Abstract
Theranostics play an important role in cancer treatment due to its realized real-time tracking of therapeutic efficacy in situ. In this work, we have designed and synthesized a terpyridine-modified pillar [5]arenes (TP5). By the coordination of terpyridine and Zn2+, the complex TP5/Zn was obtained. Then, supramolecular amphiphile can be constructed by using host–guest complexation between a polyethylene glycol contained guest (PM) and TP5/Zn. Combining the fluorescence properties from the terpyridine group and the amphiphilicity from the system, the obtained TP5/Zn/PM can further be self-assembled into fluorescent particles with diameters of about 150 nm in water. The obtained particles can effectively load anti-cancer drugs and realize living cell imaging and a precise release of the drugs.
1. Introduction
Pillar[n]arenes are one type of classical macrocycles, which are synthesized from the hydroquinone-derivatives connected at 2,5-position by methylene groups [1,2,3,4]. Pillar[n]arenes have a rigid hydrophobic cavity with an adjustable size, which endows the pillar[n]arenes with rich host–guest properties [5,6,7,8]. In recent years, the synthesis, functionalized derivation, host–guest properties and applications of pillar[n]arenes have attracted much attention [9,10,11,12,13,14,15,16,17,18], especially in the aspect of constructing pillar[n]arene-based controllable drug release systems for cancer therapy [19,20,21,22,23,24,25]. For example, Prof. Pei constructed tumor microenvironment responsive supramolecular glyco-nanovesicles based on a diselenium-bridged pillar [5]arene dimer for targeted chemotherapy [19]. Prof. Fan fabricated multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy [24]. Prof. Wang and coworkers prepared a dual acid-responsive bola-type supramolecular vesicle by the complexation between a water-soluble pillar [5]arene (WP5) and an acid-sensitive guest molecule (G) containing the 2,4,8,10-tetraoxaspiro [5.5]undecane moiety for an efficient intracellular anticancer drug delivery [26]. Our group prepared the cationic water-soluble pillar [5]arene-modified Cu2–xSe nanoparticles for targeting photothermal therapy in the NIR-II window [25].
Terpyridine not only has a strong fluorescence signal under suitable excitation conditions, but also is used as a general ligand to form transition metal complexes; this provides a new idea for the preparation of living cell fluorescent imaging biomaterials [27,28]. After the coordination of ligands with metal ions, the binding mode and ability of the metal complexes with the target are affected by the structural effect of the ligands and the electronic effect of the metal ions, and as the introduction of metal ions can produce a synergistic effect, the activity of the complex is increased [29]. On the other hand, some transition metal ions are very important to the human body, for example, zinc plays an indispensable role in many life processes: it can promote the metabolism of various substances in the body, promote the proliferation of the lymphatic system, enhance the resistance to viruses and bacteria, etc. [30,31].
Herein, we have designed and synthesized a terpyridine-modified-pillar [5]arene (TP5). By the coordination of terpyridine and Zn2+, pillar [5]arene-Zn complexes (TP5/Zn) were obtained. Then, by using a host–guest complexation between TP5/Zn and a guest molecule which contains polyethylene glycol (PM), the supramolecular amphiphile (TP5/Zn/PM) was constructed successfully. TP5/Zn/PM can further self-assemble into fluorescent particles with a diameter of about 150 nm due to its combined amphiphilic nature and fluorescence properties from the terpyridine group. The obtained fluorescent particles can load and control the release of anti-cancer drugs effectively to realize the precise release of drugs and living cell imaging. This work may provide a new way for scientists to construct nano-theranostics through dynamic host–guest interactions.
2. Results and Discussion
2.1. Characterization of TP5
As described in Scheme 1, TP5 was prepared from a mono alkyl bromide-modified pillar [5]arene (BrP5) and terpyridine in CH3CN with KI as the catalyst. The structure of TP5 was fully characterized by conducting 1H NMR (see Figure S1 in Supplementary Materials), 13C-NMR (see Figure S2 in Supplementary Materials), MS (see Figure S3 in Supplementary Materials) and single crystal X-ray analyses. No proton signal was observed below 0 ppm from the 1H NMR spectra of TP5s, indicating that the alkyl chain is outside the cavity of pillar [5]arene. The single crystal structure of TP5 clearly shows that the alkyl chain is not penetrated into the cavity, which is consistent with the NMR results (Figure 1).
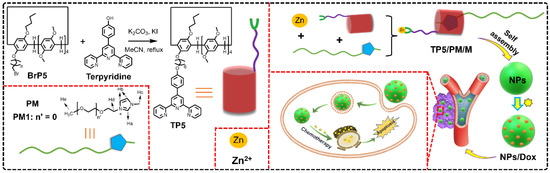
Scheme 1.
Synthesis route to terpyridine-modified pillar [5]arenes (TP5) and cartoon representation of construction of supramolecular amphiphile based on TP5, guest molecule contains polyethylene glycol (PM) and Zn ions for cancer therapy.
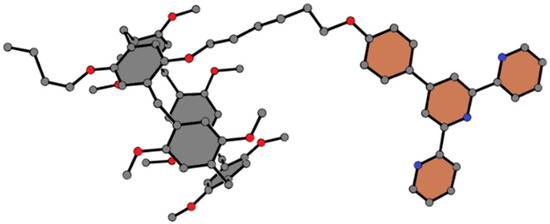
Figure 1.
Single crystal structure of TP5. Carbon atoms are represented in gray, oxygen atoms in red and nitrogen atoms in purple. Hydrogen atoms were omitted for clarity.
2.2. Coordination and Host–Guest Recognition
As we all know, terpyridine is a classical tridentate ligand, which can coordinate with a variety of metal ions to form metal complexes [27]. On the other hand, Zn2+ ions play an indispensable role in many life processes. In order to explore the recognition performance of TP5 on Zn2+ ions, the fluorescence of TP5 was investigated after adding Zn2+ ions. As shown in Figure S4, compared with TP5, the fluorescence intensity decreased sharply after the addition of ZnCl2. Then the fluorescence titration of Zn(II) with TP5 showed a continuous decrease with the increase in the Zn(II):TP5 ratio (from 0:10 to 10:0 µM). The fluorescence titration curve revealed that the fluorescence intensity at 406 nm decreased linearly on increasing the ratio of the Zn(II) ions (Figure 2a). The method of continuous variation (Job’s plot) was also performed to prove the 1:1 stoichiometry (Figure 2b). All the above results indicated that TP5 and Zn2+ could form a stable 1:1 complex (TP5/Zn).
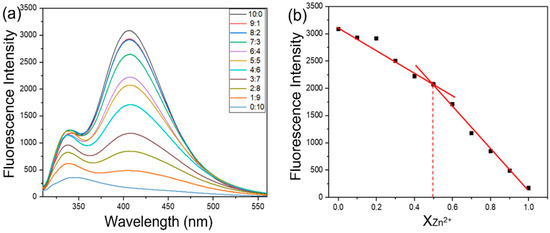
Figure 2.
(a) The fluorescence titration of Zn(II) with TP5, [TP5] + [Zn2+] = 2 × 10−5 M. (b) Job’s plot curve from spectra (a).
The free cavity in TP5/Zn endowed it with a unique host–guest property. PM1 was chosen as the model guest to investigate the host–guest interaction between TP5/Zn and the guest molecule which contains polyethylene glycol. The proton NMR spectrum of an equimolar solution of TP5/Zn and guest PM1 showed that the complex is in fast exchange on the proton NMR time scale (see Figure S5 in Supplementary Materials). Protons Ha, Hb, Hc and Hd on guest PM1 shifted upfield after complexation while no obvious chemical shift changes were observed for He after complexation. These phenomena suggested that PM1 was threaded through the cavity of TP5/Zn to form a [2]pseudorotaxane with the imidazolium part located in the cavity and the tail (He) out of the cavity. Furthermore, the Job’s plot analysis revealed that a stoichiometry system between TP5/Zn and PM1 in the complex can be determined at 330 nm (see Figure S6 in Supplementary Materials). As expected, when the molar fraction of the complex sensor was 0.5, the absorbance reached a maximum, which demonstrates that the interaction between TP5/Zn and PM1 forms a 1:1 complex.
2.3. Construction and Characterization of Nano-Theranostics
After the establishment of the new host–guest recognition motif in aqueous solution, we further applied it to construct the supramolecular amphiphile TP5/Zn/PM. The guest molecule PM has two advantages, the first is that it has a strong complexing ability with pillar [5]arene, and the second is that it endows the system with amphiphilicity, which makes the system form stable assemblies. The critical aggregate concentration (CAC) in water was determined to be about 2.32 × 10−6 mol/L from the change of water surface tension (Figure 3a). Furthermore, the dynamic light scattering (DLS) experiment performed with a 2.50 × 10−6 M aqueous solution of TP5/Zn/PM over a scattering angle of 90°, showed a narrow size distribution (Figure 3b). The average hydrodynamic diameter (Dh) of TP5/Zn/PM was observed to be about 150 nm. Transmission electron microscopy (TEM) was used to investigate the morphology of TP5/Zn/PM-based assemblies in water. As shown in Figure 3c, when TP5/Zn/PM was dissolved in water, it self-assembled into spherical structures with a diameter of about 140 nm immediately. In addition, the SEM image showed that TP5/Zn/PM self-assembled into particles in water, which consisted with the TEM results (Figure 3d). TP5/Zn/PM self-assembled into particles in water possibly due to a larger curvature of its membrane, and a membrane with a larger curvature could form micelles easily. Furthermore, the zeta potential of hollow vesicles was −42.3 ± 4.78 mV, indicating that the vesicles were very stable in solution [32].
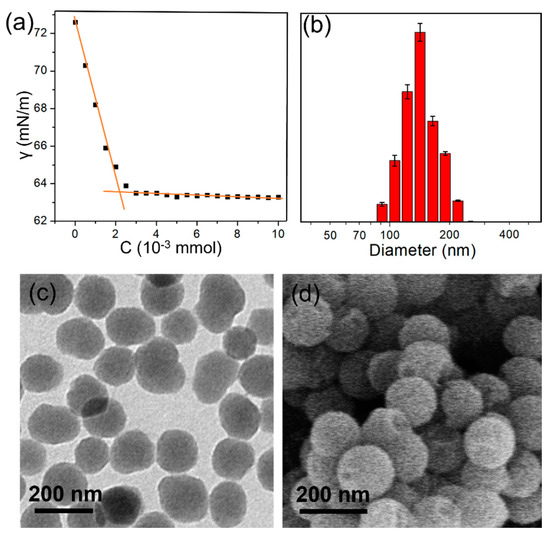
Figure 3.
(a) Water surface tension as a function of the concentration of TP5/Zn/PM. (b) DLS of TP5/Zn/PM self-assembly in water. [C] = 2.50 × 10−6 M. (c) TEM and (d) SEM images of TP5/Zn/PM self-assembly in water [C] = 2.50 × 10−6 M.
2.4. Cell Imaging
Then, the Dox loading and in vitro release were carried out to check whether TP5/Zn/PM-based particles can be used as a drug carrier. The particles entrapped Dox effectively with an encapsulation efficiency of 195 μg/mg (Figure S7), indicating that TP5/Zn/PM-based particles are satisfactory drug-loaded materials. The Dox release experiments were investigated in PBS with pH 7.4, 6.0 and 4.7, respectively (Figure S8). Then, the UV-Vis spectra was used to monitor the release of Dox against time. After 10 h, the total release rate was 6.9% at pH 7.4, 45.9% at pH 6.0 and 62.1% at pH 4.7, respectively. As we all know, the microenvironment of tumor tissue is acidic due to an excess in expressed lactic acid and CO2 in the metabolites of tumor cells [33,34]. Dox loading particles can suspend the Dox release in normal cells, and it is found that this pH-responsive Dox release is a slow process under acidic conditions. Therefore, Dox loading particles can prolong dosing time and reduce toxicity.
Cellular uptake ability is an important parameter for the therapeutic effects of nanomaterials. [35,36,37] On the other hand, the terpyridine unit in pillar [5]arene has a strong fluorescence signal under a suitable excitation, so we can utilize a laser scanning confocal microscope (CLSM) to investigate the internalization of the obtained materials by HeLa cells. The HeLa cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM). The medium was supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The HeLa cells were seeded in 96-well plates (5 × 104 cell mL−1, 0.1 mL per well) for 24 h at 37 °C in 5% CO2. Then the cells were incubated in TP5/Zn/PM, Dox and TP5/Zn/PM/Dox for 4 h, respectively. The medium was then removed, and the cells were washed 3 times with a phosphate buffer. Finally, the cells were observed by fluorescence microscopy. As shown in Figure 4, the cells that were treated with TP5/Zn/PM exhibited a bright blue fluorescence emission in the cytoplasm, while those treated with Dox exhibited a bright red fluorescence emission in the nucleus. However, the cells that were incubated with TP5/Zn/PM/Dox showed both a significant blue and red fluorescence. All the above results confirmed that all the obtained pillar [5]arene-based materials not only can be uptaken by HeLa cells efficiently, but also can be applied in living cell imaging.
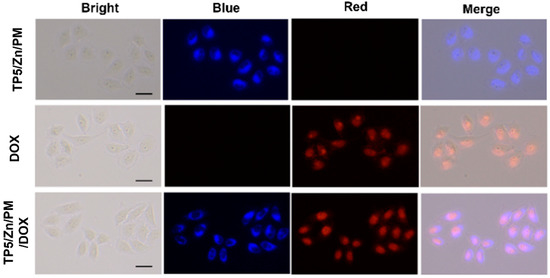
Figure 4.
Fluorescent images of HeLa cells treated with TP5/Zn/PM, Dox and TP5/Zn/PM/Dox for 4 h, respectively.
2.5. In Vitro Cancer Therapy
The HeLa cells were also selected to investigate the cancer therapy effect of TP5/Zn/PM/Dox in vitro. After incubating with different groups, their viabilities were investigated via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays [38,39,40]. As shown in Figure 5a, when the HeLa cells cultivated with TP5/Zn, PM and TP5/Zn/PM (concentration from 0–56 μg mL−1), the viabilities of the cells were all above 97%, indicating that our material itself has a good biocompatibility. For the cells in the TP5/Zn/PM/Dox and Dox groups, the cell viabilities all decreased as the concentration increased, and the cytotoxicity of TP5/Zn/PM/Dox was higher than that of Dox at the same concentration (Figure 5b). Nevertheless, the cell viability of TP5/Zn/PM/Dox was only 4% when the concentration increased to 60 µg/mL, exhibiting the largest cytotoxicity toward the HeLa cells.
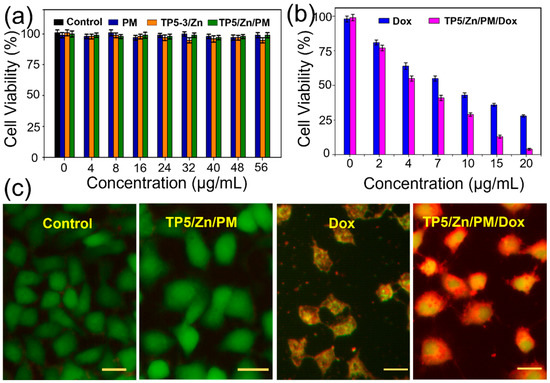
Figure 5.
(a) The viabilities of HeLa cells cultivated with different concentration of PM, TP5/Zn and TP5/Zn/PM. (b) The viabilities of HeLa cells cultivated with different concentration of Dox and TP5/Zn/PM/Dox. (c) Fluorescence images of calcein-AM (live cells, green) and PI (dead cells, red) contained HeLa cells after different treatments. Scale bar = 20 µm.
At last, to check the cancer therapy effect of TP5/Zn/PM/Dox, live (green) and dead (red) cells were differentiated by calcein acetoxymethyl (calcein-AM) and propidium iodide (PI) staining. In the control and TP5/Zn/PM groups, the cells exhibited a green fluorescence, indicating that they are living well. On the contrary, when treated with free Dox, the cells exhibited an orange fluorescence. However, when treated with TP5/Zn/PM/Dox, almost all of the cells showed a bright red fluorescence, indicating that all of the cells died. These results clearly confirmed the satisfied therapeutic effect of TP5/Zn/PM/Dox.
3. Materials and Methods
All reagents were commercially available and used as supplied without further purification. Solvents were either employed as purchased or dried according to the procedures described in the literature. The 1H- or 13C-NMR spectra were recorded with a Bruker Avance DMX 400 spectrophotometer (Bruker, Bremen, Germany) with use of the deuterated solvent as the lock and the residual solvent or TMS as the internal reference. The solid-state nuclear magnetic resonance (NMR) spectra were recorded on a BRUKER 400WB AVANCE III spectrometer. A scanning electron microscopy (SEM) investigation was carried out on a JEOL 6390LV instrument (ZEISS, Oberkochen, Germany). The transmission electron microscopy (TEM) images were obtained using a Talos F200X instrument with an accelerating voltage of 80 kV (FEI, Hillsboro, OR, USA). UV-Vis spectroscopy was measured on a Shimadzu UV-2501 PC UV-Vis spectrometer (Shimadzu, Kyoto, Japan). The HeLa cells was purchased from Tongpai (Shanghai, China) Biotechnology Co., Ltd.
4. Conclusions
In brief, a terpyridine-modified pillar [5]arene (TP5) has been synthesized. Terpyridine was not only complexed with Zn ions but also endowed TP5 with fluorescent properties. At the same time, the cavity of pillar [5]arene can complex with the guest molecule PM to form a stable supramolecular amphiphile. The resulting TP5/Zn/PM can further self-assemble into fluorescent particles. The obtained fluorescent particles can load and control the release of anti-cancer drugs effectively, which realized the precise release of drugs and living cell imaging. This work may provide a new way for scientists to construct nano-theranostics through dynamic host–guest interactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27196428/s1, Figures S1–S3: Characterization of TP5; Table S1: Information of crystal data for TP5; Figure S4: Fluorescence spectra of TP5 and TP5/Zn; Figure S5: Partial 1H NMR (CDCl3, 400 MHz, R.T.) of PM1, PM1 + TP5/Zn and TP5/Zn; Figure S6: UV-Vis spectra of TP5/Zn and PM1 with different ratio; Figure S7: UV-Vis absorption spectra (H2O, RT) of different concentrations of Dox; Figure S8: Drug release curves of TP5/Zn/PM-based particles in PBS solutions of different pH values at 37 °C. BrP5 and terpyridine are prepared according references. References [41,42] are cited in the supplementary materials.
Author Contributions
Conceptualization, Y.Z. and L.Y.; methodology, Y.Z.; validation, L.Y., L.M. and Y.H.; formal analysis, Y.Z.; writing—original draft preparation, C.-G.Y. and Y.Y.; writing—review and editing, Y.Y.; supervision, Y.Y.; funding acquisition, C.-G.Y. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21871227), the Six Talent Peak Projects in Jiangsu Province (XCL-085).
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
No applicable.
Acknowledgments
We also thank Nantong University Analysis & Testing Center for characterization.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.; Nakamoto, Y. para-Bridged Symmetrical Pillar [5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Kou, Y.; Liang, J.; Chen, Z.; Wang, L.; Meier, H. A Facile and Efficient Preparation of Pillararenes and a Pillarquinone. Angew. Chem. Int. Ed. 2009, 48, 9721–9723. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, C.; Jing, X.; Yao, Y. Rim-differentiated pillar [5]arenes. Chin. Chem. Lett. 2021, 32, 3322–3330. [Google Scholar] [CrossRef]
- Sun, G.; Qian, W.; Jiao, J.; Han, T.; Shi, Y.; Hu, X.; Wang, L. A highly efficient artificial light-harvesting system with two-step sequential energy transfer based on supramolecular self-assembly. J. Mater. Chem. A 2020, 8, 9590–9596. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, X.; Wang, S.; Zhu, Z.; Cen, M.; Ou, C.; Zhao, Q.; Yan, Q.; Wang, J.; Yao, Y. Pillar [5]arene-Based 3D Hybrid Supramolecular Polymer for Green Catalysis in Water. Inorg. Chem. 2021, 60, 2883–2887. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, Y.; Dai, D.; Yang, Y.; Wu, B.; Yang, X. Stabilization of Grignard reagents by a pillar [5]arene host-Schlenk equilibria and Grignard reactions. Chem. Commun. 2020, 56, 1381–1384. [Google Scholar] [CrossRef]
- Lee, E.; Park, I.; Ju, H.; Kim, S.; Jung, J.; Habata, Y.; Lee, S. Formation of a Pillar [5]arene-Based Two-Dimensional Poly-Pseudo-Rotaxane: Threading and Crosslinking by the Same Guest Molecules. Angew. Chem. Int. Ed. 2019, 58, 11296–11300. [Google Scholar] [CrossRef]
- Guo, H.; Ye, J.; Zhang, Z.; Wang, Y.; Yuan, X.; Ou, C.; Ding, Y.; Yan, C.; Wang, J.; Yao, Y. Pillar [5]arene-Based [2]Rotaxane: Synthesis, Characterization, and Application in a Coupling Reaction. Inorg. Chem. 2020, 59, 11915–11919. [Google Scholar] [CrossRef]
- Wan, K.; Gao, S.; Fang, X.; Xu, M.; Yang, Y.; Xue, M. Oxacalix [4]arene-bridged pillar [5]arene dimers: Syntheses, planar chirality and construction of chiral rotaxanes. Chem. Commun. 2020, 56, 10155–10158. [Google Scholar] [CrossRef]
- Kaizerman-Kane, D.; Hadar, M.; Tal, N.; Dobrovetsky, R.; Zafrani, Y.; Cohen, Y. pH-Responsive Pillar [6]arene-based Water-Soluble Supramolecular Hexagonal Boxes. Angew. Chem. Int. Ed. 2019, 58, 5302–5306. [Google Scholar] [CrossRef]
- Hou, C.; Liu, L.; Meng, S.; Wu, Y.; Xie, M.; Shan, Y.; He, P.; Sun, P.; Liao, X. Hybrid vesicles of pillar [5]arene/silica: Host-guest complexation and application in pH-triggered release. Chin. Chem. Lett. 2021, 32, 214–217. [Google Scholar] [CrossRef]
- Nazarova, A.; Padnya, P.; Cragg, P.J.; Stoikov, I. [1]Rotaxanes based on phosphorylated pillar [5]arenes. New J. Chem. 2022, 46, 2033–2037. [Google Scholar] [CrossRef]
- Lou, X.; Yang, Y. Pyridine-Conjugated Pillar [5]arene: From Molecular Crystals of Blue Luminescence to Red-Emissive Coordination Nanocrystals. J. Am. Chem. Soc. 2021, 143, 11976–11981. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Ma, J.; Liang, W.; Xiao, C.; Wu, W.; Zhou, D.; Yao, J.; Sun, W.; Sun, J.; Gao, G.; et al. Guest-Binding-Induced Interhetero Hosts Charge Transfer Crystallization: Selective Coloration of Commonly Used Organic Solvents. J. Am. Chem. Soc. 2021, 143, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Gong, W.; Hassan, M.; Qu, W.; Liu, L.; Ning, G. Guest induced morphology transitions of star shaped pillar [5]arene trimer via endo host-guest and “exo-wall” electron-transfer interactions. Chem. Lett. 2021, 32, 371–374. [Google Scholar] [CrossRef]
- Yakimova, L.; Guralnik, E.; Shurpik, D.; Evtugyn, V.; Osin, Y.; Subakaeva, E.; Sokolova, E.; Zelenikhin, P.; Stoikov, I. Morphology, structure and cytotoxicity of dye-loaded lipid nanoparticles based on monoamine pillar [5]arenes. Mater. Chem. Front. 2020, 4, 2962–2970. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, T.; Fan, Y.; Qu, W.; Zhu, W.; Ma, X.; Yao, H.; Zhang, Y.; Lin, Q. A pillar [5]arene-based and OH− dependent dual-channel supramolecular chemosensor for recyclable CO2 gas detection: High sensitive and selective off-on-off response. Dye. Pigment. 2020, 174, 108073. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.; Zhang, Z.; Wang, J.; Yuan, X.; Zhao, Q.; Ding, Y.; Yao, Y. CO2 and photo-controlled reversible conversion of supramolecular assemblies based on water soluble pillar [5]arene and coumarin-containing guest. Chin. Chem. Lett. 2021, 32, 349–352. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Chen, Z.; Hu, X.; Pu, L.; Pei, Z.; Pei, Y. Tumor microenvironment responsive supramolecular glyco-nanovesicles based on diselenium-bridged pillar [5]arene dimer for targeted chemotherapy. Chem. Commun. 2020, 56, 10642–10645. [Google Scholar] [CrossRef]
- Muhammed, M.; Cruz, L.; Emwas, A.; El-Zohry, A.; Moosa, B.; Mohammed, O.; Khashab, N. Pillar [5]arene-Stabilized Silver Nanoclusters: Extraordinary Stability and Luminescence Enhancement Induced by Host-Guest Interactions. Angew. Chem. Int. Ed. 2019, 58, 15665–15670. [Google Scholar] [CrossRef]
- Jiao, Y.; Lan, S.; Ma, D. Ultra-stable and multistimuli-responsive nanoparticles coated with zwitterionic pillar[n]arene for enhanced cellular uptake. Chin. Chem. Lett. 2021, 32, 1025–1028. [Google Scholar] [CrossRef]
- Xiao, T.; Qi, L.; Zhong, W.; Lin, C.; Wang, R.; Wang, L. Stimuli-responsive nanocarriers constructed from pillar[n]arene-based supra-amphiphiles. Mater. Chem. Front. 2019, 3, 1973–1993. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Shi, B.; Shangguan, L.; Tong, W.; Yu, G.; Mao, Z.; Huang, F. Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tian, L.; Xu, J.; Xia, B.; Li, J.; Lu, F.; Lu, X.; Wang, W.; Huang, W.; Fan, Q. Multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy. Chem. Commun. 2018, 54, 10328–10331. [Google Scholar] [CrossRef] [PubMed]
- Cen, M.; Ding, Y.; Wang, J.; Yuan, X.; Lu, B.; Wang, Y.; Yao, Y. Cationic Water-Soluble Pillar [5]arene-Modified Cu2–xSe Nanoparticles: Supramolecular Trap for ATP and Application in Targeted Photothermal Therapy in the NIR-II Window. ACS Macro Lett. 2020, 9, 1558–1562. [Google Scholar] [CrossRef]
- Sun, G.; He, Z.; Hao, M.; Zuo, M.; Xu, Z.; Hu, X.; Zhu, J.; Wang, L. Dual acid-responsive bola-type supramolecular vesicles for efficient intracellular anticancer drug delivery. J. Mater. Chem. B 2019, 7, 3944–3949. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newkome, G. Terpyridine-based metallosupramolecular constructs: Tailored monomers to precise 2D-motifs and 3D-metallocages. Chem. Soc. Rev. 2018, 47, 3991–4016. [Google Scholar] [CrossRef]
- Ghosh, B.; Topić, F.; Sahoo, P.; Mal, P.; Linnera, J.; Kalenius, E.; Tuononen, H.; Rissanen, K. Synthesis, structure and photophysical properties of a highly luminescent terpyridine-diphenylacetylene hybrid fluorophore and its metal complexes. Dalton Trans. 2015, 44, 254–267. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Wang, Z.; Liu, R.; Luo, B.; Yang, D.; Chen, H.; Pan, L.; Ma, Z. Copper chloride complexes with substituted 4′-phenyl-terpyridine ligands: Synthesis, characterization, antiproliferative activities and DNA interactions. Dalton Trans. 2021, 50, 8243–8257. [Google Scholar] [CrossRef]
- Ohalloran, T. Transition Metals in Control of Gene Expression. Science 1993, 261, 715–725. [Google Scholar] [CrossRef]
- Berg, J.; Shi, Y. The Galvanization of Biology: A Growing Appreciation for the Roles of Zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, L.; Hou, M.; Chen, Q.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Phase-Change Material Packaged within Hollow Copper Sulfide Nanoparticles Carrying Doxorubicin and Chlorin e6 for Fluorescence-Guided Trimodal Therapy of Cancer. ACS Appl. Mater. Interfaces 2019, 11, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.; Kerckhoff, W.; Vaupel, P.; Müller-Klieser, W. Effect of CO2 and lactic acid on intracellular pH of ascites tumor cells. Resp. Physiol. 1981, 45, 273–285. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Cen, M.; Jing, D.; Bei, J.; Huang, Y.; Zhang, J.; Lu, B.; Wang, Y.; Yao, Y. GOx-assisted synthesis of pillar [5]arene based supramolecular polymeric nanoparticles for targeted/synergistic chemo-chemodynamic cancer therapy. J. Nanobiotechnol. 2022, 20, 33. [Google Scholar] [CrossRef]
- Tian, X.; Zuo, M.; Niu, P.; Velmurugan, K.; Wang, K.; Zhao, Y.; Wang, L.; Hu, X. Orthogonal Design of a Water-Soluble meso-Tetraphenylethene-Functionalized Pillar [5]arene with Aggregation-Induced Emission Property and Its Therapeutic Application. ACS Appl. Mater. Interfaces 2021, 13, 37466–37474. [Google Scholar] [CrossRef]
- Zhou, W.-L.; Chen, Y.; Lin, W.; Liu, Y. Luminescent lanthanide–macrocycle supramolecular assembly. Chem. Commun. 2021, 57, 11443–11456. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Shi, L.; Cen, M.; Wang, J.; Ding, Y.; Yao, Y. Platinum(II) Metallatriangle: Construction, Coassembly with Polypeptide, and Application in Combined Cancer Photodynamic and Chemotherapy. Inorg. Chem. 2021, 60, 7627–7631. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, W.; Cheng, M.; Zhang, S.; Kovaleva, E.G. Controlled release of drug molecules by pillararene-modified nanosystems. Chem. Commun. 2022, 58, 3255–3269. [Google Scholar] [CrossRef]
- Tang, M.; Liu, Y.-H.; Xu, X.-M.; Zhang, Y.-M.; Liu, Y. Dual-responsive drug release and fluorescence imaging based on disulfide-pillar [4]arene aggregate in cancer cells. Bioorgan. Med. Chem. 2022, 57, 116649. [Google Scholar] [CrossRef]
- Yang, K.; Qi, S.; Yu, X.; Bai, B.; Zhang, X.; Mao, Z.; Huang, F.; Yu, G. A Hybrid Supramolecular Polymeric Nanomedicine for Cascade-Amplified Synergetic Cancer Therapy. Angew. Chem. Int. Ed. 2022, 61, e202203786. [Google Scholar]
- Yao, Y.; Li, J.; Dai, J.; Chi, X.; Xue, M. A water-soluble pillar[6]arene: Synthesis, host–guest chemistry, controllable self-assembly, and application in controlled release. RSC Adv. 2014, 4, 9039–9043. [Google Scholar] [CrossRef]
- Shi, B.; Jie, K.; Zhou, Y.; Xia, D.; Yao, Y. Formation of fluorescent supramolecular polymeric assemblies via orthogonal pillar[5]arene-based molecular recognition and metal ion coordination. Chem. Commun. 2015, 51, 4503–4506. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).