Cobalt Incorporated Graphitic Carbon Nitride as a Bifunctional Catalyst for Electrochemical Water-Splitting Reactions in Acidic Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Compounds
2.2. Instruments
3. Results and Discussion
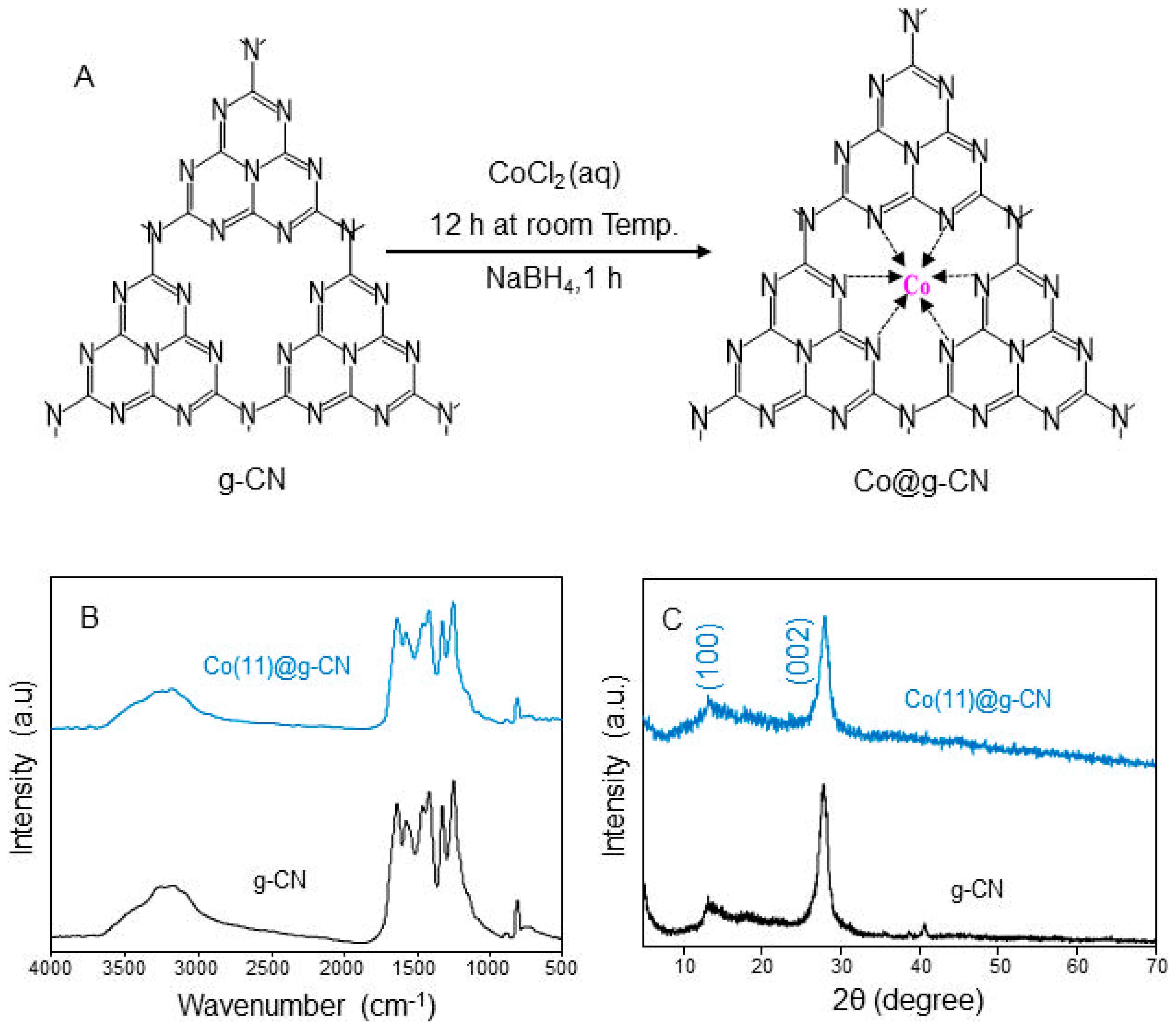
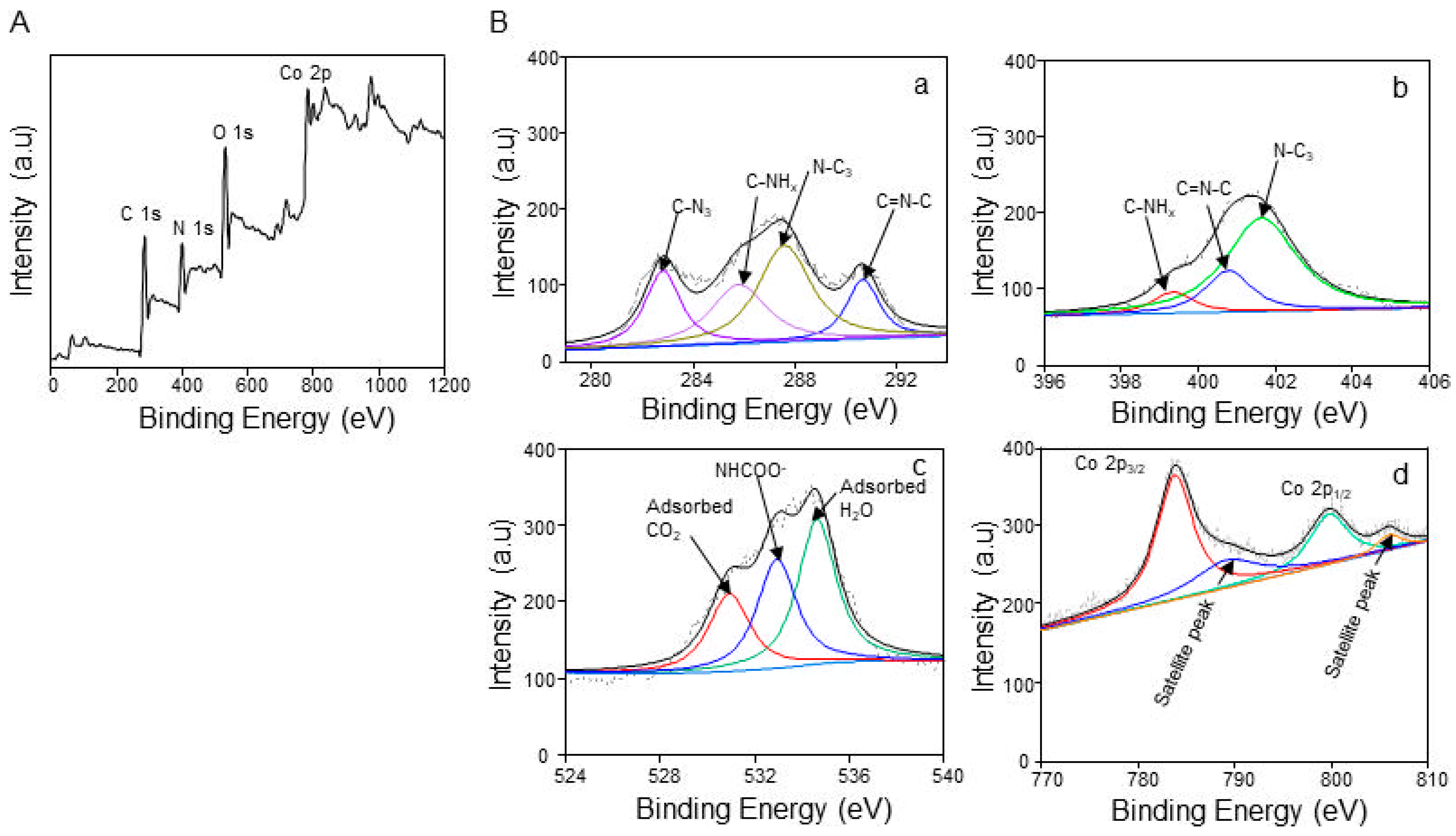
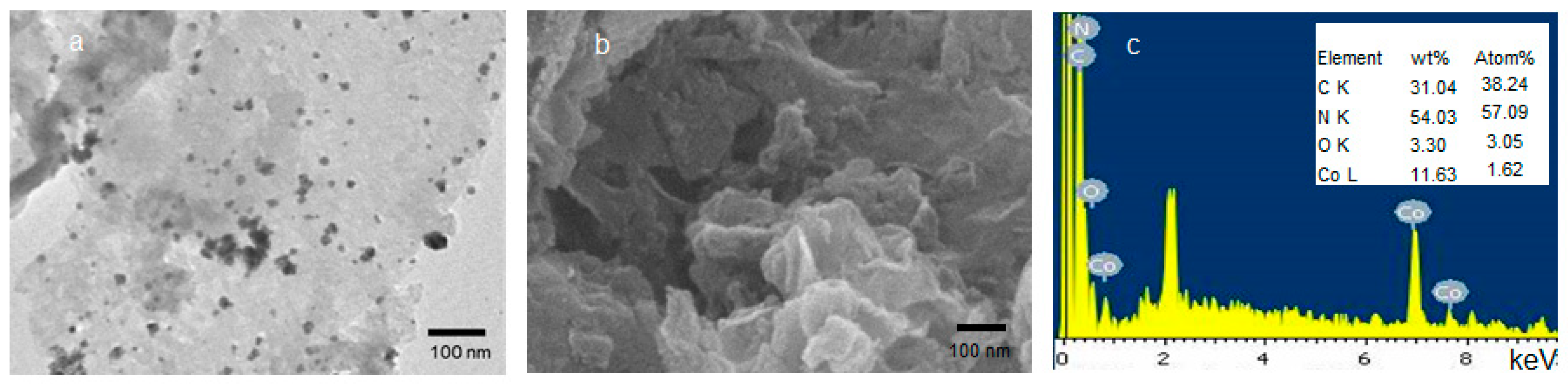
3.1. Structural Characteristics of Co@g-CN
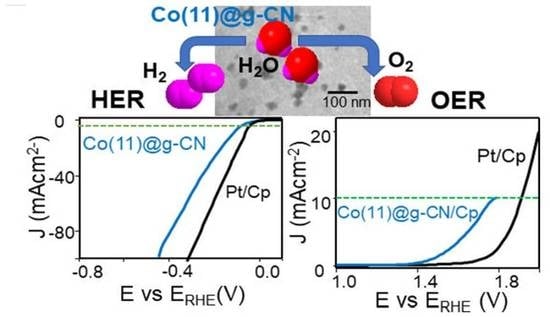
3.2. Electrochemical Water-Splitting Activity of Co@g-CN Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Devadas, B.; Chang, C.C.; Imae, T. Hydrogen evolution reaction efficiency of carbon nanohorn incorporating molybdenum sulfide and polydopamine/palladium nanoparticles. J. Taiwan Inst. Chem. Eng. 2019, 102, 378–386. [Google Scholar] [CrossRef]
- Itagi, M.; Bhat, Z.M.; Thimmappa, R.; Pandit, D.; Devendrachari, M.C.; Sannegowda, L.K.; Thotiyl, M.O. An Electrochemical Valorization Fuel Cell for Simultaneous Electroorganic and Hydrogen Fuel Syntheses. J. Phys. Chem. C 2020, 124, 11284–11292. [Google Scholar] [CrossRef]
- Aftab, S.; Shah, A.; Nisar, J.; Ashiq, M.N.; Akhter, M.S.; Shah, A.H. Marketability Prospects of Microbial Fuel Cells for Sustainable Energy Generation. Energy Fuels 2020, 34, 9108–9136. [Google Scholar] [CrossRef]
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.T.; Chen, L.; Weng, C.C.; Yuan, G.G.; Yuan, Z.Y. Well-Defined Mo2C Nanoparticles Embedded in Porous N-Doped Carbon Matrix for Highly Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 33276–33286. [Google Scholar] [CrossRef] [PubMed]
- Joyner, J.; Oliveira, E.F.; Yamaguchi, H.; Kato, K.; Vinod, S.; Galvao, D.S.; Salpekar, D.; Roy, S.; Martinez, U.; Tiwary, C.S. Graphene Supported MoS2 Structures with High Defect Density for an Efficient HER Electrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 12629–12638. [Google Scholar] [CrossRef] [PubMed]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Lee, J.-W.; Lee, J.-H.; Bajgai, J.; Lee, K.-J.; Park, S.-J. Photocatalytic hydrogen evolution via water splitting: A short review. Catalysts 2018, 8, 655–674. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Huang, Y.; Fu, J. Recent advances in electrocatalysts for neutral and large-current-density water electrolysis. Nano. Energy 2020, 80, 105545. [Google Scholar] [CrossRef]
- Jo, W.-K.; Moru, S.; Tonda, S. Cobalt-Coordinated Sulfur-Doped Graphitic Carbon Nitride on Reduced Graphene Oxide: An Efficient Metal–(N,S)–C-Class Bifunctional Electrocatalyst for Overall Water Splitting in Alkaline Media. ACS Sustain. Chem. Eng. 2019, 7, 15373–15384. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, C.Q.; Liu, W.; Hung, S.F.; Bin Yang, H.; Gao, J.; Cai, W.; Chen, H.M.; Li, J.; Liu, B. Coordination engineering of iridium nanocluster bifunctional electrocatalyst for highly efficient and pH-universal overall water splitting. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Wu, D.; Chen, Z.-n.; Sun, F.; Zhang, H.; Chen, Z.; Cao, M.; Zhuang, W.; Cao, R. Highly Active and Stable Water Splitting in Acidic Media Using a Bifunctional Iridium/Cucurbit[6]uril Catalyst. ACS Energy Lett. 2019, 4, 1301–1307. [Google Scholar] [CrossRef]
- Kwak, I.H.; Kwon, I.S.; Debela, T.T.; Abbas, H.G.; Park, Y.C.; Seo, J.; Ahn, J.P.; Lee, J.H.; Park, J.; Kang, H.S. Phase Evolution of Re1−xMoxSe2 Alloy Nanosheets and Their Enhanced Catalytic Activity toward Hydrogen Evolution Reaction. ACS Nano. 2020, 14, 11995–12005. [Google Scholar] [CrossRef] [PubMed]
- Henckel, D.A.; Lenz, O.M.; Krishnan, K.M.; Cossairt, B.M. Improved HER Catalysis through Facile, Aqueous Electrochemical Activation of Nanoscale WSe2. Nano Lett 2018, 18, 2329–2335. [Google Scholar] [CrossRef]
- Devadas, B.; Imae, T. Hydrogen evolution reaction efficiency by low loading of platinum nanoparticles protected by dendrimers on carbon materials. Electrochem. Commun. 2016, 72, 135–139. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, T.; Cheng, Y.; Liao, F.; Wu, K.; Shao, M. Ir/g-C3N4/Nitrogen-Doped Graphene Nanocomposites as Bifunctional Electrocatalysts for Overall Water Splitting in Acidic Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 39161–39167. [Google Scholar] [CrossRef]
- Wahab, M.A.; Joseph, J.; Atanda, L.; Sultana, U.K.; Beltramini, J.N.; Ostrikov, K.; Will, G.; O’Mullane, A.P.; Abdala, A. Nanoconfined Synthesis of Nitrogen-Rich Metal-Free Mesoporous Carbon Nitride Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 1439–1447. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Teng, X.; Meyer, T.J.; Chen, Z. CoP Nanoframes as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. ACS. Catal. 2019, 10, 412–419. [Google Scholar] [CrossRef]
- Chanda, D.; Tufa, R.A.; Birdja, Y.Y.; Basu, S.; Liu, S. Hydrothermally/electrochemically decorated FeSe on Ni-foam electrode: An efficient bifunctional electrocatalysts for overall water splitting in an alkaline medium. Int. J. Hydrogen Energy 2020, 45, 27182–27192. [Google Scholar] [CrossRef]
- Zulqarnain, M.; Shah, A.; Khan, M.A.; Jan Iftikhar, F.; Nisar, J. FeCoSe2 Nanoparticles Embedded in g-C3N4: A Highly Active and Stable bifunctional electrocatalyst for overall water splitting. Sci. Rep. 2020, 10, 6328. [Google Scholar] [CrossRef]
- Ejeta, S.Y.; Imae, T. Photodegradation of pollutant pesticide by oxidized graphitic carbon nitride catalysts. J. Photochem. Photobiol. A 2021, 404, 112955–112966. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Gao, Q.; Zhang, S.; Ge, C.; Yang, S.; Zhong, X.; Fang, Y. Modified Graphitic Carbon Nitride Nanosheets for Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2019, 12, 4996–5006. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Li, J.; Zhao, X.; Yang, E.-C.; Zhao, X.-J. Cobalt-doped graphitic carbon nitride with enhanced peroxidase-like activity for wastewater treatment. RSC Adv. 2016, 6, 35568–35576. [Google Scholar] [CrossRef]
- Chen, P.-W.; Li, K.; Yu, Y.-X.; Zhang, W.-D. Cobalt-doped graphitic carbon nitride photocatalysts with high activity for hydrogen evolution. Appl. Surf. Sci. 2017, 392, 608–615. [Google Scholar] [CrossRef]
- Wu, W.; Ruan, Z.; Li, J.; Li, Y.; Jiang, Y.; Xu, X.; Li, D.; Yuan, Y.; Lin, K. In Situ Preparation and Analysis of Bimetal Co-doped Mesoporous Graphitic Carbon Nitride with Enhanced Photocatalytic Activity. Nanomicro. Lett. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.J.; Imae, T.; Ujihara, M.; Huang, S.-J.; Wu, P.-H.; Liu, S.-B. Poly(amido amine) dendrimer-incorporated organoclays as efficient adsorbents for capture of NH3 and CO2. Chem. Eng. J. 2017, 312, 118–125. [Google Scholar] [CrossRef]
- Dai, H.; Gao, X.; Liu, E.; Yang, Y.; Hou, W.; Kang, L.; Fan, J.; Hu, X. Synthesis and characterization of graphitic carbon nitride sub-microspheres using microwave method under mild condition. Diam. Relat. Mater. 2013, 38, 109–117. [Google Scholar] [CrossRef]
- Efa, M.T.; Imae, T. Effects of carbon dots on ZnO nanoparticle-based dye-sensitized solar cells. Electrochim. Acta 2019, 303, 204–210. [Google Scholar] [CrossRef]
- Etefa, H.F.; Imae, T.; Yanagida, M. Enhanced Photosensitization by Carbon Dots Co-adsorbing with Dye on p-Type Semiconductor (Nickel Oxide) Solar Cells. ACS. Appl. Mater. Interfaces 2020, 12, 18596–18608. [Google Scholar] [CrossRef]
- Li, J. Investigation of hydrogen evolution on electrodeposited Ni/P coated carbon paper electrode in microbial fuel cell. Int. J. Electrochem. Sci. 2019, 14, 7582–7593. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Li, S.; Simke, J.R.J.; Schmidt, J.; Thomas, A. Bifunctional Electrocatalysts for Overall Water Splitting from an Iron/Nickel-Based Bimetallic Metal-Organic Framework/Dicyandiamide Composite. Angew Chem. Int Ed. Engl. 2018, 57, 8921–8926. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Song, H.; Li, X.; Zhang, X.; Gao, B.; Zheng, Y.; Huo, K.; Chu, P.K. Hierarchical 0D-2D Co/Mo Selenides as Superior Bifunctional Electrocatalysts for Overall Water Splitting. Front. Chem. 2020, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, J.; An, L.; Lu, M.; Sun, K.; Zhao, Y.Q.; Gao, D.; Cheng, F.; Xi, P. FeS2/CoS2 Interface Nanosheets as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Small 2018, 14, e1801070. [Google Scholar] [CrossRef]
- Dong, C.; Liu, X.; Wang, X.; Yuan, X.; Xu, Z.; Dong, W.; Riaz, M.S.; Li, G.; Huang, F. Hierarchical Ni/NiTiO3 derived from NiTi LDHs: A bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2017, 5, 24767–24774. [Google Scholar] [CrossRef]
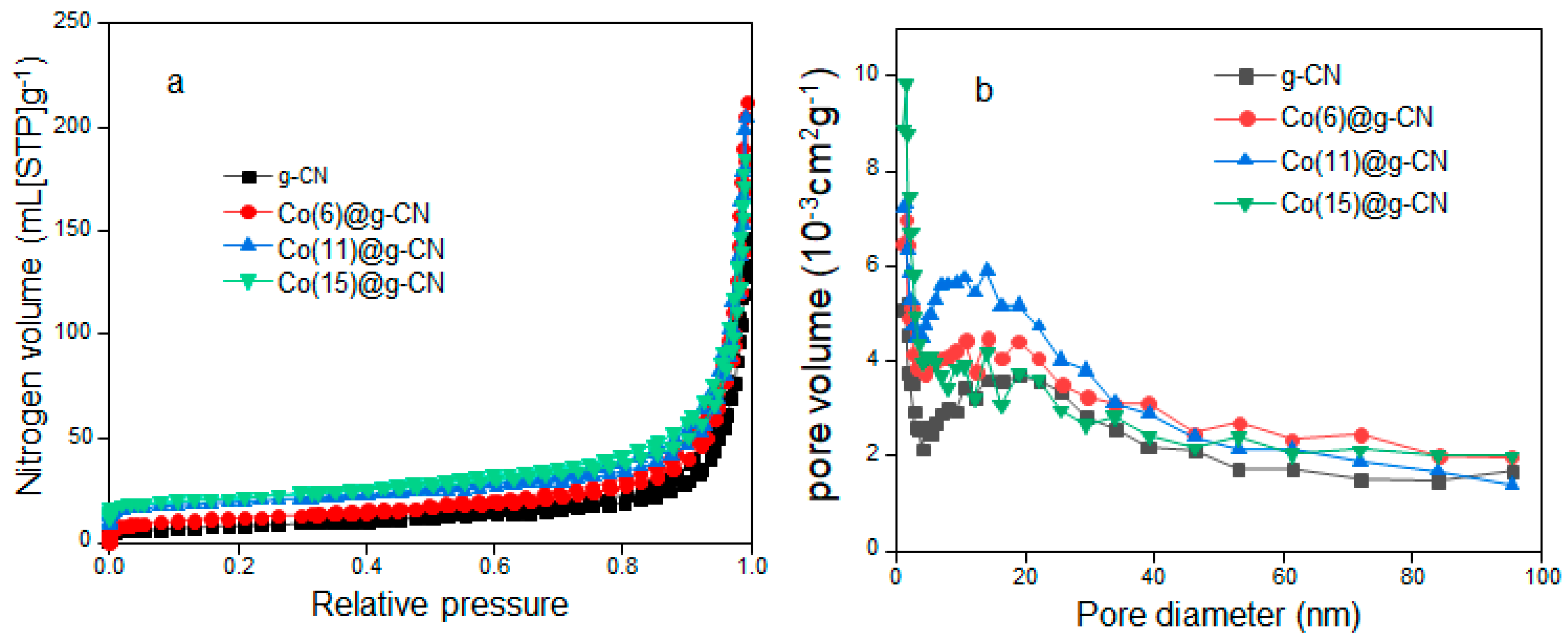
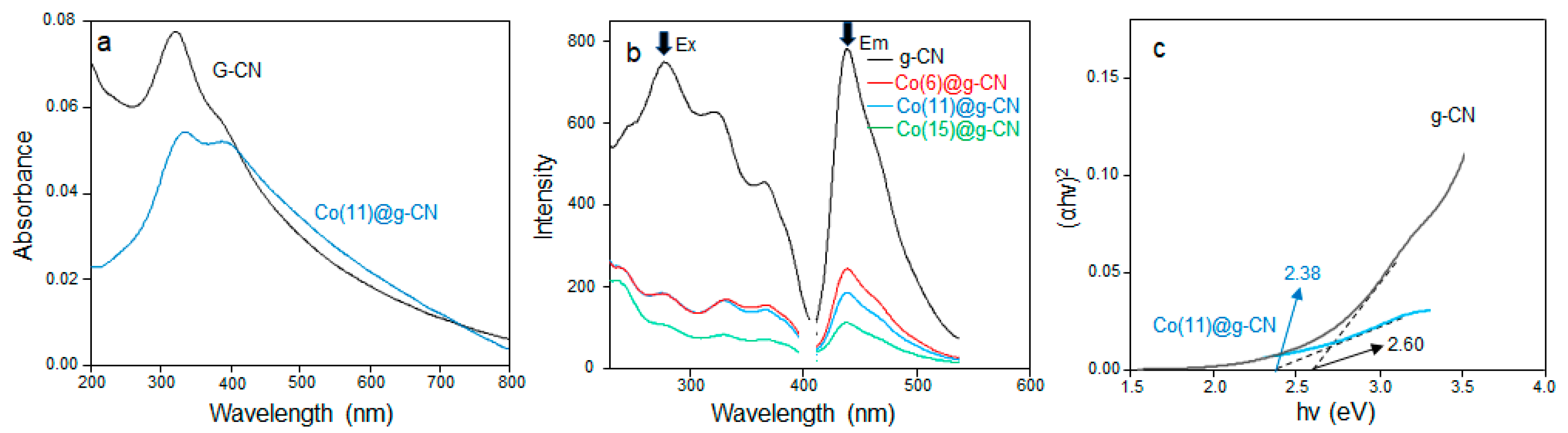
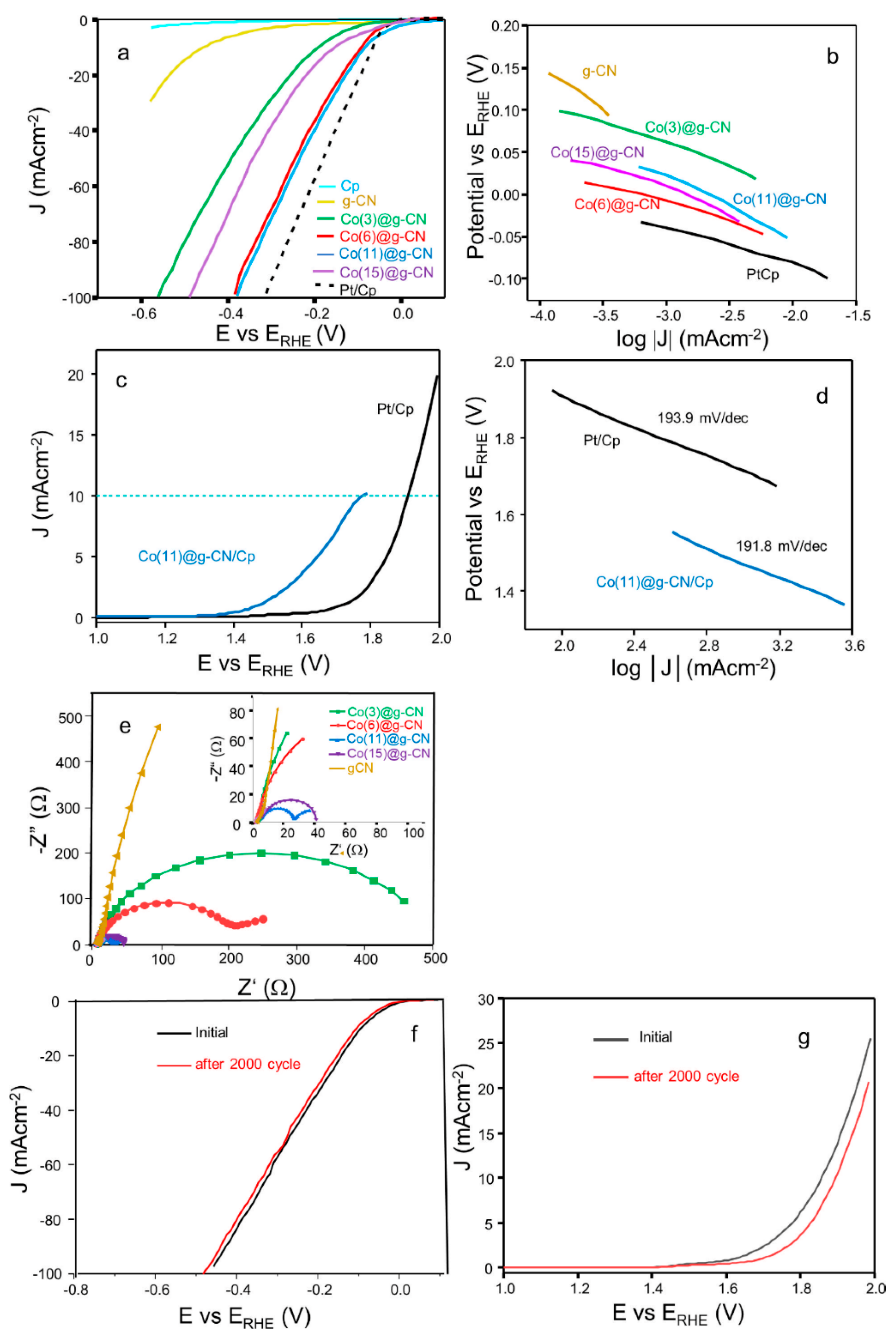
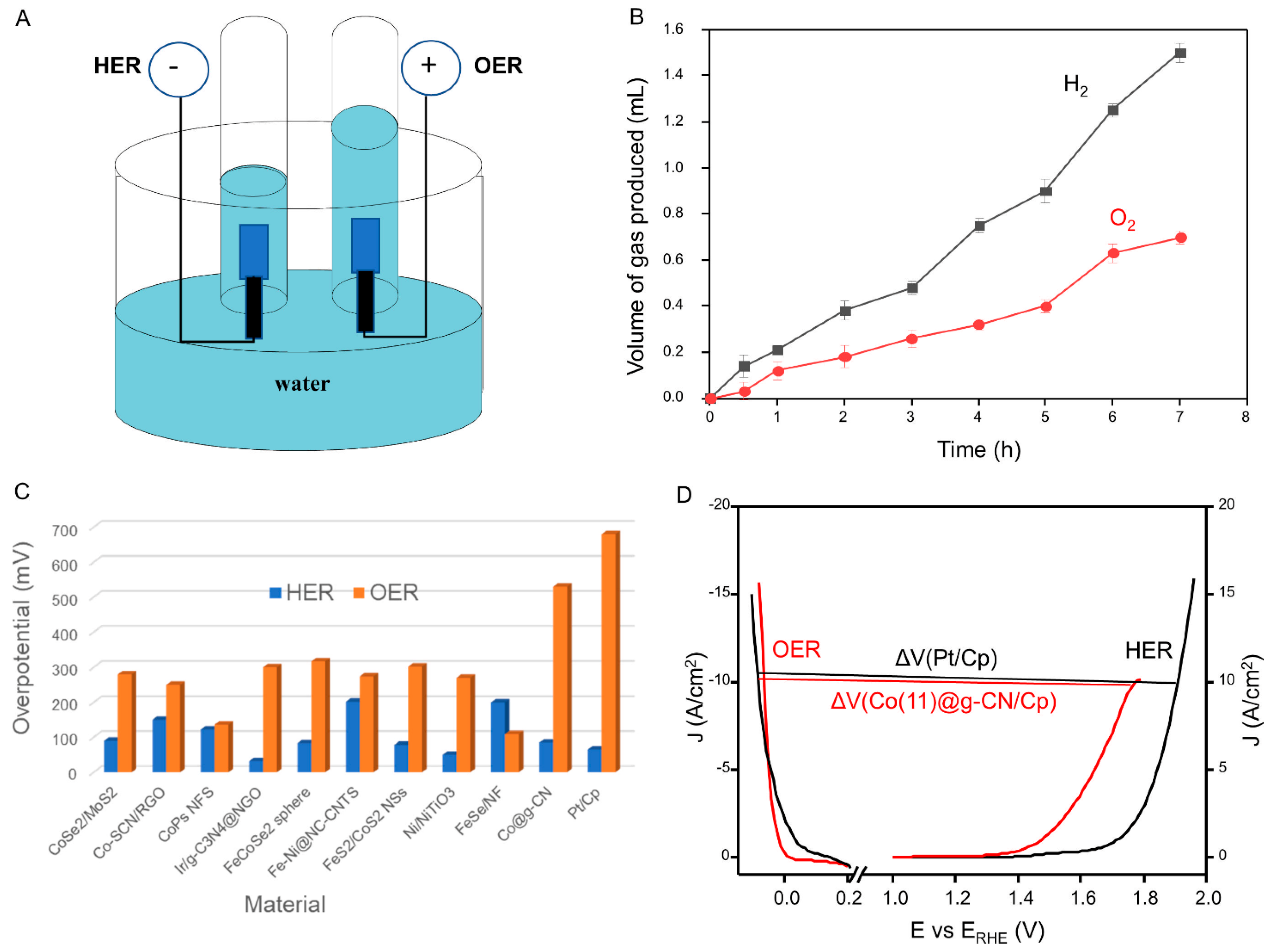
| Catalyst | Specific Surface Area (m2·g−1) | Pore Volume (cm2·g−1) | Pore Diameter (nm) |
|---|---|---|---|
| g-CN | 28.34 | 0.224 | 31.64 |
| Co(6)@g-CN | 41.26 | 0.293 | 28.44 |
| Co(11)@g-CN | 46.05 | 0.299 | 25.97 |
| Co(15)@g-CN | 38.12 | 0.266 | 27.87 |
| Catalyst | HER Overpotential at 10 mVcm−2 (mV) | HER Tafel Slope (mV/dec.) | OER Overpotential at 10 mVcm−2 (mV) | OER Tafel Slope (mV/dec.) | Rs (Ω) | Rct (Ω) |
|---|---|---|---|---|---|---|
| g-CN/Cp | 459 | 106.4 | - | - | 4.45 | 6057.8 |
| Co(3)@g-CN/Cp | 88 | 110.2 | - | - | 1.92 | 517.3 |
| Co(6)@g-CN/Cp | 96 | 69.9 | - | - | 2.15 | 203.8 |
| Co(11)@g-CN/Cp | 85 | 44.2 | 530 | 191.8 | 2.96 | 27.0 |
| Co(15)@g-CN/Cp | 65 | 102.4 | - | - | 2.45 | 40.8 |
| Pt/Cp | 65 | 38.1 | 680 | 193.9 | - | - |
| Catalyst * | Medium | HER Tafel Slope (mv/dec.) | HER Overpotential at 10 mVcm−2 (mV) | OER Tafel Slope (mv/dec.) | OER Overpotential at 10 mVcm−2 (mV) | Overpotential Voltage Difference (ΔV) (V) | Reference |
|---|---|---|---|---|---|---|---|
| Co-SCN/RGO | Basic | 94 | 150 | 96 | 250 | 1.63 | [10] |
| Ir/g-C3N4/NGS | Acidic | 22 | 28 | 72.8 | 300 | 1.56 | [16] |
| 0D-2D CoSe2/MoSe2 | Basic | 84.8 | 90 | 86.8 | 280 | 1.63 | [32] |
| CoP NFs | Acidic, Basic | 35.5 | 122 | 49.6 | 323 | 1.65 | [18] |
| FeCoSe2@g-C3N4 | Acidic, Basic | - | 83 | - | 360 | - | [20] |
| MIL-88-Fe/Ni | Basic | 33.8 | 202 | 45.5 | 274 | - | [31] |
| FeS2/CoS2 | Basic | 44 | 78.2 | 42 | 302 | 1.47 | [33] |
| Ni/NiTiO3 | Basic | 118 | 50 | 62.2 | 270 | 1.65 | [34] |
| FeSe@NF | Basic | 145 | 200 | 1.85 | [19] | ||
| Pt/Cp | Acidic | 38.1 | 65 | 193.9 | 680 | 1.98 | This work |
| Co(11)@g-CN | Acidic | 44.2 | 85 | 191 | 530 | 1.84 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejeta, S.Y.; Imae, T. Cobalt Incorporated Graphitic Carbon Nitride as a Bifunctional Catalyst for Electrochemical Water-Splitting Reactions in Acidic Media. Molecules 2022, 27, 6445. https://doi.org/10.3390/molecules27196445
Ejeta SY, Imae T. Cobalt Incorporated Graphitic Carbon Nitride as a Bifunctional Catalyst for Electrochemical Water-Splitting Reactions in Acidic Media. Molecules. 2022; 27(19):6445. https://doi.org/10.3390/molecules27196445
Chicago/Turabian StyleEjeta, Shibiru Yadeta, and Toyoko Imae. 2022. "Cobalt Incorporated Graphitic Carbon Nitride as a Bifunctional Catalyst for Electrochemical Water-Splitting Reactions in Acidic Media" Molecules 27, no. 19: 6445. https://doi.org/10.3390/molecules27196445
APA StyleEjeta, S. Y., & Imae, T. (2022). Cobalt Incorporated Graphitic Carbon Nitride as a Bifunctional Catalyst for Electrochemical Water-Splitting Reactions in Acidic Media. Molecules, 27(19), 6445. https://doi.org/10.3390/molecules27196445