Supramolecular Tools to Improve Wound Healing and Antioxidant Properties of Abietic Acid: Biocompatible Microemulsions and Emulgels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dimensional Characteristics and Viscosity Properties of Microemulsions and Emulgels
2.2. Prerequisites for the Use of Microemulsions and Emulgels Loaded with Abietic Acid in Biomedical Applications
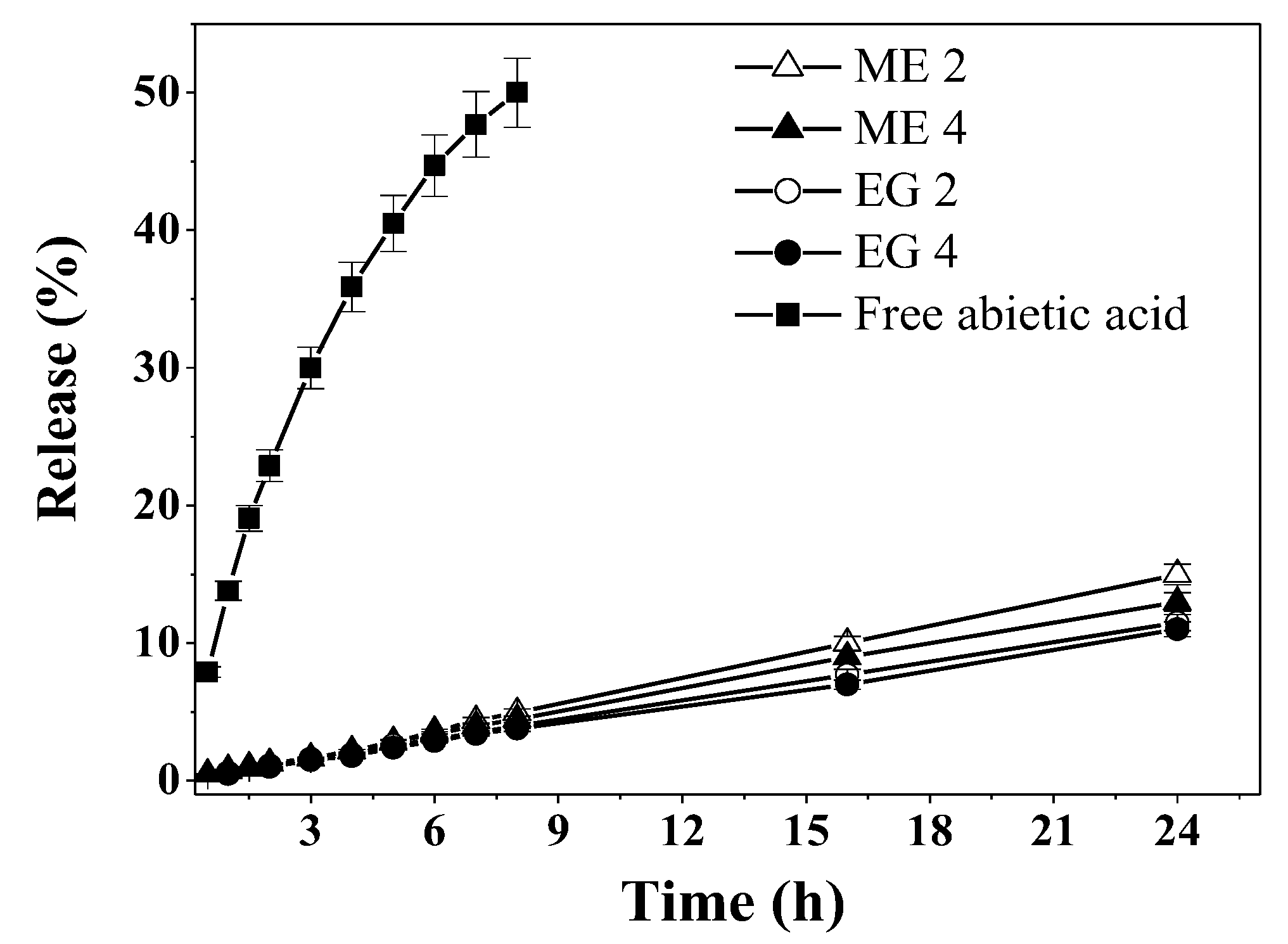
2.2.1. Sustained Release of Abietic Acid
2.2.2. Antioxidant Properties of Abietic Acid Containing Compositions
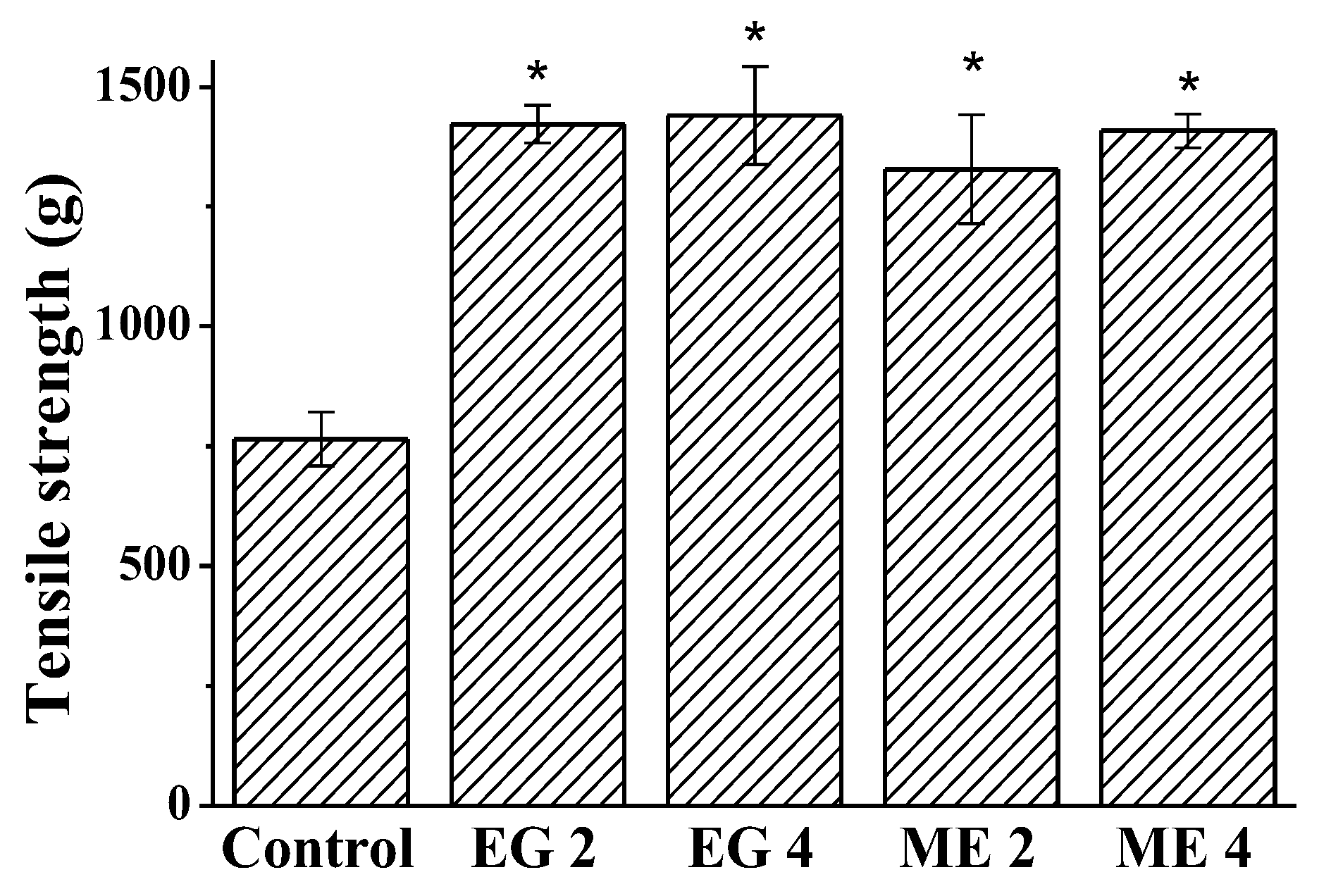
2.2.3. Wound-Healing Activity of Microemulsions and Emulgels
3. Materials and Methods
3.1. Materials
3.2. Preparation of Microemulsions and Emulgels
3.3. Particle Size and ζ-Potential Determination
3.4. Rheological Studies of Microemulsions and Emulgels
3.5. Determination of Abietic Acid Solubility
3.6. Dialysis
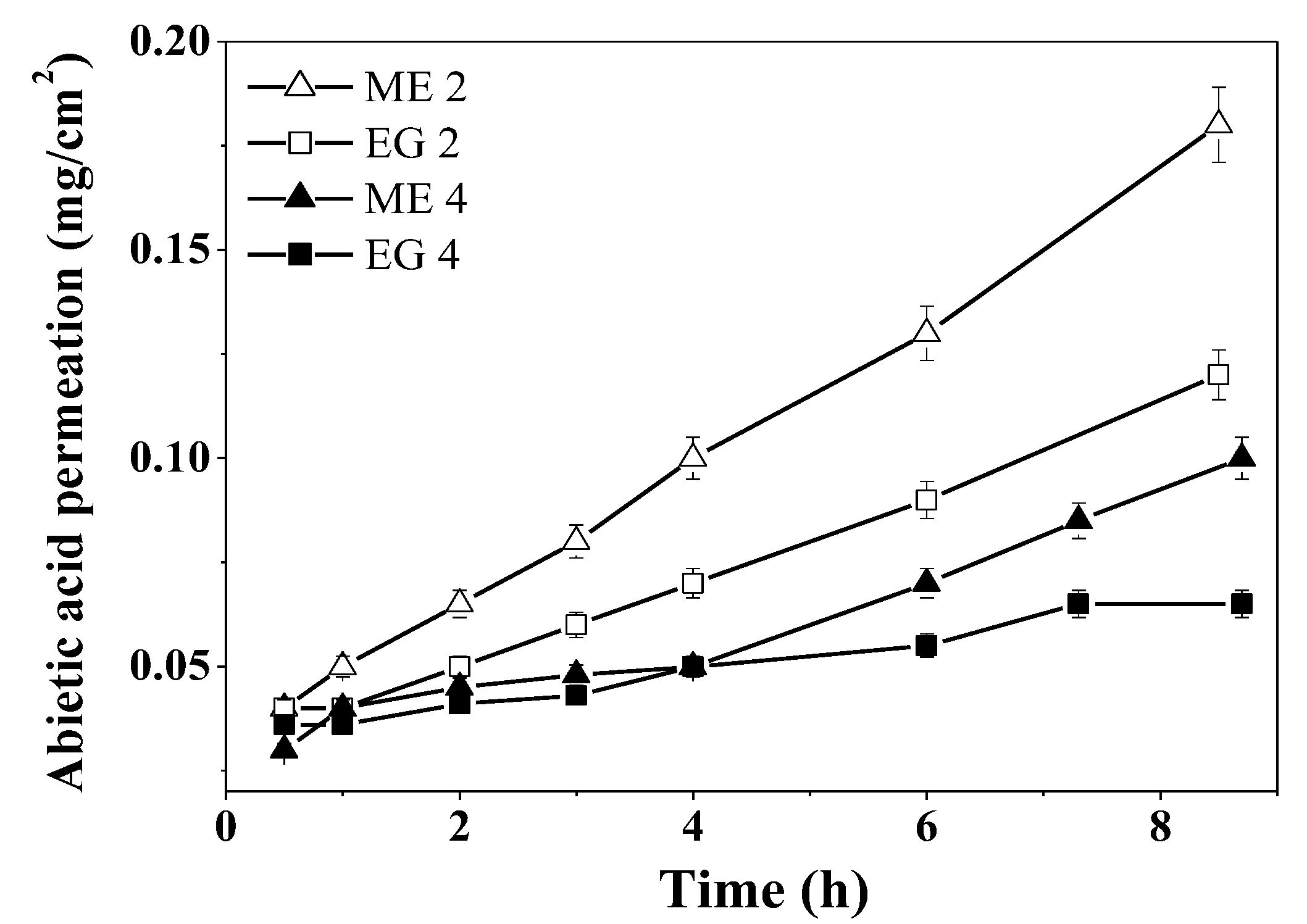
3.7. Abietic Acid Diffusion and Permeation Studies
3.8. Antioxidant Activity
3.9. Incision Wound Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kashapov, R.; Ibragimova, A.; Pavlov, R.; Gabdrakhmanov, D.; Kashapova, N.; Burilova, E.; Zakharova, L.; Sinyashin, O. Nanocarriers for biomedicine: From lipid formulations to inorganic and hybrid nanoparticles. Int. J. Mol. Sci. 2021, 22, 7055. [Google Scholar] [CrossRef] [PubMed]
- Moulahoum, H.; Ghorbanizamani, F.; Zihnioglu, F.; Timur, S. Surface biomodification of liposomes and polymersomes for efficient targeted drug delivery. Bioconjugate Chem. 2021, 32, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-assembling drug formulations with tunable permeability and biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Küpeli Akkol, E. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of pinus species by in vivo and in vitro experimental Models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef]
- Fana, S.E.; Ahmadpour, F.; Rasouli, H.R.; Tehrani, S.S.; Maniati, M. The effects of natural compounds on wound healing in iranian traditional medicine: A comprehensive review. Complement. Ther. Clin. Pract. 2021, 42, 101275. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Liu, L.; Kotiw, M.; Benkendorff, K. Review of anti-inflammatory, immune-modulatory and wound healing properties of molluscs. J. Ethnopharmacol. 2018, 210, 156–178. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Yang, S.-F.; Hsieh, Y.-H.; Hung, C.-H.; Chu, S.-C.; Yang, S.-H.; Chen, P.-N. The inhibitory effect of abietic acid on melanoma cancer metastasis and invasiveness in vitro and in vivo. Am. J. Chin. Med. 2015, 43, 1697–1714. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Liu, Q.; Dai, J. Abietic acid suppresses non-small-cell lung cancer cell growth via blocking IKKβ/NF-ΚB signaling. Onco. Targets. Ther. 2019, 12, 4825–4837. [Google Scholar] [CrossRef]
- Gao, Y.; Zhaoyu, L.; Xiangming, F.; Chunyi, L.; Jiayu, P.; Lu, S.; Jitao, C.; Liangcai, C.; Jifang, L. Abietic acid attenuates allergic airway inflammation in a mouse allergic asthma model. Int. Immunopharmacol. 2016, 38, 261–266. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.K.; Lee, D.-S.; Yoo, J.-E.; Shin, M.-S.; Yamabe, N.; Kim, S.-N.; Lee, S.; Kim, K.H.; Lee, H.-J.; et al. Abietic acid isolated from pine resin (Resina Pini) enhances angiogenesis in HUVECs and accelerates cutaneous wound healing in mice. J. Ethnopharmacol. 2017, 203, 279–287. [Google Scholar] [CrossRef]
- Fernández, M.A.; Tornos, M.P.; García, M.D.; de las Heras, B.; Villar, A.M.; Sáenz, M.T. Anti-inflammatory activity of abietic acid, a diterpene isolated from Pimenta Racemosa Var. Grissea. J. Pharm. Pharmacol. 2010, 53, 867–872. [Google Scholar] [CrossRef] [PubMed]
- el Saved, F.; Manzur, F.; Bayle, P.; Marguery, M.S.; Bazex, J. Contact urticaria from abietic acid. Contact Dermatitis 1995, 32, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Ahmad, J.; Alasmary, M.Y.; Akhter, S.; Aslam, M.; Pathak, K.; Jamil, P.; Abdullah, M.M. Nanoemulgel as an approach to improve the biopharmaceutical performance of lipophilic drugs: Contemporary research and application. J. Drug Delivery Sci. Technol. 2022, 72, 103420. [Google Scholar] [CrossRef]
- Ajazuddin; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Ray, S.; Rahman, M.; Shaharyar, A.; Bhowmik, R.; Bera, R.; Karmakar, S. Nano-Emulgel: Emerging as a smarter topical lipidic emulsion-based nanocarrier for skin healthcare applications. Recent Pat. Antiinfect. Drug Discov. 2019, 14, 16–35. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone loaded topical nanoemulgel for wound healing: Formulation design and in-Vivo Evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef]
- Tijani, A.O.; Nunez, E.; Singh, K.; Khanna, G.; Puri, A. Transdermal route: A viable option for systemic delivery of antidepressants. J. Pharm. Sci. 2021, 110, 3129–3149. [Google Scholar] [CrossRef]
- Salem, M.A.; Bansal, A.; Khalid, S.; Sadath, A.; Najmuddin, M. Development of microemulsion based emulgel formulations of valdecoxib. Indian Drugs. 2010, 47, 31–38. [Google Scholar]
- Vandana, K.; Yalavarthi, P.; Sundaresan, C.; Sriramaneni, R.; Vadlamudi, H. In-vitro assessment and pharmacodynamics of nimesulide incorporated Aloe Vera transemulgel. Curr. Drug Discov. Technol. 2014, 11, 162–167. [Google Scholar] [CrossRef]
- Pednekar, A.; Dandagi, P.; Gadad, A.; Mastiholimath, V. Formulation and characterisation of meloxicam loaded emulgel for topical application. Int. J. Pharm. Pharm. Sci. 2015, 7, 216–222. [Google Scholar]
- Nikumbh, K.V.; Sevankar, S.G.; Patil, M.P. Formulation development, in vitro and in vivo evaluation of microemulsion-based gel loaded with Ketoprofen. Drug Deliv. 2015, 22, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shen, Y.; Wang, J.; Ouahab, A.; Zhang, T.; Tu, J. Cationic polymer-based micro-emulgel with self-preserving ability for transdermal delivery of diclofenac sodium. Drug Deliv. 2015, 22, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Sujitha, Y.S.; Muzib, Y.I. Formulation and optimization of quercetin loaded nanosponges topical gel: Ex vivo, pharmacodynamic and pharmacokinetic studies. Int. J. Appl. Pharm. 2019, 11, 156–165. [Google Scholar] [CrossRef]
- Lei, L.; He, Z.; Chen, H.; McClements, D.J.; Li, B.; Li, Y. Microstructural, rheological, and antibacterial properties of cross-linked chitosan emulgels. RSC Adv. 2015, 5, 100114–100122. [Google Scholar] [CrossRef]
- Ali Khan, B.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and evaluation of Ocimum Basilicum-based emulgel for wound healing using animal model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef]
- Gradzielski, M.; Duvail, M.; de Molina, P.M.; Simon, M.; Talmon, Y.; Zemb, T. Using microemulsions: Formulation based on knowledge of their mesostructure. Chem. Rev. 2021, 121, 5671–5740. [Google Scholar] [CrossRef] [PubMed]
- Friberg, S.E.; Bothorel, P. Microemulsions: Structure and Dynamics; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar] [CrossRef]
- Ghosh, P.; Murthy, R. Microemulsions: A potential drug delivery system. Curr. Drug Deliv. 2006, 3, 167–180. [Google Scholar] [CrossRef]
- Cecchi, L.; Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Rocco, F.; Innocenti, M.; Vanti, G.; Mulinacci, N.; Bergonzi, M.C. Formulation of a phenol-rich extract from unripe olives (Olea europaea L.) in microemulsion to improve its solubility and intestinal permeability. Molecules 2020, 25, 3198. [Google Scholar] [CrossRef]
- Elmowafy, M. Skin penetration/permeation success determinants of nanocarriers: Pursuit of a perfect formulation. Colloids Surf. B 2021, 203, 111748. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (Niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Sanchez-Lopez, E.; Santos, T.D.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Development and characterization of nanoemulsions for ophthalmic applications: Role of cationic surfactants. Materials 2021, 14, 7541. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-assembly of amphiphilic compounds as a versatile tool for construction of nanoscale drug carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Kaur, G.; Bhasin, K.K. Analysis of tween based microemulsion in the presence of TB drug rifampicin. Colloids Surf. B 2007, 60, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Koroleva, M.Y.; Kushnazarova, R.A.; Mishchenko, E.V.; Petrov, K.A.; Lenina, O.A.; Vyshtakalyuk, A.B.; Voloshina, A.D.; Zakharova, L.Y. Microemulsions and nanoemulsions modified with cationic surfactants for improving the solubility and therapeutic efficacy of loaded drug indomethacin. Nanotechnology 2022, 33, 155103. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Zakharova, L.Y. Self-assembly of mixed systems based on nonionic and carbamate-bearing cationic surfactants as a tool for fabrication of biocompatible nanocontainers. J. Mol. Liq. 2019, 292, 111407. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Voloshina, A.D.; Lenina, O.A.; Zakharova, L.Y.; Sinyashin, O.G. Carbamate-bearing surfactants: Micellization, solubilization, and biological activity. J. Mol. Liq. 2018, 269, 203–210. [Google Scholar] [CrossRef]
- Wender, P.A.; Rothbard, J.B.; Jessop, T.C.; Kreider, E.L.; Wylie, B.L. Oligocarbamate molecular transporters: Design, synthesis, and biological evaluation of a new class of transporters for drug delivery. J. Am. Chem. Soc. 2002, 124, 13382–13383. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, J.; Du, Y.; Fu, S.; Song, C.; Zhi, D.; Zhao, Y.; Chen, H.; Zhang, S.; Zhang, S. Novel carbamate-linked quaternary ammonium lipids containing unsaturated hydrophobic chains for gene delivery. Bioorg. Med. Chem. 2018, 26, 3535–3540. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Wang, Y.; Liu, W.; Yin, G.; Wang, D.; Li, J.; Shi, T.; Wang, Z. Design, synthesis, and biological evaluation of carbamate derivatives of N-Salicyloyl Tryptamine as multifunctional agents for the treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2022, 229, 114044. [Google Scholar] [CrossRef]
- Kushnazarova, R.A.; Mirgorodskaya, A.B.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Novel cationic surfactants with cleavable carbamate fragment: Tunable morphological behavior, solubilization of hydrophobic drugs and cellular uptake study. J. Mol. Liq. 2020, 318, 113894. [Google Scholar] [CrossRef]
- Khan, B.A.; Khan, A.; Khan, M.K.; Braga, V.A. Preparation and properties of high sheared Poly(Vinyl Alcohol)/Chitosan blended hydrogels films with Lawsonia inermis extract as wound dressing. Drug Deliv. Sci. Technol. 2021, 61, 102227. [Google Scholar] [CrossRef]
- Shahi, S.; Sonwane, U.; Zadbuke, N.; Tadwee, I. Design and development of diphenhydramine hydrochloride topical liposomal drug delivery system. Int. J. Pharm. Pharm. Sci. 2013, 5, 534–542. [Google Scholar]
- Safitri, F.I.; Nawangsari, D.; Febrina, D. Overview: Application of carbopol 940 in gel. In Proceedings of the International Conference on Health and Medical Sciences (AHMS 2020), Yogyakarta, Indonesia, 18 July 2020; Atlantis Press: Dordrecht, The Netherlands, 2021. [Google Scholar]
- Teaima, M.H.; Badawi, N.M.; Attia, D.A.; El-Nabarawi, M.A.; Elmazar, M.M.; Mousa, S.A. Efficacy of pomegranate extract loaded solid lipid nanoparticles transdermal emulgel against Ehrlich Ascites Carcinoma. Nanomed. Nanotechnol. Biol. Med. 2022, 39, 102466. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for improved topical delivery of retinyl palmitate: Formulation design and stability evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Düwel, S.; Hundshammer, C.; Gersch, M.; Feuerecker, B.; Steiger, K.; Buck, A.; Walch, A.; Haase, A.; Glaser, S.J.; Schwaiger, M.; et al. Imaging of pH in vivo using hyperpolarized 13C-labelled zymonic acid. Nat. Commun. 2017, 8, 15126. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Polymer and Composite Rheology, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Li, Q.; Liu, X.; Byambasuren, K.; Wang, X.; Qiu, S.; Gao, Y.; Dang, L.; Liu, Z.; Shu, Q.; Wang, Z. Revealing the effects of multi-scale molecules on α-linolenic acid-loaded W1/O/W2 microemulsion: A combined study from physical properties, antioxidant capacity and in vitro release kinetics. J. Mol. Liq. 2020, 303, 112675. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, Y.-S.; Zhao, J.-S.; Li, Z.-Y.; Chen, H.-H. A PH-sensitive curcumin loaded microemulsion-filled alginate and porous starch composite gels: Characterization, in vitro release kinetics and biological activity. Int. J. Biol. Macromol. 2021, 182, 1863–1873. [Google Scholar] [CrossRef]
- Siepmann, J. Modeling of drug release from delivery systems based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Delivery Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Elmas, A.; Akyüz, G.; Bergal, A.; Andaç, M.; Andaç, O. Mathematical modelling of drug release. Res. Eng. Struct. Mater. 2020, 6, 327–350. [Google Scholar] [CrossRef]
- Scomoroscenco, C.; Teodorescu, M.; Raducan, A.; Stan, M.; Voicu, S.N.; Trica, B.; Ninciuleanu, C.M.; Nistor, C.L.; Mihaescu, C.I.; Petcu, C.; et al. Novel gel microemulsion as topical drug delivery system for curcumin in dermatocosmetics. Pharmaceutics 2021, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Shinde, U.A.; Modani, S.H.; Singh, K.H. Design and development of repaglinide microemulsion gel for transdermal delivery. AAPS Pharm. Sci. Tech. 2018, 19, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, A.V.; Proskurnina, E.V.; Vladimirov, Y.A. Determination of antioxidants by sensitized chemiluminescence using 2.2′-Azo-Bis(2-Amidinopropane). Moscow Univ. Chem. Bull. 2012, 67, 127–132. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A Review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, Y.; Nakatsuji, T.; Liu, Y.-T.; Zouboulis, C.C.; Gallo, R.L.; Zhang, L.; Hsieh, M.-F.; Huang, C.-M. An innate bactericidal oleic acid effective against skin infection of methicillin-resistant Staphylococcus Aureus: A therapy concordant with evolutionary medicine. J. Microbiol. Biotechnol. 2011, 21, 391–399. [Google Scholar] [CrossRef]
- Yoon, B.; Jackman, J.; Valle-González, E.; Cho, N.-J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Ehrlich, H.P.; Hunt, T.K. Effects of cortisone and vitamin A on wound healing. Ann. Surg. 1968, 167, 324–328. [Google Scholar] [CrossRef]
- Aburjai, T.; Al-Janabi, R.; Al-Mamoori, F.; Azzam, H. In vivo wound healing and antimicrobial activity of Alkanna strigose. Wound Med. 2019, 25, 100152. [Google Scholar] [CrossRef]



| System | Concentration, % wt. | ||||||
|---|---|---|---|---|---|---|---|
| Oleic Acid | Tween 80 | CB-16(Bu) | PBS | Ethanol | Abietic Acid | Carbopol® 940 | |
| ME 1 | 15.7 | 19.7 | - | 37.4 | 27.2 | - | - |
| ME 2 | 15.6 | 19.6 | - | 37.2 | 27.1 | 0.5 | - |
| ME 3 | 15.7 | 16.7 | 3.0 | 37.4 | 27.2 | - | - |
| ME 4 | 15.6 | 16.6 | 3.0 | 37.2 | 27.1 | 0.5 | - |
| ME 5 | 15.5 | 16.5 | 3.0 | 37.0 | 27.0 | 1.0 | - |
| EG 1 | 7.8 | 9.8 | - | 68.4 | 13.5 | - | 0.5 |
| EG 2 | 7.7 | 9.7 | - | 68.2 | 13.4 | 0.5 | 0.5 |
| EG 3 | 7.8 | 8.3 | 1.5 | 68.4 | 13.5 | - | 0.5 |
| EG 4 | 7.7 | 8.2 | 1.5 | 68.2 | 13.4 | 0.5 | 0.5 |
| Microemulsion | First Day | A Month Later | ||
|---|---|---|---|---|
| Diameter, nm | PdI | Diameter, nm | PdI | |
| ME 1 | 122 ± 6 | 0.127 ± 0.017 | 110 ± 5 | 0.145 ± 0.018 |
| ME 2 | 118 ± 5 | 0.179 ± 0.021 | 115 ± 5 | 0.188 ± 0.019 |
| ME 3 | 60 ± 3 | 0.285 ± 0.031 | 70 ± 3 | 0.265 ± 0.033 |
| ME 4 | 62 ± 3 | 0.276 ± 0.028 | 72 ± 4 | 0.282 ± 0.030 |
| Formulation | Zero Order | First Order | Korsmeyer–Peppas | Higuchi | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | k | R2 | k | R2 | k | n | R2 | k | |
| Free abietic acid | 0.7969 | 7.41 ± 0.46 | 0.9323 | 0.10 ± 0.005 | 0.9912 | 15.0 ± 0.62 | 0.60 ± 0.02 | 0.9742 | 17.5 ± 0.39 |
| ME 2 | 0.9984 | 0.62 ± 0.005 | 0.9962 | 0.0065 ± 9.3·10−5 | 0.9988 | 0.61 ± 0.02 | 1.03 ± 0.01 | 0.7621 | 2.12 ± 0.24 |
| ME 4 | 0.9983 | 0.55 ± 0.005 | 0.990 | 0.0058 ± 4.0·10−5 | 0.9989 | 0.57 ± 0.02 | 0.97 ± 0.01 | 0.7912 | 1.91 ± 0.19 |
| EG 2 | 0.9986 | 0.48 ± 0.004 | 0.9994 | 0.0050 ± 2.7·10−5 | 0.9999 | 0.53 ± 0.001 | 0.97 ± 0.001 | 0.7829 | 1.70 ± 0.18 |
| EG 4 | 0.9976 | 0.45 ± 0.005 | 0.9979 | 0.0047 ± 5.0·10−5 | 0.9980 | 0.50 ± 0.02 | 0.97 ± 0.02 | 0.7792 | 1.61 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirgorodskaya, A.; Kushnazarova, R.; Pavlov, R.; Valeeva, F.; Lenina, O.; Bushmeleva, K.; Kuryashov, D.; Vyshtakalyuk, A.; Gaynanova, G.; Petrov, K.; et al. Supramolecular Tools to Improve Wound Healing and Antioxidant Properties of Abietic Acid: Biocompatible Microemulsions and Emulgels. Molecules 2022, 27, 6447. https://doi.org/10.3390/molecules27196447
Mirgorodskaya A, Kushnazarova R, Pavlov R, Valeeva F, Lenina O, Bushmeleva K, Kuryashov D, Vyshtakalyuk A, Gaynanova G, Petrov K, et al. Supramolecular Tools to Improve Wound Healing and Antioxidant Properties of Abietic Acid: Biocompatible Microemulsions and Emulgels. Molecules. 2022; 27(19):6447. https://doi.org/10.3390/molecules27196447
Chicago/Turabian StyleMirgorodskaya, Alla, Rushana Kushnazarova, Rais Pavlov, Farida Valeeva, Oksana Lenina, Kseniya Bushmeleva, Dmitry Kuryashov, Alexandra Vyshtakalyuk, Gulnara Gaynanova, Konstantin Petrov, and et al. 2022. "Supramolecular Tools to Improve Wound Healing and Antioxidant Properties of Abietic Acid: Biocompatible Microemulsions and Emulgels" Molecules 27, no. 19: 6447. https://doi.org/10.3390/molecules27196447
APA StyleMirgorodskaya, A., Kushnazarova, R., Pavlov, R., Valeeva, F., Lenina, O., Bushmeleva, K., Kuryashov, D., Vyshtakalyuk, A., Gaynanova, G., Petrov, K., & Zakharova, L. (2022). Supramolecular Tools to Improve Wound Healing and Antioxidant Properties of Abietic Acid: Biocompatible Microemulsions and Emulgels. Molecules, 27(19), 6447. https://doi.org/10.3390/molecules27196447








