Synthesis and Characterization of Newly Designed and Highly Solvatochromic Double Squaraine Dye for Sensitive and Selective Recognition towards Cu2+
Abstract
1. Introduction
2. Results and Discussion
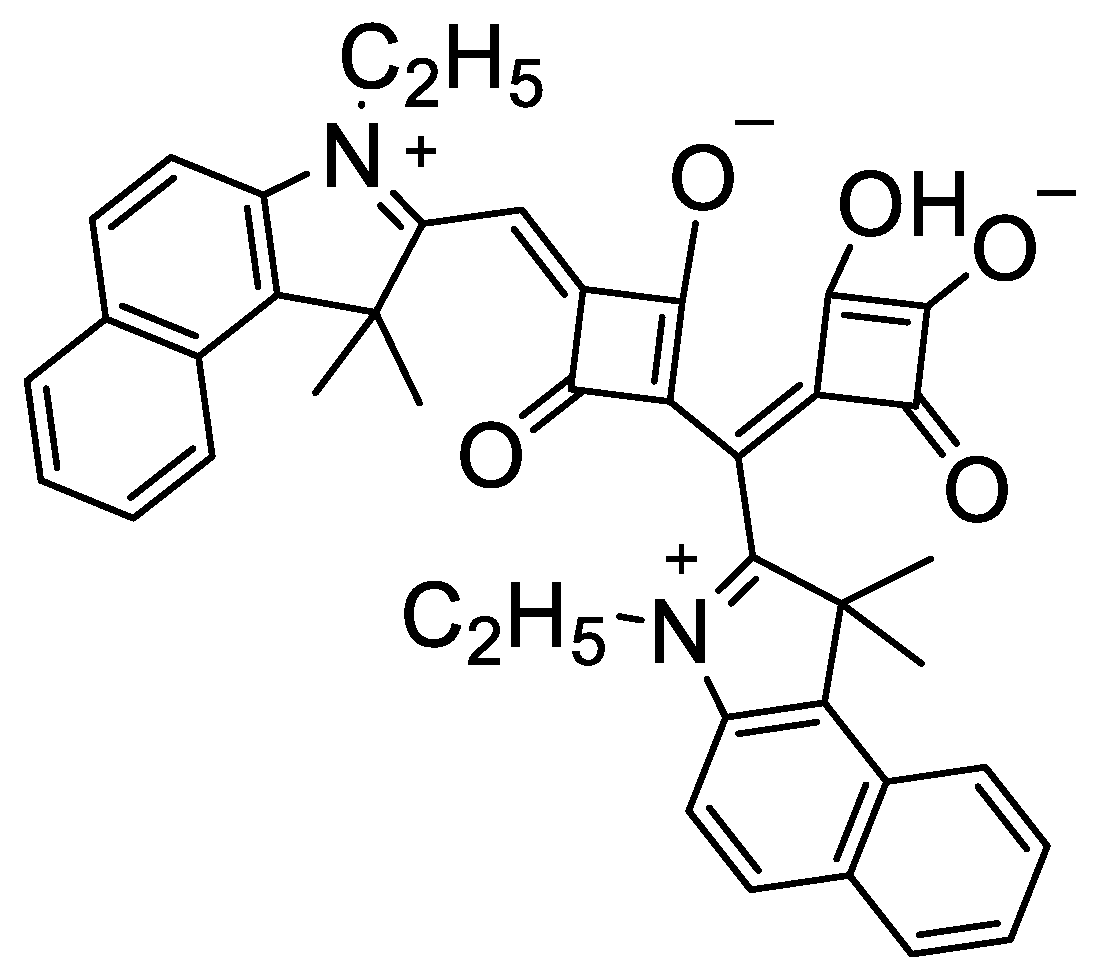
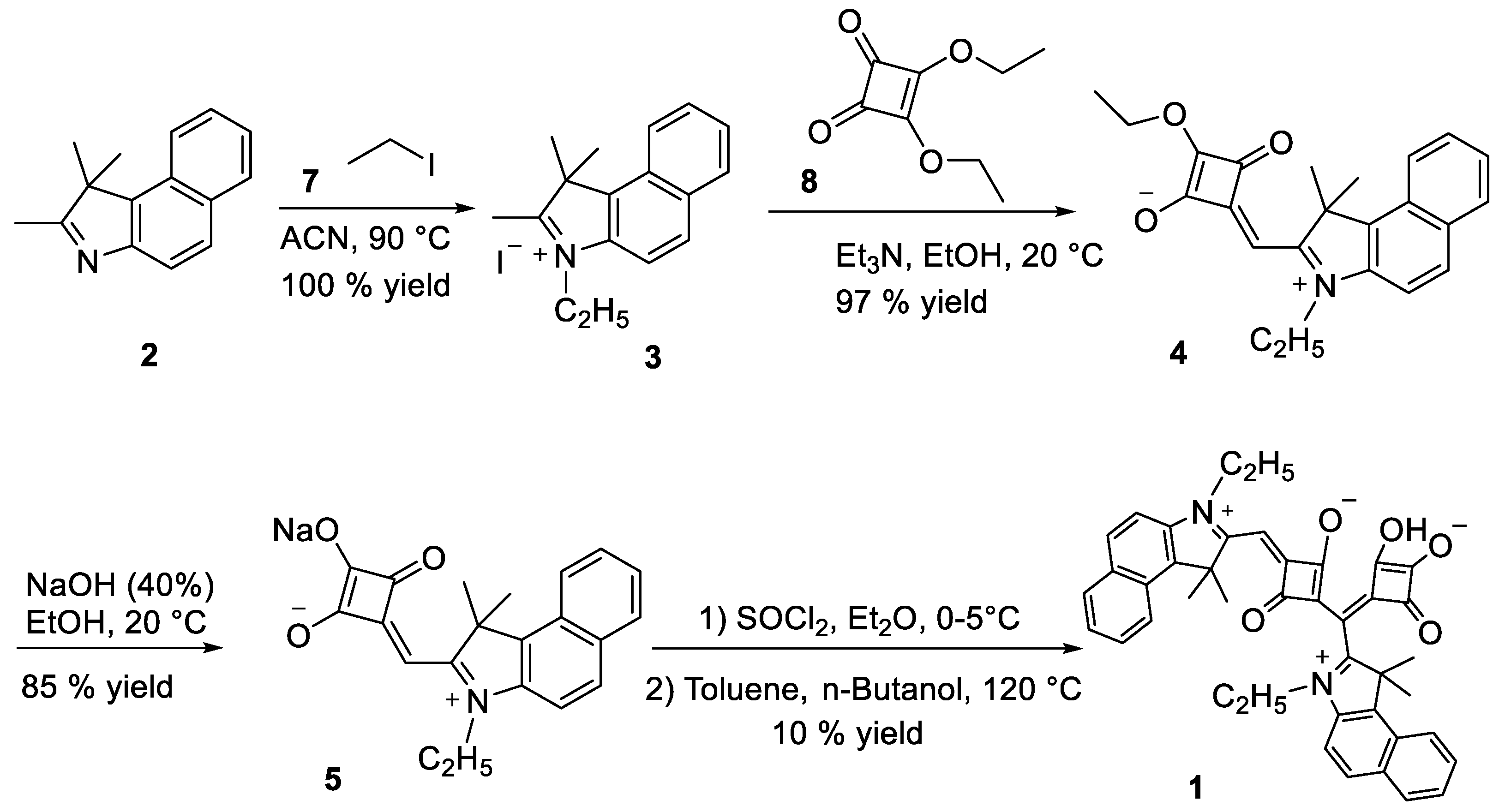
2.1. Synthesis of the Double-Squaraine (DSQ) Dye (1)
2.2. Solvatochromism in the DSQ Dye
2.3. Harnessing the Potentiality of DSQ Dye in Metal Ion Sensing
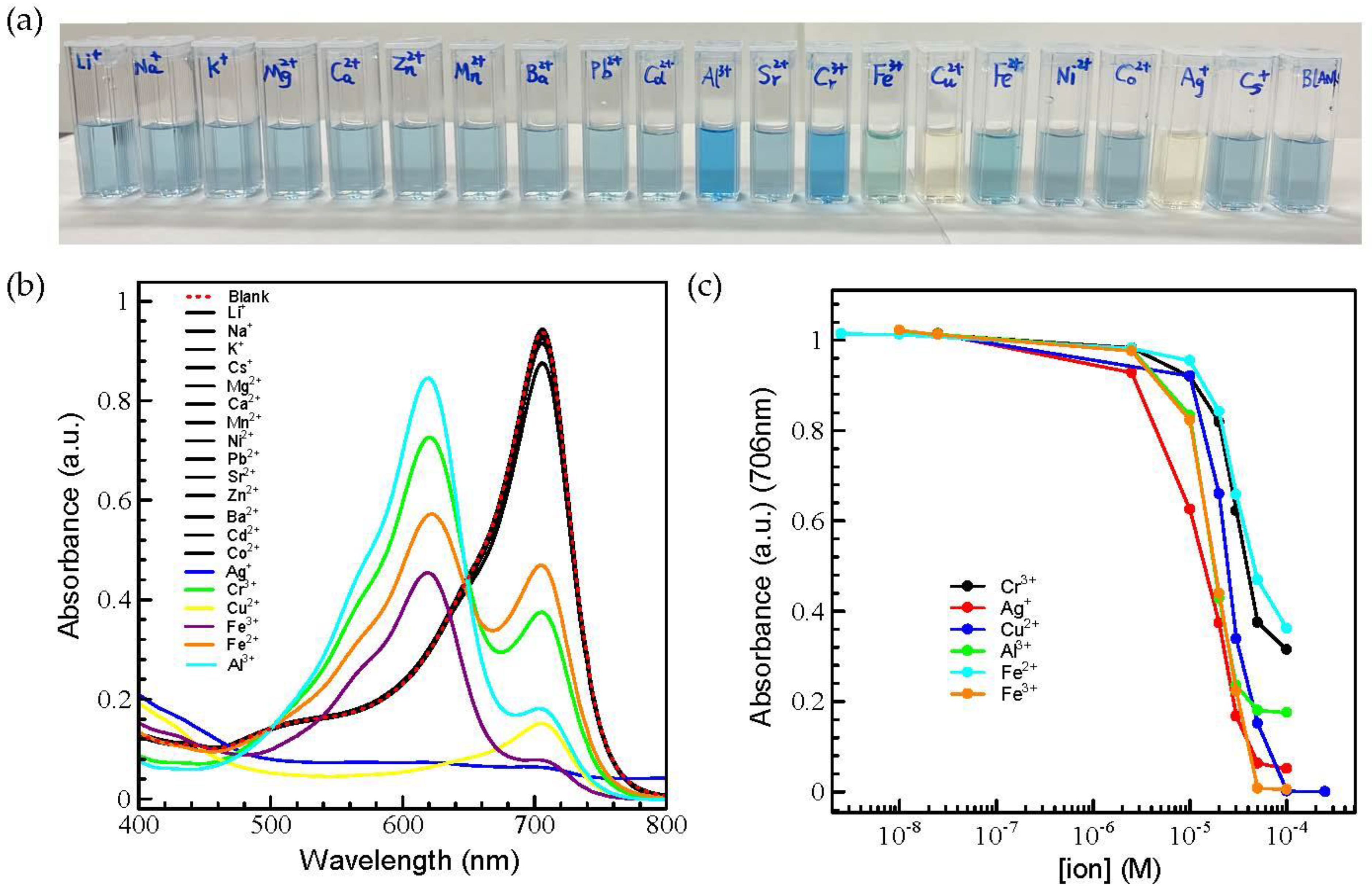
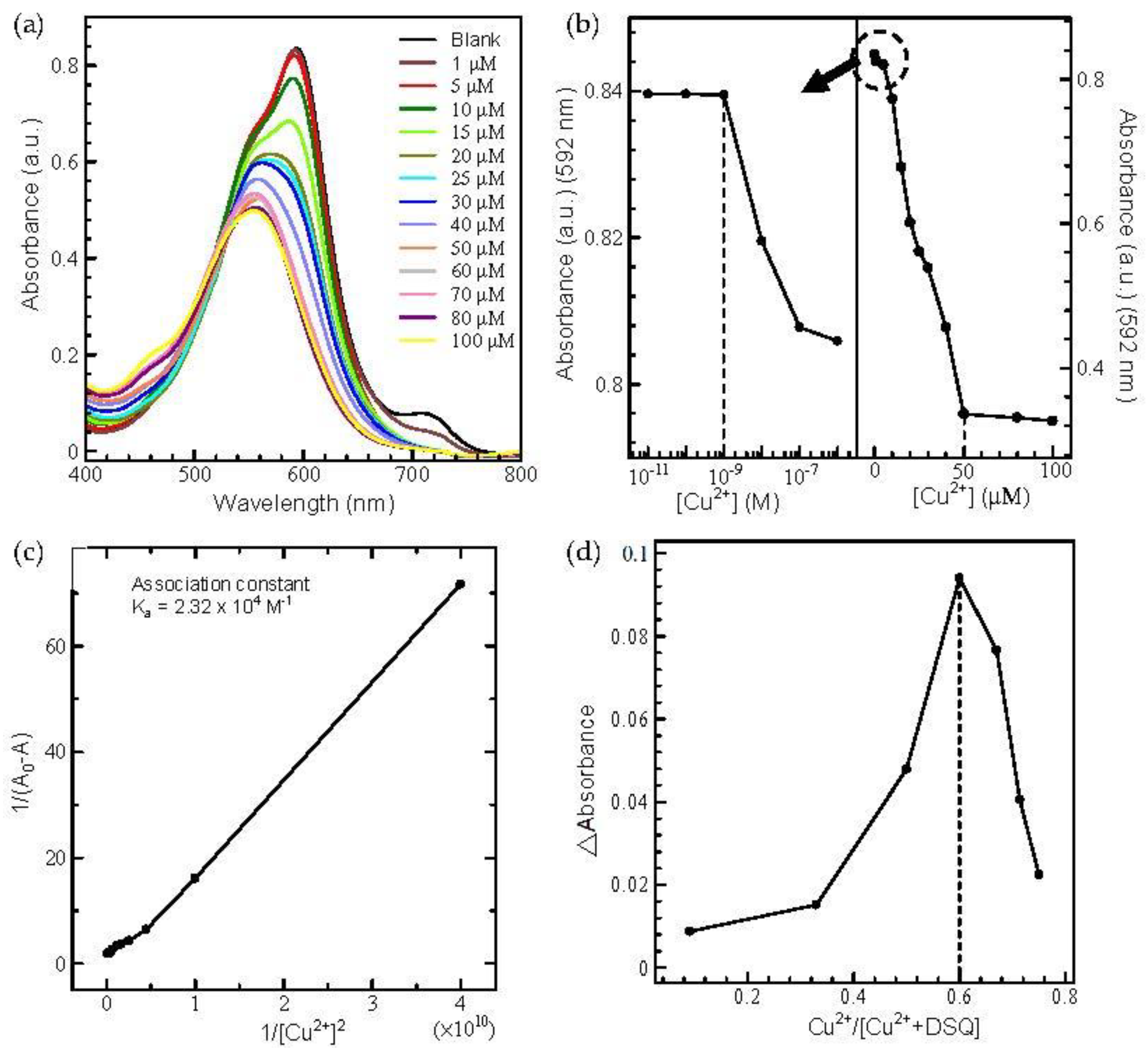
2.3.1. Investigation of Metal Ion Sensing in the DMF/Water Solvent System
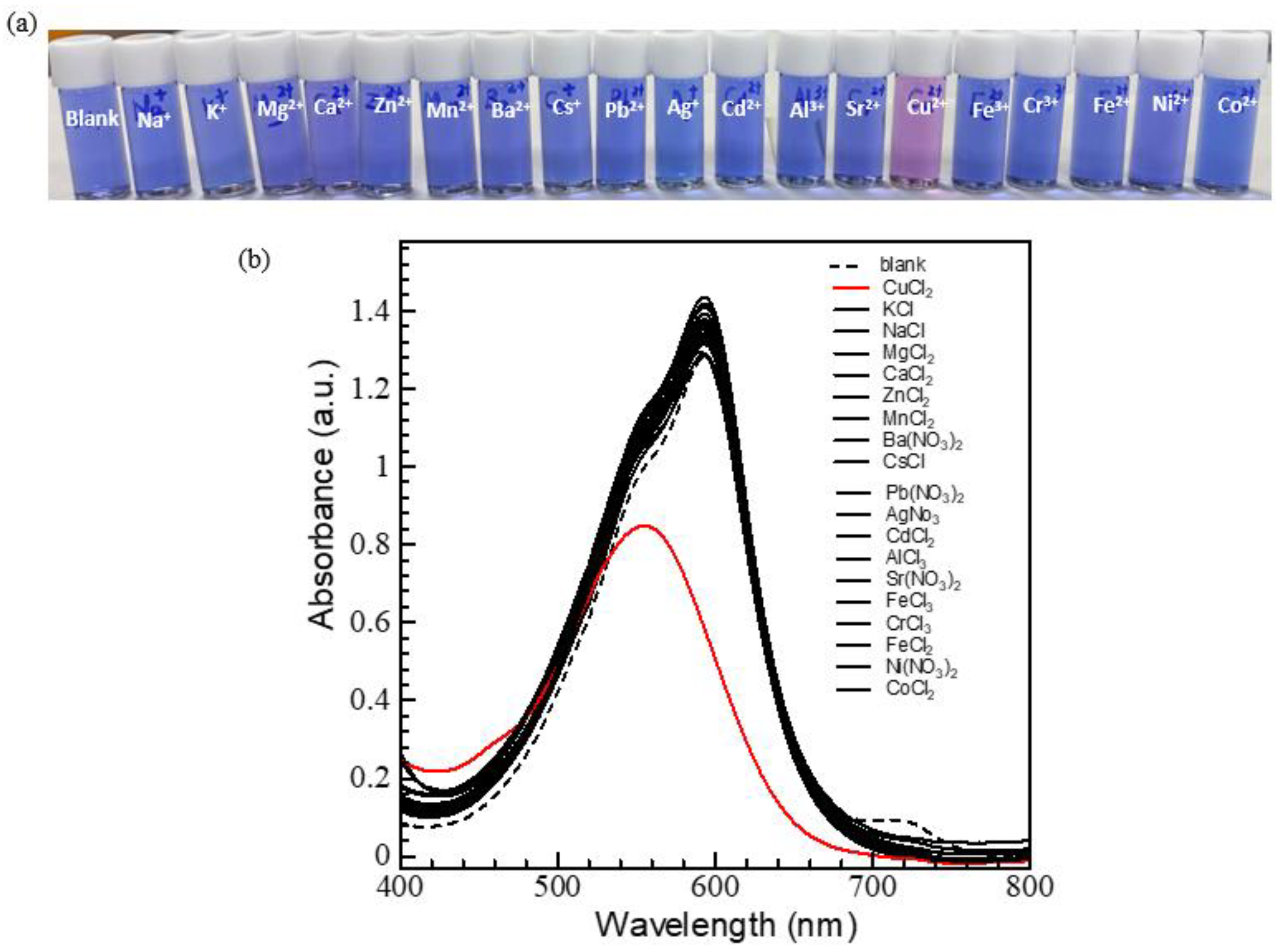
2.3.2. Selective Metal Ion Sensing Utilizing DMF/ACN Solvent System
3. Experimental
3.1. General Information
3.2. Synthetic Procedure
3.3. Preparation of Solutions for the Solvatochromism Investigation
3.4. Preparation of Stock Solutions of Metal Ions
3.5. Metal Ion Sensing by Absorption Spectral Measurement
3.6. Density Functional Theory (DFT) Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, Y.; Wang, B.; Cheng, Q.; Li, X.; Li, Z. Removal of Toxic Heavy Metal Ions (Pb, Cr, Cu, Ni, Zn, Co, Hg, and Cd) from Waste Batteries or Lithium Cells Using Nanosized Metal Oxides: A Review. J. Nanosci. Nanotechnol. 2020, 20, 7231–7254. [Google Scholar] [CrossRef]
- Chung, S.-J.; Zheng, S.; Odani, T.; Beverina, L.; Fu, J.; Padilha, L.A.; Biesso, A.; Hales, J.M.; Zhan, X.; Schmidt, K.; et al. Extended Squaraine Dyes with Large Two-Photon Absorption Cross-Sections. J. Am. Chem. Soc. 2006, 128, 14444–14445. [Google Scholar] [CrossRef]
- Petermann, R.; Tian, M.; Tatsuura, S.; Furuki, M. Synthesis of New Squaraine Dyes for Optical Switches. Dye. Pigment. 2003, 57, 43–54. [Google Scholar] [CrossRef]
- Gsänger, M.; Kirchner, E.; Stolte, M.; Burschka, C.; Stepanenko, V.; Pflaum, J.; Würthner, F. High-Performance Organic Thin-Film Transistors of J-Stacked Squaraine Dyes. J. Am. Chem. Soc. 2014, 136, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Smits, E.C.P.; Setayesh, S.; Anthopoulos, T.D.; Buechel, M.; Nijssen, W.; Coehoorn, R.; Blom, P.W.M.; de Boer, B.; de Leeuw, D.M. Near-Infrared Light-Emitting Ambipolar Organic Field-Effect Transistors. Adv. Mater. 2007, 19, 734–738. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Lin, H.; Wang, D.; Cao, E.; Liu, S.; Nie, Z.; Jia, B. Ultrafast Multi-Target Control of Tightly Focused Light Fields. Opto-Electronic Adv. 2022, 5, 210026. [Google Scholar] [CrossRef]
- Zavaleta, A.; Lykhin, A.O.; Monteiro, J.H.S.K.; Uchida, S.; Bell, T.W.; de Bettencourt-Dias, A.; Varganov, S.A.; Gallucci, J. Full Visible Spectrum and White Light Emission with a Single, Input-Tunable Organic Fluorophore. J. Am. Chem. Soc. 2020, 142, 20306–20312. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine Dyes: A Mine of Molecular Materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjug. Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Treibs, A.; Jacob, K. Cyclotrimethine Dyes Derived from Squaric Acid. Angew. Chem. Int. Ed. Engl. 1965, 4, 694. [Google Scholar] [CrossRef]
- Sprenger, H.-E.; Ziegenbein, W. Cyclobutenediylium Dyes. Angew. Chem. Int. Ed. Engl. 1968, 7, 530–535. [Google Scholar] [CrossRef]
- Sprenger, H.-E.; Ziegenbein, W. The Cyclobutenediylium Cation, a Novel Chromophore from Squaric Acid. Angew. Chem. Int. Ed. Engl. 1967, 6, 553–554. [Google Scholar] [CrossRef]
- Maahs, G.; Hegenberg, P. Syntheses and Derivatives of Squaric Acid. Angew. Chem. Int. Ed. Engl. 1966, 5, 888–893. [Google Scholar] [CrossRef]
- Laramie, M.D.; Levitz, A.; Henary, M. Cyanine and Squaric Acid Metal Sensors. Sens. Actuators B Chem. 2017, 243, 1191–1204. [Google Scholar] [CrossRef]
- Stamentović, V.; Collado, D.; Perez-Inestrosa, E. Photophysical Properties and Bioimaging Application of an Aminonaphthalimide-Squaraine Non-Conjugated System. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120546. [Google Scholar] [CrossRef] [PubMed]
- Beverina, L.; Salice, P. Squaraine Compounds: Tailored Design and Synthesis towards a Variety of Material Science Applications. Eur. J. Org. Chem. 2010, 2010, 1207–1225. [Google Scholar] [CrossRef]
- Geiger, T.; Kuster, S.; Yum, J.-H.; Moon, S.-J.; Nazeeruddin, M.K.; Grätzel, M.; Nüesch, F. Molecular Design of Unsymmetrical Squaraine Dyes for High Efficiency Conversion of Low Energy Photons into Electrons Using TiO2 Nanocrystalline Films. Adv. Funct. Mater. 2009, 19, 2720–2727. [Google Scholar] [CrossRef]
- Yum, J.-H.; Walter, P.; Huber, S.; Rentsch, D.; Geiger, T.; Nüesch, F.; De Angelis, F.; Grätzel, M.; Nazeeruddin, M.K. Efficient Far Red Sensitization of Nanocrystalline TiO2 Films by an Unsymmetrical Squaraine Dye. J. Am. Chem. Soc. 2007, 129, 10320–10321. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Zablocki, J.; Abdullaeva, O.S.; Brück, S.; Balzer, F.; Lützen, A.; Arteaga, O.; Schiek, M. Giant Intrinsic Circular Dichroism of Prolinol-Derived Squaraine Thin Films. Nat. Commun. 2018, 9, 2413. [Google Scholar] [CrossRef] [PubMed]
- Maltese, V.; Cospito, S.; Beneduci, A.; De Simone, B.C.; Russo, N.; Chidichimo, G.; Janssen, R.A.J. Electro-Optical Properties of Neutral and Radical Ion Thienosquaraines. Chem.—A Eur. J. 2016, 22, 10179–10186. [Google Scholar] [CrossRef]
- Corrente, G.A.; Parisi, F.; Maltese, V.; Cospito, S.; Imbardelli, D.; La Deda, M.; Beneduci, A. Panchromatic Fluorescence Emission from Thienosquaraines Dyes: White Light Electrofluorochromic Devices. Molecules 2021, 26, 6818. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Zhu, X.; Pan, Z.; Zhang, X.; Wang, J.; Fu, N. Self-Assembled Nanosensor Based on Squaraine Dye for Specific Recognition and Detection of Human Serum Albumin. Sens. Actuators B Chem. 2018, 255, 977–985. [Google Scholar] [CrossRef]
- Wang, G.; Xu, W.; Guo, Y.; Fu, N. Near-Infrared Squaraine Dye as a Selective Protein Sensor Based on Self-Assembly. Sens. Actuators B Chem. 2017, 245, 932–937. [Google Scholar] [CrossRef]
- McEwen, J.J.; Wallace, K.J. Squaraine Dyes in Molecular Recognition and Self-Assembly. Chem. Commun. 2009, 42, 6339–6351. [Google Scholar] [CrossRef] [PubMed]
- Gassensmith, J.J.; Baumes, J.M.; Smith, B.D. Discovery and Early Development of Squaraine Rotaxanes. Chem. Commun. 2009, 42, 6329–6338. [Google Scholar] [CrossRef] [PubMed]
- Ta, D.D.; Dzyuba, S.V. Squaraine-Based Optical Sensors: Designer Toolbox for Exploring Ionic and Molecular Recognitions. Chemosensors 2021, 9, 302. [Google Scholar] [CrossRef]
- Chenthamarakshan, C.R.; Eldo, J.; Ajayaghosh, A. Squaraine Dye Based Molecular Wires Containing Flexible Oxyethylene Chains as Sensors. Enhanced Fluorescence Response on Li+ Recognition. Macromolecules 1999, 32, 5846–5851. [Google Scholar] [CrossRef]
- Eldo, J.; Ajayaghosh, A. New Low Band Gap Polymers: Control of Optical and Electronic Properties in near Infrared Absorbing π-Conjugated Polysquaraines. Chem. Mater. 2002, 14, 410–418. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Eldo, J. A Novel Approach Toward Low Optical Band Gap Polysquaraines. Org. Lett. 2001, 3, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Nguyen, T.V.; Kuwano, Y.; Chen, X.; Miyanaga, K.; Nakazumi, H.; Yagi, S.; Soman, S.; Ajayaghosh, A. Intramolecular Exciton-Coupled Squaraine Dyes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2018, 122, 21745–21754. [Google Scholar] [CrossRef]
- Nakazumi, H.; Ohta, T.; Etoh, H.; Uno, T.; Colyer, C.L.; Hyodo, Y.; Yagi, S. Near-Infrared Luminescent Bis-Squaraine Dyes Linked by a Thiophene or Pyrene Spacer for Noncovalent Protein Labeling. Synth. Met. 2005, 153, 33–36. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Arunkumar, E.; Daub, J. A Highly Specific Ca2+-Ion Sensor: Signaling by Exciton Interaction in a Rigid-Flexible-Rigid Bichromophoric “H” Foldamer. Angew. Chem. Int. Ed. 2002, 41, 1766–1769. [Google Scholar] [CrossRef]
- Li, K.; Duan, X.; Jiang, Z.; Ding, D.; Chen, Y.; Zhang, G.-Q.; Liu, Z. J-Aggregates of Meso-[2.2]Paracyclophanyl-BODIPY Dye for NIR-II Imaging. Nat. Commun. 2021, 12, 2376. [Google Scholar] [CrossRef]
- Shen, C.; Bialas, D.; Hecht, M.; Stepanenko, V.; Sugiyasu, K.; Würthner, F. Polymorphism in Squaraine Dye Aggregates by Self-Assembly Pathway Differentiation: Panchromatic Tubular Dye Nanorods versus J-Aggregate Nanosheets. Angew. Chem. Int. Ed. 2021, 60, 11949–11958. [Google Scholar] [CrossRef]
- Wan, W.; Zeng, L.; Jin, W.; Chen, X.; Shen, D.; Huang, Y.; Wang, M.; Bai, Y.; Lyu, H.; Dong, X.; et al. A Solvatochromic Fluorescent Probe Reveals Polarity Heterogeneity upon Protein Aggregation in Cells. Angew. Chem. Int. Ed. 2021, 60, 25865–25871. [Google Scholar] [CrossRef]
- Pires, P.A.R.; El Seoud, O.A.; Machado, V.G.; de Jesus, J.C.; de Melo, C.E.A.; Buske, J.L.O.; Cardozo, A.P. Understanding Solvation: Comparison of Reichardt’s Solvatochromic Probe and Related Molecular “Core” Structures. J. Chem. Eng. Data 2019, 64, 2213–2220. [Google Scholar] [CrossRef]
- Machado, V.G.; Stock, R.I.; Reichardt, C. Pyridinium N-Phenolate Betaine Dyes. Chem. Rev. 2014, 114, 10429–10475. [Google Scholar] [CrossRef]
- Inoue, T.; Pandey, S.S.; Fujikawa, N.; Yamaguchi, Y.; Hayase, S. Synthesis and Characterization of Squaric Acid Based NIR Dyes for Their Application towards Dye-Sensitized Solar Cells. J. Photochem. Photobiol. A Chem. 2010, 213, 23–29. [Google Scholar] [CrossRef]
- Available online: https://Macro.Lsu.Edu/Howto/Solvents/Dielectric%20Constant%20.htm (accessed on 26 December 2020).
- Anandhan, K.; Cerón, M.; Perumal, V.; Ceballos, P.; Gordillo-Guerra, P.; Pérez-Gutiérrez, E.; Castillo, A.E.; Thamotharan, S.; Percino, M.J. Solvatochromism and PH Effect on the Emission of a Triphenylimidazole-Phenylacrylonitrile Derivative: Experimental and DFT Studies. RSC Adv. 2019, 9, 12085–12096. [Google Scholar] [CrossRef]
- Lu, Z.-Z.; Zhang, R.; Li, Y.-Z.; Guo, Z.-J.; Zheng, H.-G. Solvatochromic Behavior of a Nanotubular Metal–Organic Framework for Sensing Small Molecules. J. Am. Chem. Soc. 2011, 133, 4172–4174. [Google Scholar] [CrossRef]
- Pooventhiran, T.; Bhattacharyya, U.; Rao, D.J.; Chandramohan, V.; Karunakar, P.; Irfan, A.; Mary, Y.S.; Thomas, R. Detailed Spectra, Electronic Properties, Qualitative Non-Covalent Interaction Analysis, Solvatochromism, Docking and Molecular Dynamics Simulations in Different Solvent Atmosphere of Cenobamate. Struct. Chem. 2020, 31, 2475–2485. [Google Scholar] [CrossRef]
- Carlotti, B.; Flamini, R.; Kikaš, I.; Mazzucato, U.; Spalletti, A. Intramolecular Charge Transfer, Solvatochromism and Hyperpolarizability of Compounds Bearing Ethenylene or Ethynylene Bridges. Chem. Phys. 2012, 407, 9–19. [Google Scholar] [CrossRef]
- Akpe, V.; Biddle, T.J.; Madu, C.; Kim, T.H.; Brown, C.L.; Cock, I.E.; Abe, M. Cock Using New Solvatochromic Parameters to Investigate Dye–Solvent Interactions. Aust. J. Chem. 2022, 75, 206–219. [Google Scholar]
- Cao, Z.; Fan, X.; Yang, Z.; Zhang, X.; Ding, N.; Ding, Y.; Zhang, W. Solvent Directed Discrimination of Metal Ions Using a Coumarin-Pyridine Fluorescence Receptor. Sens. Actuators B Chem. 2020, 310, 127855. [Google Scholar] [CrossRef]
- Cao, Z.; Li, W.; Wan, H.; Zhou, J.; Jia, X.; Ding, Y. Rotating the C–N Bond in a Coumarin–Pyridine-Based Sensor for Pattern Recognition of Versatile Metal Ions. Anal. Chem. 2021, 93, 14256–14262. [Google Scholar] [CrossRef]
- Kütt, A.; Selberg, S.; Kaljurand, I.; Tshepelevitsh, S.; Heering, A.; Darnell, A.; Kaupmees, K.; Piirsalu, M.; Leito, I. PKa Values in Organic Chemistry—Making Maximum Use of the Available Data. Tetrahedron Lett. 2018, 59, 3738–3748. [Google Scholar] [CrossRef]
- Kim, Y.S.; Liang, K.; Law, K.Y.; Whitten, D.G. An Investigation of Photocurrent Generation by Squaraine Aggregates in Monolayer-Modified Tin Oxide (SnO2) Electrodes. J. Phys. Chem. 1994, 98, 984–988. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; et al. GAUSSIAN 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Xie, X.; Gutiérrez, A.; Trofimov, V.; Szilagyi, I.; Soldati, T.; Bakker, E. Charged Solvatochromic Dyes as Signal Transducers in PH Independent Fluorescent and Colorimetric Ion Selective Nanosensors. Anal. Chem. 2015, 87, 9954–9959. [Google Scholar] [CrossRef]
- Xie, X.; Szilagyi, I.; Zhai, J.; Wang, L.; Bakker, E. Ion-Selective Optical Nanosensors Based on Solvatochromic Dyes of Different Lipophilicity: From Bulk Partitioning to Interfacial Accumulation. ACS Sens. 2016, 1, 516–520. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.-M.; Han, J. Fluorescent Chemosensors for Copper(II) Ion: Structure, Mechanism and Application. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 78–103. [Google Scholar] [CrossRef]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper Homeostasis and Neurodegenerative Disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and Amyotrophic Lateral Sclerosis). Chem. Rev. 2006, 106, 1995–2044. [Google Scholar] [CrossRef]
- Bertini, I.; Rosato, A. Menkes Disease. Cell. Mol. Life Sci. 2008, 65, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Bush, A.I.; Cherny, R.A. Copper in the Brain and Alzheimer’s Disease. JBIC J. Biol. Inorg. Chem. 2010, 15, 61–76. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Mei, J.; Zhou, M.; Zhang, Y.; Liang, Q.; Xu, S.; Li, Z. Colorimetric and Fluorescent Probe for Highly Selective and Sensitive Recognition of Cu2+ and Fe3+ Based on Asymmetric Squaraine Dye. Inorg. Chem. Commun. 2022, 142, 109592. [Google Scholar] [CrossRef]
- Ma, X.; Sun, R.; Cheng, J.; Liu, J.; Gou, F.; Xiang, H.; Zhou, X. Fluorescence Aggregation-Caused Quenching versus Aggregation-Induced Emission: A Visual Teaching Technology for Undergraduate Chemistry Students. J. Chem. Educ. 2016, 93, 345–350. [Google Scholar] [CrossRef]
- Huang, Y.; Xing, J.; Gong, Q.; Chen, L.-C.; Liu, G.; Yao, C.; Wang, Z.; Zhang, H.-L.; Chen, Z.; Zhang, Q. Reducing Aggregation Caused Quenching Effect through Co-Assembly of PAH Chromophores and Molecular Barriers. Nat. Commun. 2019, 10, 169. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Sharma, S.; Pandey, S.S. Synthesis and Characterization of Newly Designed and Highly Solvatochromic Double Squaraine Dye for Sensitive and Selective Recognition towards Cu2+. Molecules 2022, 27, 6578. https://doi.org/10.3390/molecules27196578
Tang L, Sharma S, Pandey SS. Synthesis and Characterization of Newly Designed and Highly Solvatochromic Double Squaraine Dye for Sensitive and Selective Recognition towards Cu2+. Molecules. 2022; 27(19):6578. https://doi.org/10.3390/molecules27196578
Chicago/Turabian StyleTang, Linjun, Shubham Sharma, and Shyam S. Pandey. 2022. "Synthesis and Characterization of Newly Designed and Highly Solvatochromic Double Squaraine Dye for Sensitive and Selective Recognition towards Cu2+" Molecules 27, no. 19: 6578. https://doi.org/10.3390/molecules27196578
APA StyleTang, L., Sharma, S., & Pandey, S. S. (2022). Synthesis and Characterization of Newly Designed and Highly Solvatochromic Double Squaraine Dye for Sensitive and Selective Recognition towards Cu2+. Molecules, 27(19), 6578. https://doi.org/10.3390/molecules27196578





