d-Orbital Aromaticity. Angew. Chem. Int. Ed. 2005, 44, 7251–7254. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.J.; Averkiev, B.B.; Zubarev, D.Y.; Wang, L.S.; Boldyrev, A.I. δ Aromaticity in [Ta3O3]−. Angew. Chem. Int. Ed. 2007, 46, 4277–4280. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Pennington, W.T.; Robinson, G.H. A Metallic System with Aromatic Character. Synthesis and Molecular Structure of Na2[(Mes2C6H3)Ga]3 (Mes = 2,4,6-Me3C6H2): The First Cyclogallane. J. Am. Chem. Soc. 1995, 117, 7578–7579. [Google Scholar] [CrossRef]
- Blanchard, S.; Fensterbank, L.; Gontard, G.; Lacote, E.; Maestri, G.; Malacria, M. Synthesis of Triangular Tripalladium Cations as Noble-Metal Analogues of the Cyclopropenyl Cation. Angew. Chem. Int. Ed. 2014, 53, 1987–1991. [Google Scholar] [CrossRef]
- Robilotto, T.J.; Bacsa, J.; Gray, T.G.; Sadighi, J.P. Synthesis of a Trigold Monocation: An Isolobal Analogue of [H3]+. Angew. Chem. Int. Ed. 2012, 51, 12077–12080. [Google Scholar] [CrossRef]
- Freitag, K.; Gemel, C.; Jerabek, P.; Oppel, I.M.; Seidel, R.W.; Frenking, G.; Banh, H.; Dilchert, K.; Fischer, R.A. The s-Aromatic Clusters [Zn3]+ and [Zn2Cu]: Embryonic Brass. Angew. Chem. Int. Ed. 2015, 54, 4370–4374. [Google Scholar] [CrossRef]
- Boronski, J.T.; Seed, J.A.; Hunger, D.; Woodward, A.W.; van Slageren, J.; Wooles, A.J.; Natrajan, L.S.; Kaltsoyannis, N.; Liddle, S.T. A crystalline tri-thorium cluster with σ-aromatic metal–metal bonding. Nature 2021, 598, 72–75. [Google Scholar] [CrossRef]
- Braunschweig, H.; Dewhurst, R.D.; Gessner, V.H. Transition metal borylene complexes. Chem. Soc. Rev. 2013, 42, 3197–3208. [Google Scholar] [CrossRef]
- Borthakur, R.; Saha, K.; Kar, S.; Ghosh, S. Recent advances in transition metal diborane(6), diborane(4) and diborene(2) chemistry. Coord. Chem. Rev. 2019, 399, 213021. [Google Scholar] [CrossRef]
- Kaur, U.; Saha, K.; Gayen, S.; Ghosh, S. Contemporary developments in transition metal boryl complexes: An overview. Coord. Chem. Rev. 2021, 446, 214106. [Google Scholar] [CrossRef]
- Saha, K.; Kaur, U.; Kar, S.; Mondal, B.; Joseph, B.; Antharjanam, P.K.S.; Ghosh, S. Trithia-diborinane and Bis(bridging-boryl) Complexes of Ruthenium Derived from a [BH3(SCHS)]− Ion. Inorg. Chem. 2019, 58, 2346–2353. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Maguire, J.A. Metallacarboranes of d- and f-block metals. In Comprehensive Organometallic Chemistry III, 3rd ed.; Crabtree, R.H., Mingos, D.M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 3, pp. 175–264. [Google Scholar] [CrossRef]
- Kar, S.; Ghosh, S. Borane Polyhedra beyond Icosahedron. In 50th Anniversary of Electron Counting Paradigms for Polyhedral Molecules. Structure and Bonding; Mingos, D.M.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 187, pp. 109–138. [Google Scholar] [CrossRef]
- De, A.; Zhang, Q.-F.; Mondal, B.; Cheung, L.F.; Kar, S.; Saha, K.; Varghese, B.; Wang, L.-S.; Ghosh, S. [(Cp2M)2B9H11] (M = Zr or Hf): Early transition metal ‘guarded’ heptaborane with strong covalent and electrostatic bonding. Chem. Sci. 2018, 9, 1976–1981. [Google Scholar] [CrossRef]
- Kar, S.; Bairagi, S.; Joshi, G.; Jemmis, E.D.; Ghosh, S. Metal stabilized [B8H8]2− derivatives with dodecahedral structure in solid- and solution-state: [(Cp2MBH3)2B8H6], (Cp = ƞ5-C5H5; M = Zr (1-Zr) and Hf (1-Hf)). Chem. Eur. J. 2021, 27, 15634–15637. [Google Scholar] [CrossRef]
- Roy, D.K.; Mondal, B.; Shankhari, P.; Anju, R.S.; Geetharani, K.; Mobin, S.M.; Ghosh, S. Supraicosahedral Polyhedra in Metallaboranes: Synthesis and Structural Characterization of 12-, 15- and 16-Vertex Rhodaboranes. Inorg. Chem. 2013, 52, 6705–6712. [Google Scholar] [CrossRef]
- Mondal, B.; Bag, R.; Ghorai, S.; Bakthavachalam, K.; Jemmis, E.D.; Ghosh, S. Synthesis, Structure, Bonding and Reactivity of Metal Complexes comprising Diborane(4) and Diborene(2): [{Cp*Mo(CO)2}2{µ-η2:η2-B2H4}] and [{Cp*M(CO)2}2B2H2M(CO)4], M=Mo,W. Angew. Chem. Int. Ed. 2018, 57, 8079–8083. [Google Scholar] [CrossRef]
- Saha, K.; Ghorai, S.; Kar, S.; Saha, S.; Halder, R.; Raghavendra, B.; Jemmis, E.D.; Ghosh, S. Stabilization of Classical [B2H5]−: Structure and Bonding of [(Cp*Ta)2(B2H5)(µ-H)L2] (Cp* = η5-C5Me5; L = SCH2S). Angew. Chem. Int. Ed. 2019, 58, 17684–17689. [Google Scholar] [CrossRef]
- Duff, A.W.; Jonas, K.; Goddard, R.; Kraus, H.J.; Krueger, C. The first triple-decker sandwich with a bridging benzene ring. J. Am. Chem. Soc. 1983, 105, 5479–5480. [Google Scholar] [CrossRef]
- Rheingold, A.L.; Foley, M.J.; Sullivan, P.J. “Triple-decker sandwich” with a planar As5 ring. Synthesis and crystal structure of CpMo[µ-(h4-As5)]MoCp. J. Am. Chem. Soc. 1982, 104, 4727–4729. [Google Scholar] [CrossRef]
- Herberich, G.E.; Hausmann, I.; Klaff, N. The 30-Electron Rule for Triple-Decker Complexes –Vanadium, Niobium and Tantalum Complexes as Illustrative Examples. Angew. Chem. Int. Ed. 1989, 28, 319–320. [Google Scholar] [CrossRef]
- Bose, S.K.; Geetharani, K.; Varghese, B.; Ghosh, S. Unusual Organic Chemistry of a Metallaborane Substrate: Formation of Tantalaborane Complex with Bridging Acyl Group (m-h2). Inorg. Chem. 2010, 49, 6375–6377. [Google Scholar] [CrossRef]
- Brunner, H.; Gehart, G.; Nuber, B.; Wachter, J.; Ziegler, M.L. The Reaction of [Cp+2Nb2(B2H6)2] (Cp+ = η-EtMe4C5) with Sulfur: Stabilization of the Tetrathioborato Ligand in Novel Sulfido Niobium Clusters. Angew. Chem. Int. Ed. 1992, 31, 1021–1023. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Tatsumi, K. Synthesis of (Pentamethylcyclopentadienyl)tantalum Sulfido Complexes via C−S Bond Cleavage of Triphenylmethanethiolate and Formation of a Novel Trithioborato Ligand. Organometallics 1997, 16, 307–309. [Google Scholar] [CrossRef]
- Kar, S.; Saha, K.; Saha, S.; Bakthavachalam, K.; Dorcet, V.; Ghosh, S. Trimetallic Cubane-Type Clusters: Transition-Metal Variation as a Probe of the Roots of Hypoelectronic Metallaheteroboranes. Inorg. Chem. 2018, 57, 10896–10905. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Bairagi, S.; Saha, K.; Raghavendra, B.; Ghosh, S. Chalcogen stabilized trimetallic clusters: Synthesis, structures, and bonding of [(Cp*M)3(E)6+m(BH)n] (M = Nb or Ta; E = S or Se; m = 0 or 1 or 2; n = 0 or 1). Dalton Trans. 2019, 48, 4203–4210. [Google Scholar] [CrossRef]
- Saha, K.; Kar, S.; Ghosh, S. Chalcogen stabilized cubane-type cluster: Synthesis and structure of [(Cp*Ta)3(µ-Se)3(µ3-Se)3B(OBuCl)]. J. Indian Chem. Soc. 2018, 95, 729–734. [Google Scholar] [CrossRef]
- Bose, S.K.; Geetharani, K.; Ramkumar, V.; Mobin, S.M.; Ghosh, S. Fine Tuning of Metallaborane Geometries: Chemistry of Metallaboranes of Early Transition Metals Derived from Metal Halides and Monoborane Reagents. Chem. Eur. J. 2009, 15, 13483–13490. [Google Scholar] [CrossRef]
- Leblanc, J.-C.; Moise, C.; Volpato, F.; Brunner, H.; Gehart, G.; Wachter, J.; Nuber, B. Comparative studies in the Cp2M(η2-S2) H series (Cp = t-BuC5H4, C5MeEt; M = Nb, Ta) on the reactivity of hydrie and disulfide ligands. J. Organomet. Chem. 1995, 485, 237–242. [Google Scholar] [CrossRef]
- Schmider, H.L.; Becke, A.D. Optimized density functionals from the extended G2 test set. J. Chem. Phys. 1998, 108, 9624–9631. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO Program 3.1; University of Wisconsin: Madison, WI, USA, 1988. [Google Scholar]
- Reed, A.E.; Weinhold, F.; Curtiss, L.A. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, R. Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Wiberg, K.B. Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Okamoto, T.; Yasuda, H.; Nakamura, A.; Kai, Y.; Kanehisa, N.; Kasai, N. Synthesis and Catalysis of Novel Mono- and Bis(diene) Complexes of Niobium and X-ray Structures of Binuclear [Nb(µ-Cl)(C5H5)(s-cis-butadiene)]2 and Mononuclear Nb(C5H5)(s-cis-2,3-dimethylbutadiene)2. J. Am. Chem. Soc. 1988, 110, 5008–5017. [Google Scholar] [CrossRef]
- Lalancette, J.M.; Frêche, A.; Monteux, R. Reductions with sulfurated borohydrides. I. Preparation of sulfurated borohydrides. Can. J. Chem. 1968, 46, 2754–2757. [Google Scholar] [CrossRef]
- Lalancette, J.M.; Arnac, M. Reductions with sulfurated borohydrides. III. Borohydrides incorporating selenium and tellurium. Can. J. Chem. 1969, 47, 3695–3697. [Google Scholar] [CrossRef]
- Ryschlewitsch, G.E.; Nainan, K.C.; Miller, S.R.; Todd, L.J.; Dewkett, W.J.; Grace, M.; Beall, H.; Hawthorne, M.F.; Leyden, R. Octahydrotriborate (1-)(B3H8]) salt. Inorg. Synth. 1974, 15, 111–118. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Crystallogr. 1994, 27, 435–436. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, J.M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Dennington, I.I.; Keith, R.T.; Millam, J.; Eppinnett, K.; Hovell, W.L.; Gilliland, R. GaussView, Version 3.09; Semichem Inc.: Shawnee, KS, USA, 2003. [Google Scholar]
- Zhurko, G.A. Available online: https://www.chemcraftprog.com (accessed on 24 April 2022).
- 11B{1H} NMR spectra were processed with a backward linear prediction algorithm to eliminate the broad 11B background signal of the NMR tube
- Led, J.J.; Gesmar, H. Application of the linear prediction method to NMR spectroscopy. Chem. Rev. 1991, 91, 1413–1426. [Google Scholar] [CrossRef]
- Yang, L.; Simionescu, R.; Lough, A.; Yan, H. Some observations relating to the stability of the BODIPY fluorophore under acidic and basic conditions. Dyes Pigm. 2011, 91, 264–267. [Google Scholar] [CrossRef]
- Weiss, R.; Grimes, R.N. Sources of Line Width in Boron-11 Nuclear Magnetic Resonance Spectra. Scalar Relaxation and Boron-Boron Coupling in B4H10 and B5H9. J. Am. Chem. Soc. 1978, 100, 1401–1405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, S.; Chatterjee, D.; Halet, J.-F.; Ghosh, S. Trimetallic Chalcogenide Species: Synthesis, Structures, and Bonding. Molecules 2022, 27, 7473. https://doi.org/10.3390/molecules27217473
Kar S, Chatterjee D, Halet J-F, Ghosh S. Trimetallic Chalcogenide Species: Synthesis, Structures, and Bonding. Molecules. 2022; 27(21):7473. https://doi.org/10.3390/molecules27217473
Chicago/Turabian StyleKar, Sourav, Debipada Chatterjee, Jean-François Halet, and Sundargopal Ghosh. 2022. "Trimetallic Chalcogenide Species: Synthesis, Structures, and Bonding" Molecules 27, no. 21: 7473. https://doi.org/10.3390/molecules27217473
APA StyleKar, S., Chatterjee, D., Halet, J.-F., & Ghosh, S. (2022). Trimetallic Chalcogenide Species: Synthesis, Structures, and Bonding. Molecules, 27(21), 7473. https://doi.org/10.3390/molecules27217473









