Microwave-Assisted Extraction of Bioactive Compounds from Lentil Wastes: Antioxidant Activity Evaluation and Metabolomic Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Microwave Irradiation Effect on the Extraction of Lentil Bioactive Compounds Endowed with Antioxidant Activity and Solvent Screening
2.2. Chemical Assays for the Measurement of the Antioxidant Activity
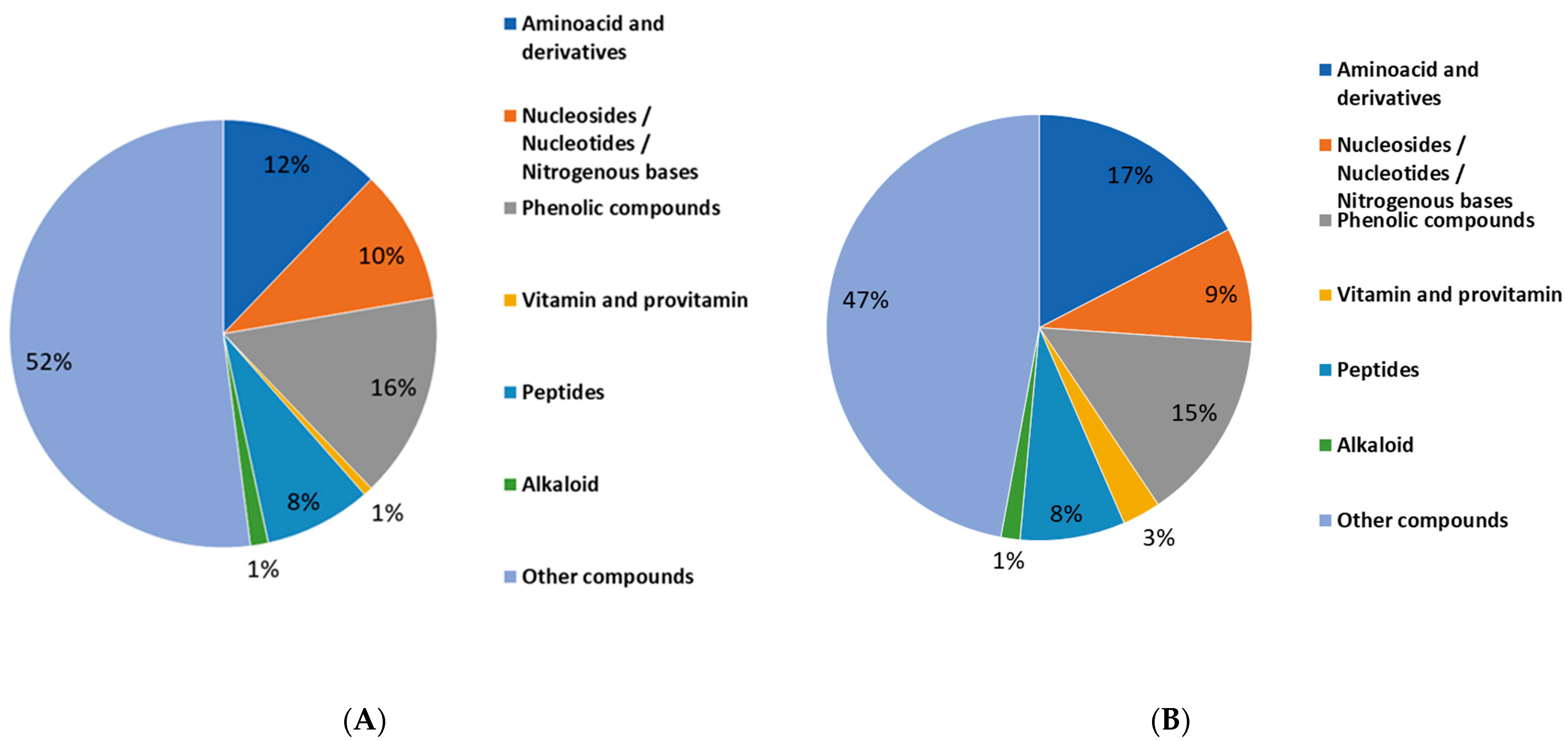
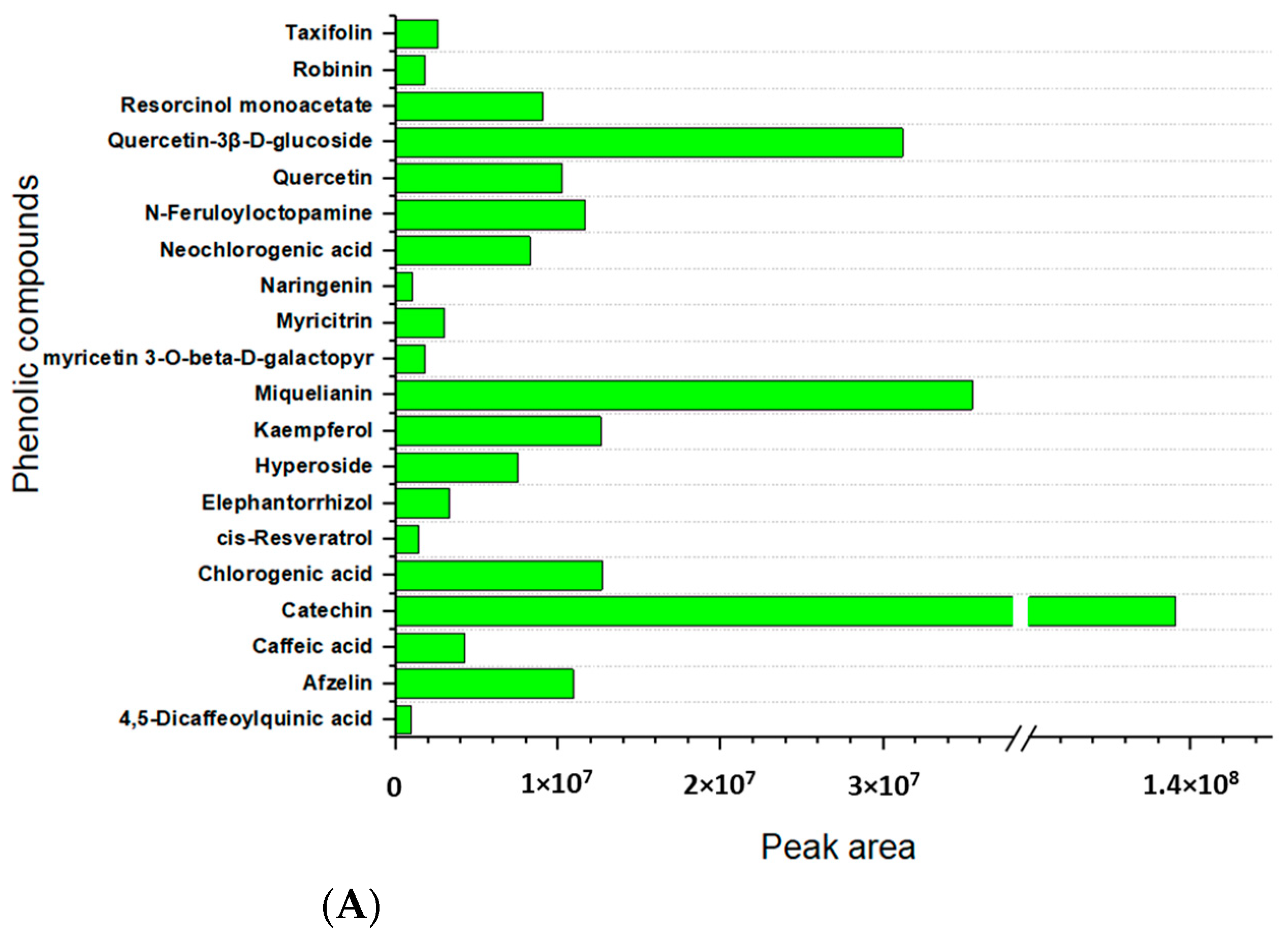
2.3. Metabolites Profiling of Lentil Hulls
3. Materials and Methods
3.1. Lentil Materials
3.2. Chemicals
3.3. Microwave-Assisted Extraction (MAE)
3.4. Conventional Extraction
3.5. Neuroblastoma Cell-Based Assays
3.5.1. Cell Viability
3.5.2. ROS Generation and Antioxidant Effects
3.5.3. Statistical Analysis
3.6. Chemical Assays
3.6.1. Determination of Total Phenolic Content (TPC)
3.6.2. Determination of Total Flavonoids Content (TFC)
3.6.3. Antioxidant Assay
DPPH Assay Procedure
ABTS Assay Procedure
3.7. Metabolite Profiling of Lentil Hulls
3.7.1. Sample Preparation
3.7.2. LC-HRMS Analysis
3.7.3. Metabolite Identification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeung, A.W.K.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jozwik, A.; Kühl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive oxygen species and their impact in neurodegenerative diseases: Literature landscape analysis. Antioxid. Redox Signal. 2020, 34, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.L.; Akanbi, T.O.; Tawiah, N.A.; Aryee, A.N.A. Valorization of seed and kernel marcs and evaluation of their antioxidant potential. Food Chem. 2022, 390, 133168. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural antioxidants from seeds and their application in meat products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef]
- Felter, S.P.; Zhang, X.; Thompson, C. Butylated hydroxyanisole: Carcinogenic food additive to be avoided or harmless antioxidant important to protect food supply? Regul. Toxicol. Pharmacol. 2021, 121, 104887. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva-Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C.; Cross, C.E. Free radicals, antioxidants, and human disease: Where are we now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar]
- Willett, W.C. Diet and health: What should we eat? Science 1994, 264, 532–537. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71S–88S. [Google Scholar] [CrossRef]
- Kushi, L.H.; Meyer, K.A.; Jacobs, D.R., Jr. Cereals, legumes, and chronic disease risk reduction: Evidence from epidemiological studies. Am. J. Clin. Nutr. 1999, 70, 451S–458S. [Google Scholar] [CrossRef]
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of Citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef]
- Moise, J.A.; Han, S.; Gudynaite-Savitch, L.; Johnson, D.A.; Miki, B.L.A. Seed coats: Structure, development, composition, biotechnology. In Vitro Cell. Dev. Biol.-Plant 2005, 41, 620–644. [Google Scholar] [CrossRef]
- Benayad, A.; Aboussaleh, Y. Mineral composition of lentils: Physiological functions, antinutritional effects, and bioavailability enhancement. J. Food Qual. 2021, 2021, 5515654. [Google Scholar] [CrossRef]
- Samaranayaka, A. Chapter 11—Lentil: Revival of poor man’s meat. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 185–196. [Google Scholar]
- Han, H.; Baik, B.-K. Antioxidant activity and phenolic content of lentils (Lens culinaris), chickpeas (Cicer arietinum L.), peas (Pisum sativum L.) and soybeans (Glycine max), and their quantitative changes during processing. Int. J. Food Sci. Technol. 2008, 43, 1971–1978. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Zieliński, H.; Piskuła, M.K. Contribution of low-molecular-weight antioxidants to the antioxidant capacity of raw and processed lentil seeds. Nahrung 2003, 47, 291–299. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Azzini, E.; Maiani, G.; Carcea, M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013, 140, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chang, S.K.C. Phenolic substance characterization and chemical and cell-based antioxidant activities of 11 lentils grown in the northern United States. J. Agric. Food Chem. 2010, 58, 1509–1517. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef]
- Singh, B.; Singh, N.; Thakur, S.; Kaur, A. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J. Food Sci. Technol. 2017, 54, 921–932. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Milani, G.; Curci, F.; Cavalluzzi, M.M.; Crupi, P.; Pisano, I.; Lentini, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Optimization of microwave-assisted extraction of antioxidants from bamboo shoots of Phyllostachys pubescens. Molecules 2020, 25, 215. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Quintieri, L.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. Antimicrobial and antibiofilm activities of citrus water-extracts obtained by microwave-assisted and conventional methods. Biomedicines 2018, 6, 70. [Google Scholar] [CrossRef]
- Roselli, M.; Lovece, A.; Bruno, C.; Cavalluzzi, M.M.; Laghezza, A.; Mercurio, A.; Lentini, G.; Corbo, F.; la Forgia, F.; Fontana, S.; et al. Antioxidant activity of Uva di Troia Canosina: Comparison of two extraction methods. Clin. Immunol. Endocr. Metab. Drugs 2015, 2, 8–12. [Google Scholar] [CrossRef]
- Sahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef]
- Chemat, F.; Cravotto, G. (Eds.) Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Food Engineering Series; Springer Science: New York, NY, USA, 2013; Volume XII, p. 238. [Google Scholar]
- Lovric, V.; Putnik, P.; Kovacevic, D.B.; Jukic, M.; Dragovic-Uzelac, V. Effect of microwave-assisted extraction on the phenolic compounds and antioxidant capacity of blackthorn flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef]
- Duenas, M.; Hernandez, T.; Estrella, I. Assessment of in vitro antioxidant capacity of the seed coat and the cotyledon of legumes in relation to their phenolic contents. Food Chem. 2006, 98, 95–103. [Google Scholar] [CrossRef]
- Kaya, E.; Tuncel, N.B.; Yılmaz Tuncel, N. The effect of ultrasound on some properties of pulse hulls. J. Food Sci. Technol. 2017, 54, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, Z.; Liu, R.; Zhang, H.; Zhu, H.; Jiang, L.; Tsao, R. Comprehensive profiling of free, conjugated and bound phenolics and lipophilic antioxidants in red and green lentil processing by-products. Food Chem. 2020, 325, 126925. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Identification and quantification of soluble and insoluble-bound phenolics in lentil hulls using HPLC-ESI-MS/MS and their antioxidant potential. Food Chem. 2020, 315, 126202. [Google Scholar] [CrossRef] [PubMed]
- Paranavitana, L.; Oh, W.Y.; Yeo, J.; Shahidi, F. Determination of soluble and insoluble-bound phenolic compounds in dehulled, whole, and hulls of green and black lentils using electrospray ionization (ESI)-MS/MS and their inhibition in DNA strand scission. Food Chem. 2021, 361, 130083. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.F.R.; Oliveira, V.B.; Falcão, D.S. Direct Alcohol Fuel Cells for portable Applications: Fundamentals, Engineering and Advances, 1st ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Novotny, N.M.; Grosfeld, J.L.; Turner, K.E.; Rescorla, F.J.; Pu, X.; Klaunig, J.E.; Hickey, R.J.; Malkas, L.H.; Sandoval, J.A. Oxidative status in neuroblastoma: A source of stress? J. Pediatr. Surg. 2008, 43, 330–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Meo, F.; Cuciniello, R.; Margarucci, S.; Bergamo, P.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Ginkgo biloba prevents oxidative stress-induced apoptosis blocking p53 activation in neuroblastoma cells. Antioxidants 2020, 9, 279. [Google Scholar] [CrossRef]
- Dal-Cim, T.; Molz, S.; Egea, J.; Parada, E.; Romero, A.; Budni, J.; Martín de Saavedra, M.D.; del Barrio, L.; Tasca, C.I.; López, M.G. Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3b pathway. Neurochem. Int. 2012, 61, 397–404. [Google Scholar] [CrossRef]
- Comert, E.D.; Mogol, B.A.; Gokmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Sumner, L.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Schymanski, E.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Rochfort, S.; Panozzo, J. Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef]
- Oomah, B.D.; Caspar, F.; Malcolmson, L.J.; Bellido, A.-S. Phenolics and antioxidant activity of lentil and pea hulls. Food Res. Int. 2011, 44, 436–441. [Google Scholar] [CrossRef]
- Duenas, M.; Hernandez, T.; Estrella, I. Phenolic composition of the cotyledon and the seed coat of lentils (Lens culinaris L.). Eur. Food Res.Technol. 2002, 215, 478–483. [Google Scholar]
- Mirali, M.; Ambrose, S.J.; Wood, S.A.; Vandenberg, A.; Purves, R.W. Development of a fast extraction method and optimization of liquid chromatography–mass spectrometry for the analysis of phenolic compounds in lentil seed coats. J. Chromatogr. B 2014, 969, 149–161. [Google Scholar] [CrossRef]
- Mirali, M.; Purves, R.W.; Vandenberg, A. Profiling the phenolic compounds of the four major seed coat types and their relation to color genes in lentil. J. Nat. Prod. 2017, 80, 1310–1317. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Rochfort, S.; Vassiliadis, S.; Maharjan, P.; Brand, J.; Panozzo, J. NMR based metabolomic analysis of health promoting phytochemicals in lentils. Metabolites 2019, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Peng, L.; Xiong, H.; Wang, J.; Tsao, R.; Peng, X.; Jiang, L.; Sun, Y. Free and bound phenolics of Laird Lentil (Lens culinaris) hulls and the anti-inflammatory activity of their digestive products via crosstalk between NF-κB and Keap1-Nrf2 signaling pathways in HT-29 cells. J. Agric. Food Chem. 2022; in press. [Google Scholar] [CrossRef]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Su, Y.; Xiong, H. Polyphenol content of Green Pea (Pisum sativum L.) hull under in vitro digestion and effects of digestive products on anti-inflammatory activity and intestinal barrier in the Caco-2/Raw264.7 coculture model. J. Agric. Food Chem. 2022, 70, 3477–3488. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Guo, F.; Pei, M.; Tsao, R.; Wang, X.; Jiang, L.; Sun, Y.; Xiong, H. Anti-inflammatory effect of lentil hull (Lens culinaris) extract via MAPK/NF-κB signaling pathways and effects of digestive products on intestinal barrier and inflammation in Caco-2 and Raw264.7 co-culture. J. Funct. Foods 2022, 92, 105044. [Google Scholar] [CrossRef]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A review on biosynthesis, analytical techniques, and pharmacological activities of trigonelline as a plant alkaloid. J. Diet. Suppl. 2018, 15, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; An, H.; Feng, L.; Liu, Q.; Wang, S.; Zhang, T. Sinapine as an active compound for inhibiting the proliferation of Caco-2 cells via downregulation of P-glycoprotein. Food Chem. Toxicol. 2014, 67, 187–192. [Google Scholar] [CrossRef]
- Available online: http://www.uwm.edu.pl/biochemia/index.php/pl/biopep (accessed on 20 July 2022).
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Saito, K.; Jin, D.H.; Ogawa, T.; Muramoto, K.; Hatakeyama, E.; Yasuhara, T.; Nokihara, K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J. Agric. Food Chem. 2003, 51, 3668–3674. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Maruyama, S.; Miyoshi, S.; Nomura, G.; Suzuki, M.; Tanaka, H.; Maeda, H. Specificity for various imino-acid-residues of a proline-specific dipeptidylcarboxypeptidase from a Streptomyces species. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1993, 1162, 72–76. [Google Scholar] [CrossRef]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship modeling of peptides containing 4-10 amino acid residues. QSAR Comb. Sci. 2006, 25, 873–880. [Google Scholar] [CrossRef]
- Rullo, M.; Cipolloni, M.; Catto, M.; Colliva, C.; Miniero, D.V.; Latronico, T.; de Candia, M.; Benicchi, T.; Linusson, A.; Giacchè, N.; et al. Probing fluorinated motifs onto dual AChE-MAO B inhibitors: Rational design, synthesis, biological evaluation, and early-ADME studies. J. Med. Chem. 2022, 65, 3962–3977. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; de Palma, A.; Giangregorio, N.; Miniero, D.-V.; Pesce, P.; Nicolotti, O.; Campagna, F.; Altomare, C.D.; Catto, M. Mannich base approach to 5-methoxyisatin 3-(4isopropylphenyl)hydrazone: A water-soluble prodrug for a multitarget inhibition of cholinesterases, beta-amyloid fibrillization and oligomer-induced cytotoxicity. Eur. J. Pharm. Sci. 2017, 109, 381–388. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Corbo, F. Processing of carob kernels to syrup by ultrasound-assisted extraction. Processes 2022, 10, 983. [Google Scholar] [CrossRef]
- Rached, I.; Barros, L.; Fernandes, I.P.; Santos-Buelga, C.; Rodrigues, A.E.; Ferchichi, A.; Barreiro, M.F.; Ferreira, I.C. Ceratonia siliqua L. hydroethanolic extract obtained by ultrasonication: Antioxidant activity, phenolic compounds profile and effects in yogurts functionalized with their free and microencapsulated forms. Food Funct. 2016, 7, 1319–1328. [Google Scholar] [CrossRef]



| IC50 (μg/mL) a | ||||||
|---|---|---|---|---|---|---|
| Entry | Sample | Solvent | Method | Yield (%) b | Antioxidant Activity c | Cytotoxicity d |
| 1 | Eston green seed | EtOAc | MAE | 0.9 | >100 | >200 |
| 2 | Eston green hull | EtOAc | MAE | 4.1 | 87 ± 2 | 110 ± 3 |
| 3 | Eston green hull | EtOAc | maceration | 4.8 | 126 ± 5 | >200 |
| 4 | Eston green hull | abs EtOH | MAE | 4.5 | 76 ± 1.3 | >200 |
| 5 | Eston green hull | H2O | MAE | 13 | 26 ± 7 | >200 |
| 6 | Crimson red hull | H2O | MAE | 12 | 10.1 ± 0.6 | >200 |
| Samples | TPC a | TFC b | ABTS c | DPPH d |
|---|---|---|---|---|
| Eston green | 7.88 ± 0.1 | 0.08 ± 0.01 | 3.43 ± 0.56 | 0.53 |
| Crimson red | 28.3 ± 0.1 | 1.89 ± 0.01 | 39.06 ± 0.73 | 0.39 |
| Class | Name | Chemical Formula | DeltaMass [ppm] | Molecular Weight (Da) | Adduct | m/z | RT (min) | Identification Level | Eston Green | Crimson Red |
|---|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | Sinapine | C16H23NO5 | 0 | 309.15762 | [M+H]+ | 310.1649 | 32.112 | ** | X | X |
| Trigonelline | C7H7NO2 | 0.05 | 137.04768 | [M–H]− | 138.055 | 2.024 | ** | X | X | |
| Aminoacids and derivatives | 2-Phenylglycine | C8H9NO2 | −0.47 | 151.06326 | [M+H]+ | 152.0705 | 7.376 | ** | X | |
| 5-Aminolevulinic acid | C5H9NO3 | −0.52 | 131.05817 | [M+H]+ | 132.0655 | 1.931 | ** | X | ||
| Asparagine | C4H8N2O3 | 0.56 | 132.05357 | [M+H]+ | 133.0609 | 1.897 | ** | X | X | |
| cis-4-Decenoyl carnitine | C17H31NO4 | −0.92 | 313.22502 | [M+H]+ | 314.2323 | 30.397 | * | X | ||
| d-Aspartic acid | C4H7NO4 | 0.07 | 133.03752 | [M+H]+ | 134.0448 | 19.36 | ** | X | ||
| d-Carnitine | C7H15NO3 | −1.14 | 161.10501 | [M+H]+ | 162.1123 | 1.951 | ** | X | ||
| Diaminopimelic acid | C7H14N2O4 | −0.1 | 190.09534 | [M+H]+ | 191.1026 | 2.069 | ** | X | ||
| dl-Arginine | C6H14N4O2 | 0.65 | 174.11179 | [M+H]+ | 175.1191 | 1.873 | ** | X | X | |
| dl-Glutamine | C5H10N2O3 | −0.27 | 146.0691 | [M+H]+ | 147.0764 | 2.021 | ** | X | X | |
| dl-Tryptophan | C11H12N2O2 | −2.75 | 204.08932 | [M–H]− | 203.082 | 21.55 | ** | X | X | |
| d-Serine | C3H7NO3 | 3.08 | 105.04292 | [M+H]+ | 106.0502 | 2.865 | ** | X | ||
| Glutinic acid | C5H4O4 | 1.21 | 128.01111 | [M+H]+ | 129.0184 | 4.388 | * | X | ||
| Glycylglycyl-N5-(phosphonoacetyl)-l-ornithine | C11H21N4O8P | 2.64 | 368.11067 | [M–H]− | 367.1034 | 47.585 | * | X | ||
| Indole-3-acetyl-l-aspartic acid | C14H14N2O5 | 1.15 | 290.09061 | [M–H]− | 289.0833 | 38.764 | ** | X | X | |
| l-2-Aminoadipic acid | C6H11N O4 | −0.5 | 161.06873 | [M+H]+ | 162.076 | 2.833 | ** | X | ||
| l-Aspartic acid | C4H7N O4 | 0.07 | 133.03752 | [M+H]+ | 134.0448 | 1.913 | ** | X | ||
| l-Glutamic acid | C5H9NO4 | 0.35 | 147.05321 | [M+H]+ | 148.0604 | 1.966 | ** | X | X | |
| l-Kynurenine | C10H12N2O3 | −0.4 | 208.08471 | [M+H]+ | 209.092 | 53.014 | ** | X | ||
| l-Pyroglutamic acid | C5H7NO3 | 0.26 | 129.04263 | [M+H]+ | 130.0499 | 4.146 | ** | X | ||
| l-Tyrosine | C9H11NO3 | −4.57 | 181.07307 | [M−H]− | 180.0658 | 5.941 | ** | X | ||
| Muramic acid | C9H17N O7 | −0.74 | 251.10031 | [M+H−H2O]+ | 234.097 | 1.966 | ** | X | ||
| N-({(1R,2S)-2-[(2Z)-5-Hydroxy-2-penten-1-yl]-3-oxocyclopentyl}acetyl)-l -isoleucine | C18H29NO5 | −0.38 | 339.20444 | [M+H]+ | 340.2115 | 43.883 | * | X | ||
| N2-[2-(Carboxymethyl)-2-hydroxy-3-methoxy-3-oxopropanoyl]arginine | C12H20N4O8 | −1.04 | 348.12775 | [M+H]+ | 349.135 | 3.966 | * | X | ||
| N3,N4-Dimethyl-l -arginine | C8H18N4O2 | −0.27 | 202.14292 | [M+H]+ | 203.1502 | 2.08 | ** | X | ||
| N6,N6,N6-Trimethyl-l-lysine | C9H20N2O2 | −0.02 | 188.15247 | [M+H]+ | 189.1598 | 1.793 | ** | X | X | |
| N6-Acetyl-l-lysine | C8H16N2O3 | −0.59 | 188.11598 | [M+H]+ | 189.1233 | 3.015 | ** | X | ||
| N-Acetyl-dl-glutamic acid | C7H11NO5 | −4.18 | 189.06293 | [M−H]− | 188.0557 | 4.651 | ** | X | ||
| N-Acetyl-dl-tryptophan | C13H14N2O3 | −0.6 | 246.10029 | [M−H]− | 245.093 | 47.529 | ** | X | X | |
| N-Acetyldopamine | C10H13NO3 | −0.04 | 195.08954 | [M+H]+ | 196.0968 | 19.554 | ** | X | ||
| N-Acetylornithine | C7H14N2O3 | 0.19 | 174.10048 | [M+H]+ | 175.1078 | 2.429 | ** | X | X | |
| O-Acetylserine | C5H9NO4 | −0.65 | 147.05306 | [M+H]+ | 148.0603 | 2.837 | ** | X | ||
| trans-3-Indoleacrylic acid | C11H9NO2 | 0.35 | 187.06339 | [M+H]+ | 188.0707 | 20.891 | ** | X | X | |
| Nucleosides/Nucleotides/Nitrogenous bases | 2′-Deoxyadenosine | C10H13N5O3 | −1.1 | 251.10156 | [M+H]+ | 252.1088 | 7.987 | ** | X | |
| 5′-Deoxy-5′-(methylsulfinyl)adenosine | C11H15N5O4S | −1.36 | 313.08405 | [M+H]+ | 314.0913 | 6.449 | * | X | ||
| Adenine | C5H5N5 | −0.14 | 135.05448 | [M+H]+ | 136.0618 | 7.514 | ** | X | X | |
| Adenosine | C10H13N5O4 | −0.16 | 267.09671 | [M+H]+ | 268.1039 | 7.457 | ** | X | X | |
| Adenosine 5′-monophosphate | C10H14N5O7P | −0.54 | 347.0629 | [M+H]+ | 348.07 | 3.301 | ** | X | X | |
| Cytidine | C9H13N3O5 | −1.1 | 243.08525 | [M+H]+ | 244.0925 | 3.16 | * | X | ||
| Guanine | C5H5N5O | −0.3 | 151.04936 | [M+H]+ | 152.0566 | 3.178 | ** | X | X | |
| Guanosine | C10H13N5O5 | 0.34 | 283.09177 | [M−H]− | 282.0847 | 8.841 | ** | X | ||
| Hypoxanthine | C5H4N4O | −0.01 | 136.03851 | [M+H]+ | 137.0458 | 8.598 | ** | X | X | |
| Succinyladenosine | C14H17N5O8 | 0.44 | 383.10788 | [M−H]− | 382.1009 | 22.063 | * | X | ||
| Thymidine | C10H14N2O5 | −0.59 | 242.09013 | [M−H]− | 241.0828 | 12.561 | * | X | ||
| Thymidine 5′-monophosphate | C10H15N2O8P | 0.35 | 322.05672 | [M−H]− | 321.0494 | 5.655 | ** | X | ||
| UDP-N-acetylglucosamine | C17H27N3O17P2 | 1.98 | 607.08277 | [M−H]− | 606.0755 | 1.98 | ** | X | X | |
| Uridine | C9H12N2O6 | −0.13 | 244.06951 | [M−H]− | 243.0622 | 5.204 | ** | X | X | |
| Uridine monophosphate (UMP) | C9H13N2O9P | 0.59 | 324.03606 | [M−H]− | 323.0288 | 2.09 | ** | X | X | |
| Xanthine | C5H4N4O2 | −0.51 | 152.03335 | [M+H]+ | 153.0406 | 11.922 | ** | X | X | |
| Xanthosine | C10H12N4O6 | 0.71 | 284.07589 | [M−H]− | 283.0686 | 11.276 | ** | X | X | |
| Phenolic compounds | 2-Hydroxycinnamic acid | C9H8O3 | −0.28 | 164.0473 | [M+H]+ | 165.0546 | 46.303 | ** | X | |
| 4,5-Dicaffeoylquinic acid | C25H24O12 | 1.39 | 516.12749 | [M−H]− | 515.1202 | 52.802 | ** | X | X | |
| 4-Coumaric acid | C9H8O3 | −0.41 | 164.04728 | [M+H]+ | 165.0546 | 23.362 | ** | X | ||
| Afzelin | C21H20O10 | −0.52 | 432.10542 | [M+H]+ | 433.1127 | 47.434 | ** | X | X | |
| Astragalin | C21H20O11 | 0.47 | 448.10077 | [M−H]− | 447.0937 | 53.359 | ** | X | ||
| Caffeic acid | C9H8O4 | −0.65 | 180.04214 | [M+H]+ | 181.0494 | 50.345 | ** | X | ||
| Catechin | C15H14O6 | 0.55 | 290.0792 | [M−H]− | 289.0721 | 27.964 | ** | X | X | |
| Chlorogenic acid | C16H18O9 | 0.52 | 354.09527 | [M−H]− | 353.0881 | 31.332 | ** | X | ||
| cis-Resveratrol | C14H12O3 | −2.01 | 228.07819 | [M+H]+ | 229.0855 | 48.31 | ** | X | X | |
| Elephantorrhizol | C15H14O8 | −1.31 | 322.06844 | [M+H]+ | 323.0757 | 44.924 | * | X | ||
| Esculetin | C9H6O4 | −0.66 | 178.02649 | [M+H]+ | 179.0338 | 42.794 | ** | X | ||
| Gallocatechin-4beta-ol | C15H14O8 | −1.02 | 322.06854 | [M+H]+ | 323.0758 | 44.976 | * | X | ||
| Gentisic acid | C7H6O4 | −0.8 | 154.02649 | [M+H−H2O]+ | 137.0232 | 31.442 | ** | X | ||
| Hyperoside | C21H20O12 | −0.93 | 464.09504 | [M+H]+ | 465.1023 | 52.357 | ** | X | ||
| Isovanillic acid | C8H8O4 | −0.42 | 168.04219 | [M+H]+ | 169.0495 | 16.696 | ** | X | ||
| Kaempferol | C15H10O6 | −1.14 | 286.04741 | [M+H]+ | 287.0547 | 47.431 | ** | X | X | |
| Kaempferol 3-(6″-p-coumarylgalactoside) | C30H26O13 | −1.13 | 594.13667 | [M+H]+ | 595.144 | 15.879 | * | X | ||
| Kaempferol-3-Galactoside-6″-Rhamnoside-3‴-Rhamnoside | C33H40O19 | 0.14 | 740.21648 | [M−H]− | 739.2098 | 52.091 | ** | X | ||
| Miquelianin | C21H18O13 | 0.99 | 478.07521 | [M−H]− | 477.0682 | 52.087 | ** | X | X | |
| myricetin 3-O-beta-d-galactopyranoside | C21H20O13 | 1.24 | 480.09099 | [M−H]− | 479.0837 | 50.067 | * | X | ||
| Myricitrin | C21H20O12 | 1.51 | 464.09617 | [M−H]− | 463.0889 | 51.46 | ** | X | X | |
| Naringenin | C15H12O5 | 0.28 | 272.06855 | [M−H]− | 271.0615 | 51.598 | ** | X | ||
| Naringeninchalcone | C15H12O5 | −1.2 | 272.06815 | [M+H]+ | 273.0754 | 49.806 | ** | X | ||
| Neochlorogenic acid | C16H18O9 | 1.27 | 354.09553 | [M−H]− | 353.0883 | 35.142 | ** | X | X | |
| N-Feruloyloctopamine | C18H19NO5 | 0.03 | 329.12633 | [M−H]− | 328.1193 | 52.074 | * | X | ||
| Nictoflorin | C27H30O15 | −0.37 | 594.15825 | [M+H]+ | 595.1653 | 50.122 | * | X | ||
| Quercetin | C15H10O7 | −1.12 | 302.04231 | [M+H]+ | 303.0496 | 53.336 | ** | X | X | |
| Quercetin-3β-d-glucoside | C21H20O12 | 1.42 | 464.09614 | [M−H]− | 463.0889 | 52.363 | ** | X | X | |
| Resorcinol monoacetate | C8H8O3 | −0.2 | 152.04731 | [M+H−H2O]+ | 135.0441 | 51.362 | ** | X | ||
| Robinin | C33H40O19 | −1.28 | 740.21543 | [M+H]+ | 741.2227 | 52.068 | ** | X | X | |
| Rutin | C27H30O16 | 1.02 | 610.15401 | [M−H]− | 609.1467 | 52.435 | ** | X | ||
| Taxifolin | C15H12O7 | −1.17 | 304.05795 | [M+H]+ | 305.0652 | 51.306 | ** | X | ||
| Peptides | ala-glu-trp | C19H24N4O6 | −2.99 | 404.16838 | [M−H]− | 403.1611 | 47.923 | * | X | |
| ala-ser-thr-tyr | C19H28N4O8 | −2.1 | 440.18979 | [M−H]− | 439.1825 | 46.227 | * | X | ||
| ala-trp-ala-pro | C22H29N5O5 | −2.03 | 443.21597 | [M−H]− | 442.2087 | 45.78 | * | X | ||
| alpha-d-Ko | C8H14O9 | −1.33 | 254.06344 | [M−H]− | 253.0562 | 1.908 | * | X | ||
| asn-tyr-tyr | C22H26N4O7 | −2.51 | 458.179 | [M−H]− | 457.1717 | 51.829 | * | X | ||
| asp-asp-his-glu | C19H26N6O11 | 0.79 | 514.16636 | [M−H]− | 513.1591 | 51.013 | * | X | ||
| asp-asp-phe-pro | C22H28N4O9 | −1.2 | 492.18504 | [M−H]− | 491.1779 | 51.002 | * | X | X | |
| asp-glu-glu-thr | C18H28N4O12 | −0.77 | 492.16999 | [M−H]− | 491.1623 | 2.087 | * | X | ||
| cys-glu-pro-asp | C17H26N4O9S | −2.97 | 462.14067 | [M+2H]2+ | 232.0776 | 46.847 | * | X | ||
| gln-trp-tyr | C25H29N5O6 | −1.06 | 495.21126 | [M−H]− | 494.204 | 29.916 | * | X | ||
| gln-tyr-ser | C17H24N4O7 | −2.61 | 396.16347 | [M−H]− | 395.1562 | 30.578 | * | X | ||
| gln-tyr-trp | C25H29N5O6 | −1.0 | 495.21129 | [M−H]− | 494.204 | 24.865 | * | X | ||
| glu-asp-phe-ile | C24H34N4O9 | −1.9 | 522.23158 | [M−H]− | 521.2243 | 52.253 | * | X | ||
| glu-gly-glu-glu | C17H26N4O11 | −2.12 | 462.15883 | [M+FA−H]− | 507.1569 | 2.059 | * | X | ||
| glu-trp-glu | C21H26N4O8 | −2.19 | 462.17405 | [M−H]− | 461.1668 | 44.783 | * | X | ||
| gly-ser-ser-phe | C17H24N4O7 | −2.68 | 396.16344 | [M−H]− | 395.1562 | 33.391 | * | X | ||
| l-Alanyl-l-proline | C8H14N2O3 | −0.47 | 186.10035 | [M+H]+ | 187.1076 | 2.091 | ** | X | ||
| l-Glutathione (reduced) | C10H17N3O6S | 0.45 | 307.08395 | [M−H]− | 306.0767 | 53.364 | ** | X | ||
| phe-ser-tyr-ala | C24H30N4O7 | −1.4 | 486.21077 | [M−H]− | 485.2035 | 45.812 | * | X | ||
| pro-pro-pro-ser | C18H28N4O6 | −2.98 | 396.19971 | [M−H]− | 395.1924 | 46.35 | * | X | ||
| thr-ser-ser-tyr | C19H28N4O9 | −1.22 | 456.18507 | [M−H]− | 455.1778 | 15.261 | * | X | ||
| val-trp-leu-glu | C27H39N5O7 | −2.43 | 545.28362 | [M−H]− | 544.2767 | 53.568 | * | X | ||
| Vitamins and provitamin | Choline | C5H13NO | 3.95 | 103.10012 | [M+H]+ | 104.1074 | 1.878 | ** | X | |
| Nicotinamide | C6H6N2O | 1.44 | 122.04819 | [M+H]+ | 123.0555 | 3.533 | ** | X | ||
| Panthenol | C9H19NO4 | −1.86 | 205.13103 | [M−H]− | 204.1236 | 14.547 | ** | X | X | |
| Pantothenic acid | C9H17NO5 | −1.41 | 219.11036 | [M−H]− | 218.103 | 14.391 | ** | X | X |
| Class | Sequence (Three-Letter Code) | Sequence (One Letter Code) | Molecular Weight (Da) | Eston Green | Crimson Red | ID Bioactive Peptide | Activity | Sequence Bioactive Peptide |
|---|---|---|---|---|---|---|---|---|
| Peptides | ala-glu-trp | AEW | 404.1684 | X | - | |||
| ala-ser-thr-tyr | ASTY | 440.1898 | X | - | ||||
| ala-trp-ala-pro | AWAP | 443.2160 | X | - | ||||
| asn-tyr-tyr | NYY | 458.1790 | X | 7965 | Antioxidant | NYY | ||
| asp-asp-his-glu | DDHE | 514.1664 | X | - | ||||
| asp-asp-phe-pro | DDFP | 492.1850 | X | X | - | |||
| asp-glu-glu-thr | DEET | 492.1700 | X | - | ||||
| cys-glu-pro-asp | CEDP | 462.1407 | X | - | ||||
| gln-trp-tyr | QWY | 495.2113 | X | - | ||||
| gln-tyr-ser | QYS | 396.1635 | X | - | ||||
| gln-tyr-trp | QYW | 495.2113 | X | 9197 | Antioxidant peptide | FFRSKLLSDGAAAAKGALLPQYW | ||
| 9198 | Alpha-amylase inhibitor | FFRSKLLSDGAAAAKGALLPQYW | ||||||
| 9199 | Antioxidant peptide | RCMAFLLSDGAAAAQQLLPQYW | ||||||
| 9200 | Alpha-amylase inhibitor | RCMAFLLSDGAAAAQQLLPQYW | ||||||
| glu-asp-phe-ile | EDFI | 522.2316 | X | - | ||||
| glu-gly-glu-glu | EGEE | 462.1588 | X | - | ||||
| glu-trp-glu | EWE | 462.1741 | X | 8237 8238 | Antioxidant | AIEWEGIESGSVEQA, IEWEGIESGSVEQA | ||
| gly-ser-ser-phe | GSSF | 396.1634 | X | - | ||||
| ala-pro | AP | 186.1004 | X | 3177 | Dipeptidyl peptidase IV inhibitor (DPP IV inhibitor) | AP | ||
| LL-Glutathione (reduced) | GSH | 307.0840 | X | 9035 | ACE inhibitor | GSH | ||
| 9954 | Antioxidant peptide | |||||||
| phe-ser-tyr-ala | FSYA | 486.2108 | X | - | ||||
| pro-pro-pro-ser | PPPS | 396.1997 | X | 3778 | Dipeptidyl carboxypeptidase inhibitor | PPPS | ||
| thr-ser-ser-tyr | TSSY | 456.1851 | X | - | ||||
| val-trp-leu-glu | VWLE | 545.2836 | X | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalluzzi, M.M.; Lamonaca, A.; Rotondo, N.P.; Miniero, D.V.; Muraglia, M.; Gabriele, P.; Corbo, F.; De Palma, A.; Budriesi, R.; De Angelis, E.; et al. Microwave-Assisted Extraction of Bioactive Compounds from Lentil Wastes: Antioxidant Activity Evaluation and Metabolomic Characterization. Molecules 2022, 27, 7471. https://doi.org/10.3390/molecules27217471
Cavalluzzi MM, Lamonaca A, Rotondo NP, Miniero DV, Muraglia M, Gabriele P, Corbo F, De Palma A, Budriesi R, De Angelis E, et al. Microwave-Assisted Extraction of Bioactive Compounds from Lentil Wastes: Antioxidant Activity Evaluation and Metabolomic Characterization. Molecules. 2022; 27(21):7471. https://doi.org/10.3390/molecules27217471
Chicago/Turabian StyleCavalluzzi, Maria Maddalena, Antonella Lamonaca, Natalie Paola Rotondo, Daniela Valeria Miniero, Marilena Muraglia, Paola Gabriele, Filomena Corbo, Annalisa De Palma, Roberta Budriesi, Elisabetta De Angelis, and et al. 2022. "Microwave-Assisted Extraction of Bioactive Compounds from Lentil Wastes: Antioxidant Activity Evaluation and Metabolomic Characterization" Molecules 27, no. 21: 7471. https://doi.org/10.3390/molecules27217471
APA StyleCavalluzzi, M. M., Lamonaca, A., Rotondo, N. P., Miniero, D. V., Muraglia, M., Gabriele, P., Corbo, F., De Palma, A., Budriesi, R., De Angelis, E., Monaci, L., & Lentini, G. (2022). Microwave-Assisted Extraction of Bioactive Compounds from Lentil Wastes: Antioxidant Activity Evaluation and Metabolomic Characterization. Molecules, 27(21), 7471. https://doi.org/10.3390/molecules27217471













