Interactions of Isoquinoline Alkaloids with Transition Metals Iron and Copper
Abstract
1. Introduction
2. Results
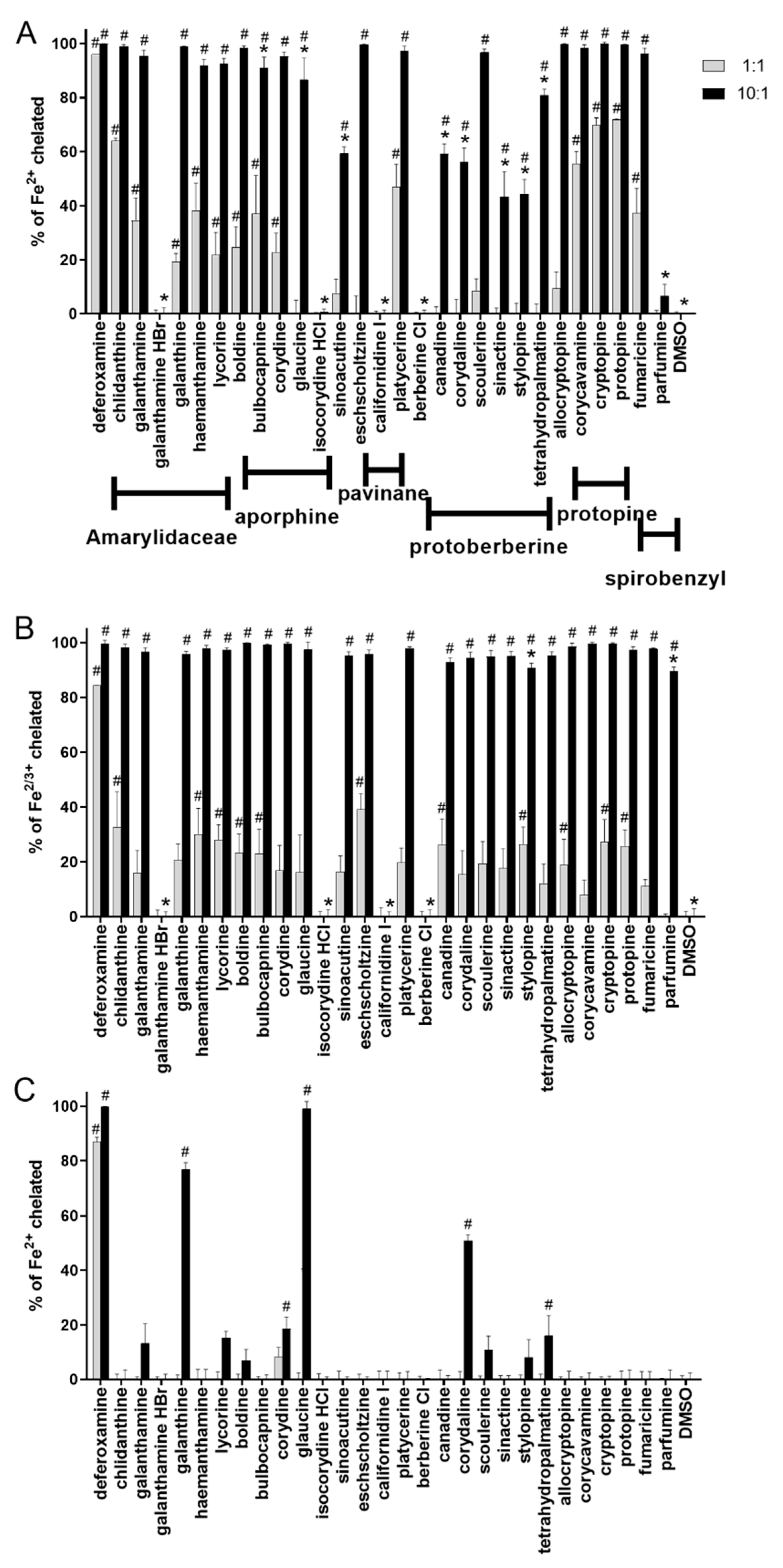
2.1. Iron Chelation in Non-Buffered Conditions: All Tested Alkaloids Chelated iron
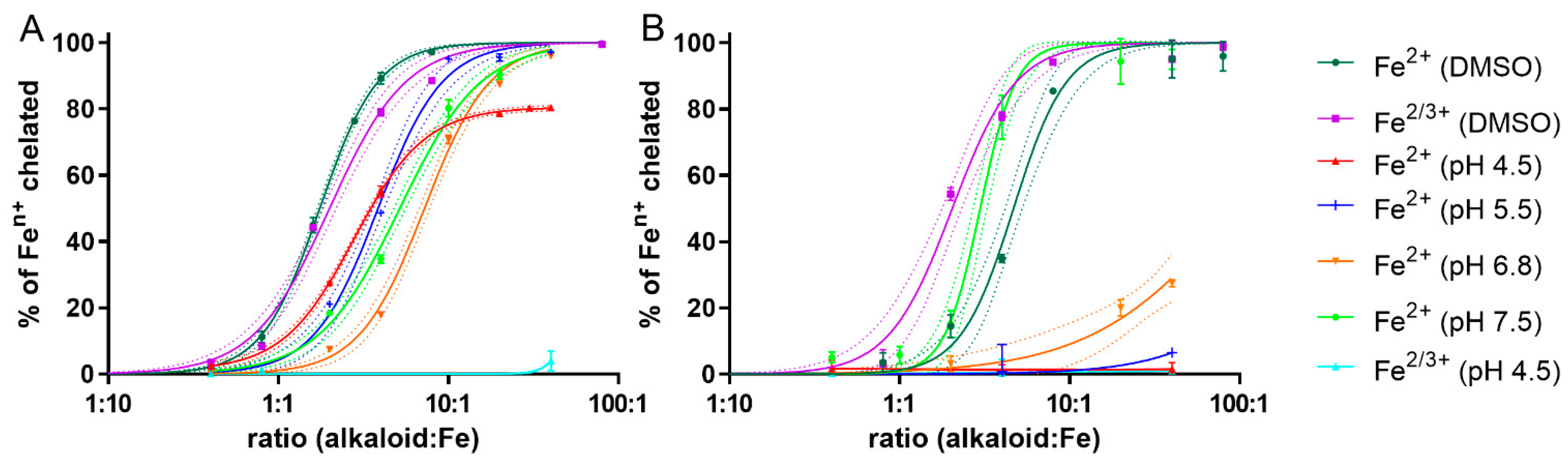
2.2. Ferrous Chelation under Different pH Conditions: Only Galantine and Glaucine Were Active at Slightly Acidic pH 6.8
2.3. Confirmation of the Ferric Chelation Effect of Glaucine in Non-Competitive Conditions
2.4. Copper Chelation: None of the Tested Alkaloids Significantly Chelated Cupric or Cuprous Ions
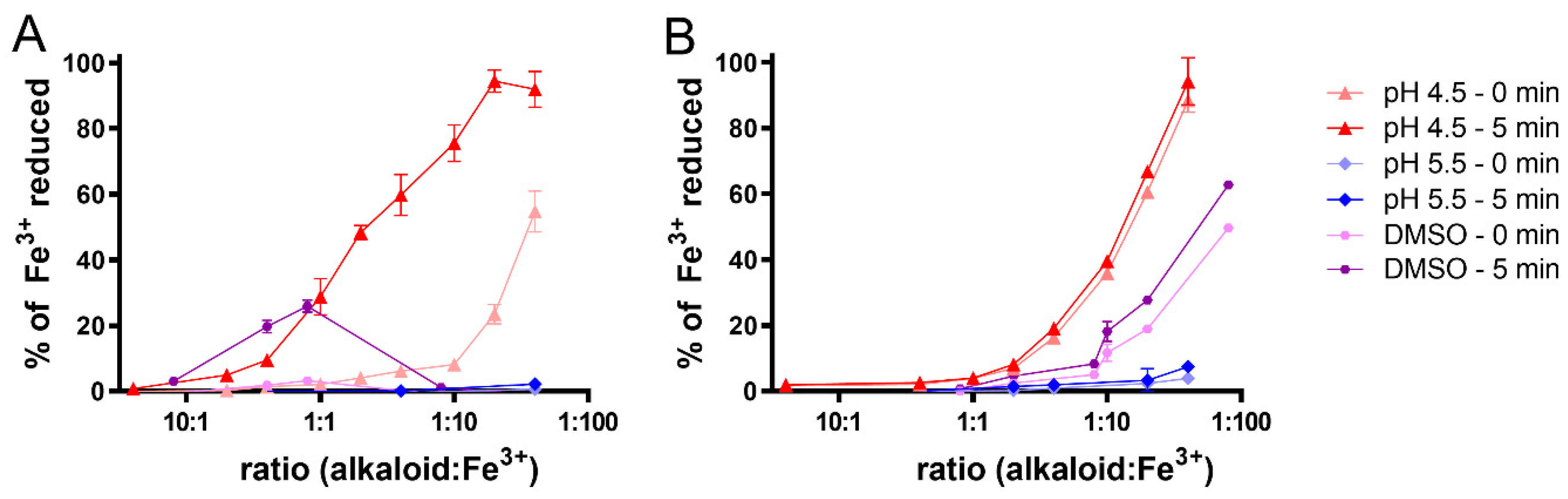
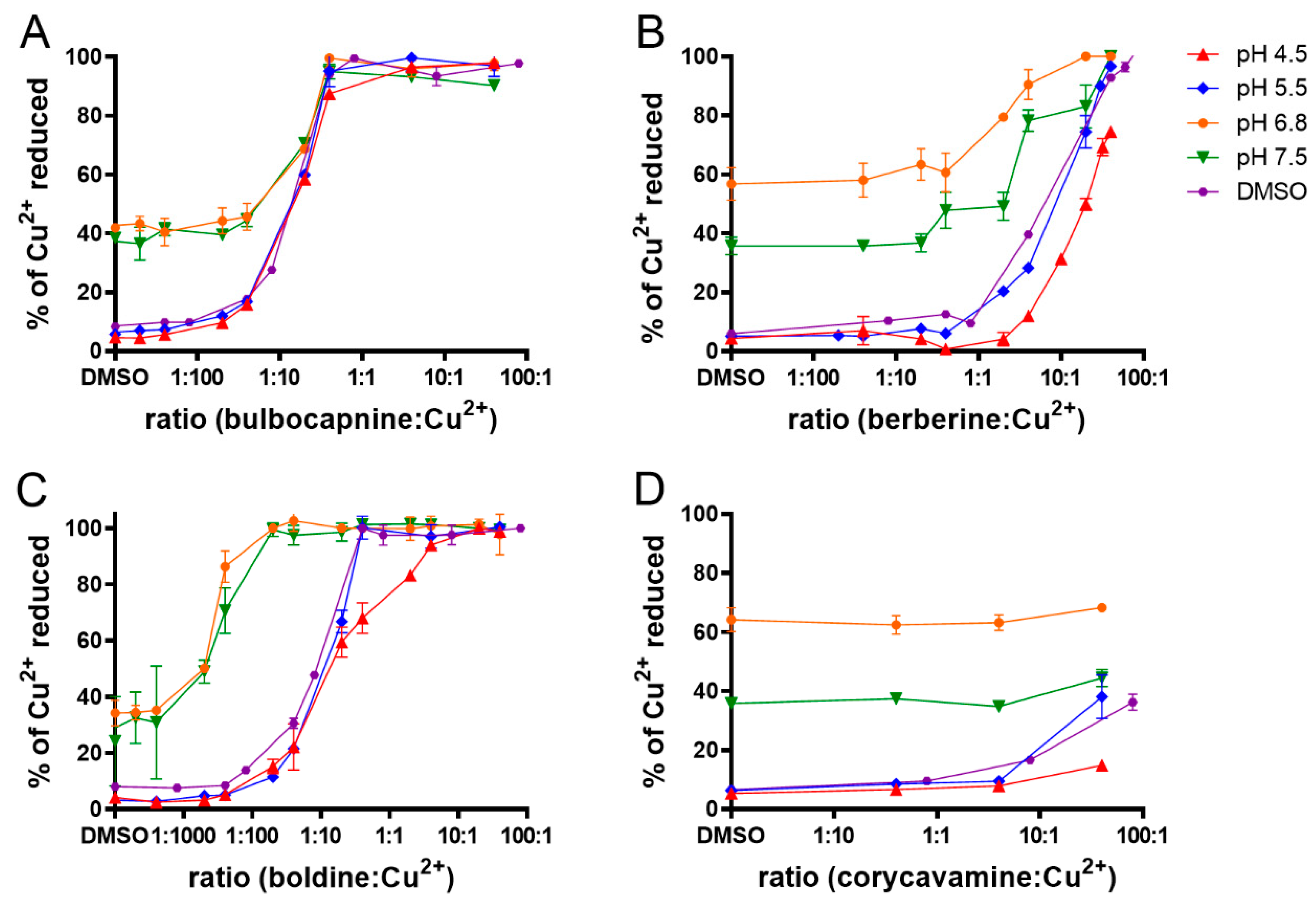
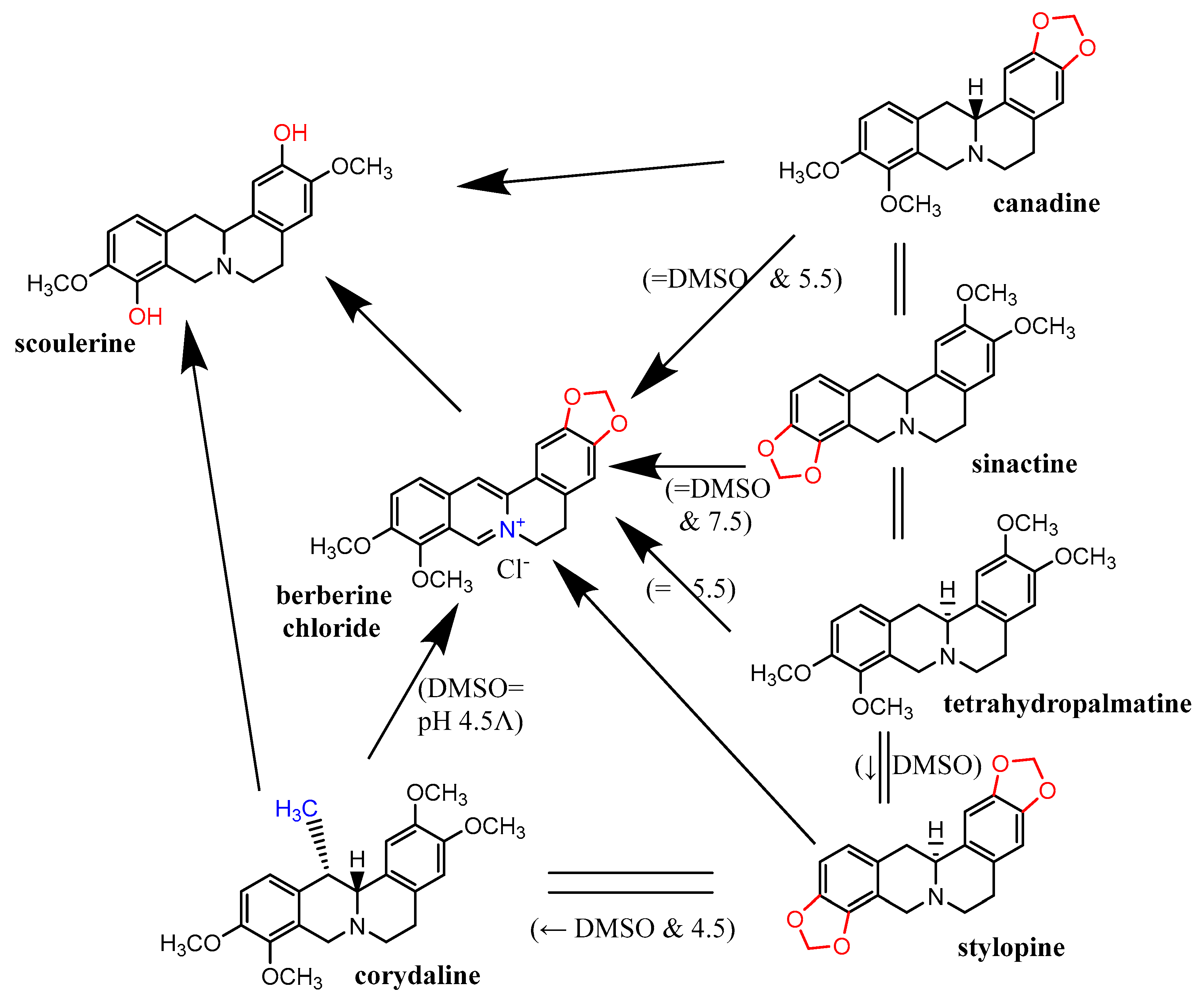
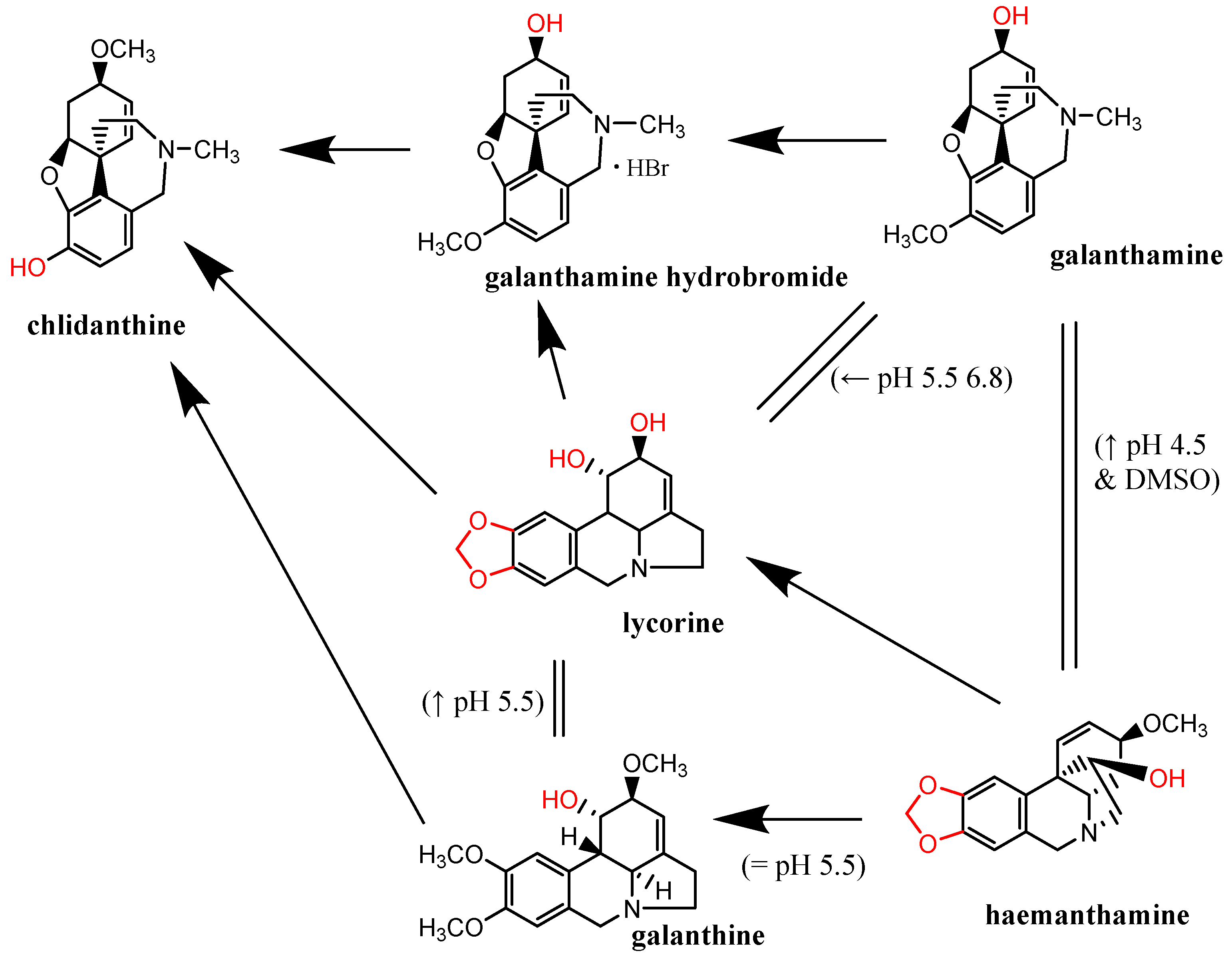
2.5. Metal Reduction: Most Alkaloids Reduced Ferric and, in Particular, Cupric Ions
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Iron/Copper Chelation-and-Reduction Determination
4.2.1. Reagent and Stock-Solution Preparation
4.2.2. Ferrozine Assay
4.2.3. Hematoxylin Assay
4.2.4. BCS Assay
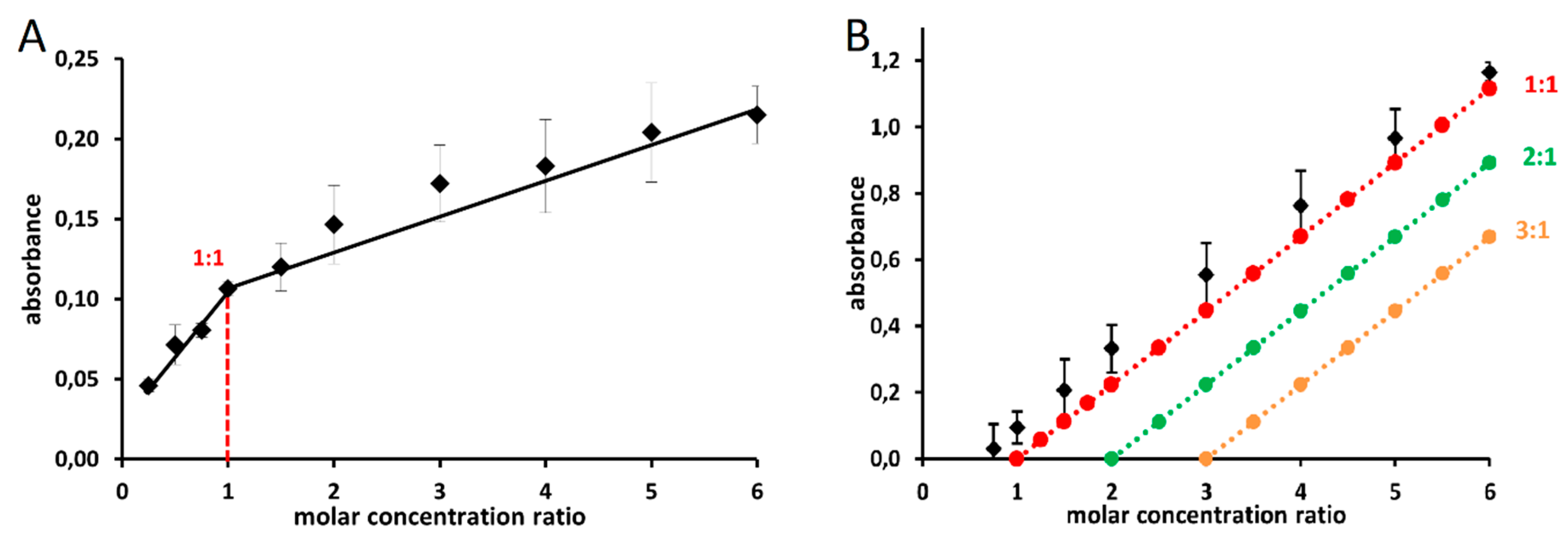
4.2.5. Assessment of Iron Stoichiometry
Job’s Method
Complementary Method
4.2.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Derry, P.J.; Hegde, M.L.; Jackson, G.R.; Kayed, R.; Tour, J.M.; Tsai, A.L.; Kent, T.A. Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog. Neurobiol. 2020, 184, 101716. [Google Scholar] [CrossRef]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Chevion, M.; Jiang, Y.; Har-El, R.; Berenshtein, E.; Uretzky, G.; Kitrossky, N. Copper and iron are mobilized following myocardial ischemia: Possible predictive criteria for tissue injury. Proc. Natl. Acad. Sci. USA 1993, 90, 1102–1106. [Google Scholar] [CrossRef]
- Rhaman, M.M.; Islam, M.R.; Akash, S.; Mim, M.; Noor alam, M.; Nepovimova, E.; Valis, M.; Kuca, K.; Sharma, R. Exploring the role of nanomedicines for the therapeutic approach of central nervous system dysfunction: At a glance. Front. Cell Dev. Biol. 2022, 10, 1780. [Google Scholar] [CrossRef]
- Cao, J.; Hou, J.; Ping, J.; Cai, D. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 64. [Google Scholar] [CrossRef]
- Yuksel, J.M.; Noviasky, J.; Britton, S. Aducanumab for Alzheimer’s Disease: Summarized Data From EMERGE, ENGAGE, and PRIME Studies. Sr. Care Pharm. 2022, 37, 329–334. [Google Scholar] [CrossRef]
- Metz, C.N.; Pavlov, V.A. Treating disorders across the lifespan by modulating cholinergic signaling with galantamine. J. Neurochem. 2020, 158, 1359–1380. [Google Scholar] [CrossRef]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef]
- Shang, X.-F.; Yang, C.-J.; Morris-Natschke, S.L.; Li, J.-C.; Yin, X.-D.; Liu, Y.-Q.; Guo, X.; Peng, J.-W.; Goto, M.; Zhang, J.-Y.; et al. Biologically active isoquinoline alkaloids covering 2014–2018. Med. Res. Rev. 2020, 40, 2212–2289. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kolnagou, A.; Kontoghiorghe, C.N.; Mourouzidis, L.; Timoshnikov, V.A.; Polyakov, N.E. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines 2020, 7, 45. [Google Scholar] [CrossRef]
- Gaur, K.; Vázquez-Salgado, A.M.; Duran-Camacho, G.; Dominguez-Martinez, I.; Benjamín-Rivera, J.A.; Fernández-Vega, L.; Sarabia, L.C.; García, A.C.; Pérez-Deliz, F.; Méndez Román, J.A.; et al. Iron and Copper Intracellular Chelation as an Anticancer Drug Strategy. Inorganics 2018, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, T.P.; Dharmasivam, M.; Dai, C.C.; Richardson, D.R. Innovative therapies for neuroblastoma: The surprisingly potent role of iron chelation in up-regulating metastasis and tumor suppressors and down-regulating the key oncogene, N-myc. Pharmacol. Res. 2021, 173, 105889. [Google Scholar] [CrossRef]
- Macáková, K.; Afonso, R.; Saso, L.; Mladěnka, P. The influence of alkaloids on oxidative stress and related signaling pathways. Free Radic. Biol. Med. 2019, 134, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Shirwaikar, A.; Shirwaikar, A.; Rajendran, K.; Punitha, I.S. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006, 29, 1906–1910. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef]
- Yoon, M.A.; Jeong, T.S.; Park, D.S.; Xu, M.Z.; Oh, H.W.; Song, K.B.; Lee, W.S.; Park, H.Y. Antioxidant effects of quinoline alkaloids and 2,4-di-tert-butylphenol isolated from Scolopendra subspinipes. Biol. Pharm. Bull. 2006, 29, 735–739. [Google Scholar] [CrossRef]
- Zahari, A.; Ablat, A.; Omer, N.; Nafiah, M.A.; Sivasothy, Y.; Mohamad, J.; Khan, M.N.; Awang, K. Ultraviolet-visible study on acid-base equilibria of aporphine alkaloids with antiplasmodial and antioxidant activities from Alseodaphne corneri and Dehaasia longipedicellata. Sci. Rep. 2016, 6, 21517. [Google Scholar] [CrossRef]
- Nasrullah, A.A.; Zahari, A.; Mohamad, J.; Awang, K. Antiplasmodial alkaloids from the bark of Cryptocarya nigra (Lauraceae). Molecules 2013, 18, 8009–8017. [Google Scholar] [CrossRef]
- Gülçin, I.; Elias, R.; Gepdiremen, A.; Chea, A.; Topal, F. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: Cepharanthine and fangchinoline. J. Enzym. Inhib. Med. Chem. 2010, 25, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kim, H.Y.; Kang, K.S.; Yokozawa, T.; Park, J.H. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch. Pharm. Res. 2009, 32, 341–345. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Surh, Y.-J. Oxidative DNA damage and cytotoxicity induced by copper-stimulated redox cycling of salsolinol, a neurotoxic tetrahydroisoquinoline alkaloid. Free Radic. Biol. Med. 2001, 30, 1407–1417. [Google Scholar] [CrossRef]
- Zahari, A.; Cheah, F.K.; Mohamad, J.; Sulaiman, S.N.; Litaudon, M.; Leong, K.H.; Awang, K. Antiplasmodial and Antioxidant Isoquinoline Alkaloids from Dehaasia longipedicellata. Planta Med. 2014, 80, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Lee, G.Y.; Kim, J.; Lee, Y.M.; Kim, J.M.; Kim, Y.S.; Kim, J.S. A new pancreatic lipase inhibitor isolated from the roots of Actinidia arguta. Arch. Pharmacol. Res. 2008, 31, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Zahari, A.; Ablat, A.; Sivasothy, Y.; Mohamad, J.; Choudhary, M.I.; Awang, K. In vitro antiplasmodial and antioxidant activities of bisbenzylisoquinoline alkaloids from Alseodaphne corneri Kosterm. Asian Pac. J. Trop. Med. 2016, 9, 328–332. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Devel. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef]
- Mladenka, P.; Macakova, K.; Zatloukalova, L.; Rehakova, Z.; Singh, B.K.; Prasad, A.K.; Parmar, V.S.; Jahodar, L.; Hrdina, R.; Saso, L. In vitro interactions of coumarins with iron. Biochimie 2010, 92, 1108–1114. [Google Scholar] [CrossRef]
- Lomozová, Z.; Hrubša, M.; Conte, P.F.; Papastefanaki, E.; Moravcová, M.; Catapano, M.C.; Proietti Silvestri, I.; Karlíčková, J.; Kučera, R.; Macáková, K.; et al. The effect of flavonoids on the reduction of cupric ions, the copper-driven Fenton reaction and copper-triggered haemolysis. Food Chem. 2022, 394, 133461. [Google Scholar] [CrossRef]
- Cahlikova, L.; Hrabinova, M.; Kulhankova, A.; Benesova, N.; Chlebek, J.; Jun, D.; Novak, Z.; Macakova, K.; Kunes, J.; Kuca, K.; et al. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase activities. Nat. Prod. Commun. 2013, 8, 1541–1544. [Google Scholar] [CrossRef]
- Kulhánková, A.; Cahlíková, L.; Novák, Z.; Macáková, K.; Kuneš, J.; Opletal, L. Alkaloids from Zephyranthes robusta BAKER and their acetylcholinesterase- and butyrylcholinesterase-inhibitory activity. Chem. Biodivers. 2013, 10, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Chlebek, J.; Macakova, K.; Cahlikovi, L.; Kurfurst, M.; Kunes, J.; Opletal, L. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava (Fumariaceae). Nat. Prod. Commun. 2011, 6, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Cahlikova, L.; Macakova, K.; Kunes, J.; Kurfurst, M.; Opletal, L.; Cvacka, J.; Chlebek, J.; Blundene, G. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Eschscholzia californica (Papaveraceae). Nat. Prod. Commun. 2010, 5, 1035–1038. [Google Scholar] [CrossRef]
- Siatka, T.; Adamcova, M.; Opletal, L.; Cahlikova, L.; Jun, D.; Hrabinova, M.; Kunes, J.; Chlebek, J. Cholinesterase and Prolyl Oligopeptidase Inhibitory Activities of Alkaloids from Argemone platyceras (Papaveraceae). Molecules 2017, 22, 1181. [Google Scholar] [CrossRef]
- Chlebek, J.; Novak, Z.; Kassemova, D.; Safratova, M.; Kostelnik, J.; Maly, L.; Locarek, M.; Opletal, L.; Host’alkova, A.; Hrabinova, M.; et al. Isoquinoline Alkaloids from Fumaria officinalis L. and Their Biological Activities Related to Alzheimer’s Disease. Chem. Biodivers. 2016, 13, 91–99. [Google Scholar] [CrossRef]
- Říha, M.; Karlíčková, J.; Filipský, T.; Macáková, K.; Hrdina, R.; Mladěnka, P. Novel method for rapid copper chelation assessment confirmed low affinity of D-penicillamine for copper in comparison with trientine and 8-hydroxyquinolines. J. Inorg. Biochem. 2013, 123, 80–87. [Google Scholar] [CrossRef]
- Filipský, T.; Říha, M.; Hrdina, R.; Vávrová, K.; Mladěnka, P. Mathematical calculations of iron complex stoichiometry by direct UV–Vis spectrophotometry. Bioorg. Chem. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Job, P. Formation and stability of inorganic complexes in solution. Ann. Chim. 1928, 9, 113–203. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, M.S.; Chlebek, J.; Hošťálková, A.; Catapano, M.C.; Lomozová, Z.; Macáková, K.; Mladěnka, P. Interactions of Isoquinoline Alkaloids with Transition Metals Iron and Copper. Molecules 2022, 27, 6429. https://doi.org/10.3390/molecules27196429
Parvin MS, Chlebek J, Hošťálková A, Catapano MC, Lomozová Z, Macáková K, Mladěnka P. Interactions of Isoquinoline Alkaloids with Transition Metals Iron and Copper. Molecules. 2022; 27(19):6429. https://doi.org/10.3390/molecules27196429
Chicago/Turabian StyleParvin, Mst Shamima, Jakub Chlebek, Anna Hošťálková, Maria Carmen Catapano, Zuzana Lomozová, Kateřina Macáková, and Přemysl Mladěnka. 2022. "Interactions of Isoquinoline Alkaloids with Transition Metals Iron and Copper" Molecules 27, no. 19: 6429. https://doi.org/10.3390/molecules27196429
APA StyleParvin, M. S., Chlebek, J., Hošťálková, A., Catapano, M. C., Lomozová, Z., Macáková, K., & Mladěnka, P. (2022). Interactions of Isoquinoline Alkaloids with Transition Metals Iron and Copper. Molecules, 27(19), 6429. https://doi.org/10.3390/molecules27196429








