Geographic Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemicals
2.3. Extractions
2.4. Analysis
2.4.1. Determination of the Total Anthocyanin Content (TAC)
2.4.2. Determination of the Total Flavonoid Content (TFC)
2.4.3. Determination of the Total Polyphenol Content (TPC)
2.4.4. Determination of the Ferric-Reducing Antioxidant Power (FRAP)
2.4.5. Determination of the DPPH Free Radical Scavenging Assay (RSA)
2.4.6. Determination of Phenolic Compounds by HPLC-UV Assay
2.4.7. Determination of the Antimicrobial Activity against Pathogens
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neamtu, A.-A.; Szoke-Kovacs, R.; Mihok, E.; Georgescu, C.; Turcus, V.; Olah, N.K.; Frum, A.; Tita, O.; Neamtu, C.; Szoke-Kovacs, Z.; et al. Bilberry (Vaccinium myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a Drosophila melanogaster High-Sugar Diet Model. Antioxidants 2020, 9, 1067. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. IJMS 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, Anti-Inflammatory, and Postulated Cytotoxic Activity of Phenolic and Anthocyanin-Rich Fractions from Polana Raspberry (Rubus idaeus L.) Fruit and Juice—In Vitro Study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC–MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, I.; Ziebel, J.; Guignard, C.; Sorokin, A.; Tikhonova, O.; Dolganova, N.; Hoffmann, L.; Eyzaguirre, P.; Hausman, J.-F. Evaluation and comparison of nutritional quality and bioactive compounds of berry fruits from Lonicera caerulea, Ribes, L. species and Rubus idaeus grown in Russia. J. Berry Res. 2011, 1, 159–167. [Google Scholar] [CrossRef]
- Mattila, P.H.; Hellström, J.; Karhu, S.; Pihlava, J.-M.; Veteläinen, M. High variability in flavonoid contents and composition between different North-European currant (Ribes spp.) varieties. Food Chem. 2016, 204, 14–20. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. IJMS 2015, 16, 2352–2365. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C.H. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis. Food Control 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and In Vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Roidaki, A.; Kollia, E.; Panagopoulou, E.; Chiou, A.; Varzakas, T.; Markaki, P.; Proestos, C. Super foods and Super herbs: Antioxidant and Antifungal Activity. Curr. Res. Nutr. Food Sci. 2016, 4, 138–145. [Google Scholar] [CrossRef]
- Krisch, J.; Ördögh, L.; Galgóczy, L.; Papp, T.; Vágvölgyi, C. Anticandidal effect of berry juices and extracts from Ribes species. Open Life Sci. 2009, 4, 86–89. [Google Scholar] [CrossRef]
- Díaz-Gómez, R.; Toledo-Araya, H.; López-Solís, R.; Obreque-Slier, E. Combined effect of gallic acid and catechin against Escherichia coli. LWT—Food Sci. Technol. 2014, 59, 896–900. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.-L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.H. Berry Phenolics: Antimicrobial Properties and Mechanisms of Action Against Severe Human Pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, X.; Liu, N.; Gao, Y.; Wang, L.; Xu, G.; Li, X.; Yang, Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics 2020, 9, 533. [Google Scholar] [CrossRef]
- Šumić, Z.; Vakula, A.; Tepić, A.; Čakarević, J.; Vitas, J.; Pavlić, B. Modeling and optimization of red currants vacuum drying process by response surface methodology (RSM). Food Chem. 2016, 203, 465–475. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. CPD 2020, 26, 1917–1928. [Google Scholar] [CrossRef]
- Demir, H.; Bicim, G. Antioxidant, Phenolic Content and Antimicrobial Properties of Bilberies (Vaccinium myrtillus L.). In Agricultural and Natural Sciences, Theory, Current Researches and New Trends; Kunter, B., Keskin, N., Eds.; IPVE: Cetinje, Montenegro, 2002; pp. 57–72. [Google Scholar]
- Nedyalkov, P.; Bakardzhiyski, I.; Dinkova, R.; Shopska, V.; Kaneva, M. Influence of the time of bilberry (Vaccinium myrtillus L.) addition on the phenolic and protein profile of beer. Acta. Sci. Pol. Technol. Aliment. 2022, 21, 5–15. [Google Scholar] [CrossRef]
- Georgieva, R.; Nedyalkov, P.; Shopska, V.N.; Kaneva, M. Effect of blueberries addition during beer maturation on yeast metabolism. FSAB 2021, 4, 105–110. [Google Scholar] [CrossRef]
- Pluta, S. Gooseberry—Ribes Uva-Crispa, Sin. R. Grossularia L. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press An Imprint of Elsevier: London, UK; San Diego, CA, USA; Cambridge, UK; Oxford, UK, 2018; pp. 211–218. [Google Scholar] [CrossRef]
- Dogan, H.H.; Duman, R.; Dinç, M. Antiviral activity of Ribes uva-crispa L. extracts In Vitro. Pak. J. Pharm. Sci. 2020, 33, 1173–1178. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Ersus Bilek, S.; Uygun, Ö.; Bircan, C. Sour Cherry By-products: Compositions, Functional Properties and Recovery Potentials—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3549–3563. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of Sour Cherry (Prunus cerasus L.) Fruits for Their Polyphenol Content, Antioxidant Properties, and Nutritional Components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef]
- Krstić, T.; Suvajdžić, L.; Stojanović, S.; Crvenković-Lozanov, Z.; Dejanović, J.; Čabarkapa, I.; Velhner, M.; Stefanović, V. Antimicrobial Activity of Sour Cherry. Agro Food Ind. Hi Tech 2016, 27, 56–58. [Google Scholar]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.; Vilela, A.; Pinto, A.; Nunes, F.M.; Cosme, F.; Anjos, R. Influence of cultivar and of conventional and organic agricultural practices on phenolic and sensory profile of blackberries (Rubus fruticosus): Chemical and sensory evaluation of blackberries. J. Sci. Food Agric. 2018, 98, 4616–4624. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Ahmad, M.; Rahman, N. Antimicrobial screening of fruit, leaves, root and stem of Rubus fruticosus. J. Med. Plants Res. 2011, 5, 5920–5924. [Google Scholar]
- Piangka, N.; Ahmed, T.; Acharjee, M. Microbiological analysis for drug resistant pathogenic microorganisms with determination of the antibacterial properties found in Fragaria x ananassa (strawberry) samples. Stamford J. Microbiol. 2016, 6, 16–19. [Google Scholar] [CrossRef][Green Version]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Popescu, C.; Popescu, C.; Popescu, B.; Daas, D.; Morgovan, C.; Olah, N.K. Antimicrobial efficacy of the organic greasy oils combination—Sea buckthorn oil and maize germs oil. Farmacia 2014, 62, 743–752. [Google Scholar]
- Jaśniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea Buckthorn (Hippophae rhamnoides) for Disease Prevention and Food Production. Antioxidants 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Uddin, A.J. Functional Food—A New Notion in Modern Food Culture and A Hope During COVID-19. Int. J. Bus. Soc. Sci. Res. 2020, 8, 61–62. [Google Scholar]
- Messaoudi, O.; Gouzi, H.; El-Hoshoudy, A.N.; Benaceur, F.; Patel, C.; Goswami, D.; Boukerouis, D.; Bendahou, M. Berries anthocyanins as potential SARS-CoV–2 inhibitors targeting the viral attachment and replication; molecular docking simulation. Egypt. J. Pet. 2021, 30, 33–43. [Google Scholar] [CrossRef]
- Frum, A.; Georgescu, C.; Gligor, F.; Lengyel, E.; Stegarus, D.; Dobrea, C.; Tita, O. Identification and Quantification of Phenolic Compounds from Red Grape Pomace. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 45–52. [Google Scholar]
- Frum, A. Extraction of Anthocyanins from By-Products from the Winemaking Process. In Proceedings of the International Conference Agri-Food Sciences, Processes and Technologies, Sibiu, Romania, 25–26 May 2015. [Google Scholar]
- Cynare folium VIII. In Farmacopeea Română, Xth ed.; Editura Medicală: București, România, 1993; p. 335.
- Georgescu, C.; Bratu, I.; Tamas, M. The study of some polyphenols of Rhododendron kotschyi. Revista de Chimie 2005, 56, 779–780. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tylkowski, B.; Tsibranska, I.; Kochanov, R.; Peev, G.; Giamberini, M. Concentration of biologically active compounds extracted from Sideritis ssp. L. by nanofiltration. Food Bioprod. Process. 2011, 89, 307–314. [Google Scholar] [CrossRef]
- Popovici, C.; Saykova, I.; Tylkowski, B. Evaluation de l’activité antioxydant des composés phénoliques par laréactivité avec le radical libre DPPH. Revue de Génie Ind. 2009, 4, 25–39. [Google Scholar]
- Tița, O.; Constantinescu, M.A.; Tița, M.A.; Georgescu, C. Use of Yoghurt Enhanced with Volatile Plant Oils Encapsulated in Sodium Alginate to Increase the Human Body’s Immunity in the Present Fight Against Stress. Int. J. Environ. Res. Public Health 2020, 17, 7588. [Google Scholar] [CrossRef] [PubMed]
- Gligor, F.G.; Frum, A.; Vicaș, L.G.; Totan, M.; Roman-Filip, C.; Dobrea, C.M. Determination of a Mixture of Plantago lanceolata L. and Salvia officinalis L. by High-Performance Liquid Chromatography with Ultraviolet Detection (HPLC-UV). Anal. Lett. 2020, 53, 1391–1406. [Google Scholar] [CrossRef]
- Craciun, V.I.; Gligor, F.G.; Juncan, A.M.; Chis, A.A.; Rus, L.L. A New, Rapid and Efficient HPLC Method to Assay Resveratrol in Food Supplements. Rev. Chim. 2019, 70, 3202–3205. [Google Scholar] [CrossRef]
- Williams, D.L.; Smith, S.R.; Peterson, B.R.; Allyn, G.; Cadenas, L.; Epperson, R.T.; Looper, R.E. Growth substrate may influence biofilm susceptibility to antibiotics. PLoS ONE 2019, 14, e0206774. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Chee, H.Y. In Vitro Antifungal Activity of Equol against Candida Albicans. Mycobiology 2010, 38, 328–330. [Google Scholar] [CrossRef]
- Georgescu, C.; Mironescu, M. Obtaining, characterisation and screening of the antifungal activity of the volatile oil extracted from Thymus serpyllum. J. Environ. Prot. Ecol. 2011, 12, 2294–2302. [Google Scholar]
- BIEMER, J.J. Antimicrobial Susceptibility Testing by the Kirby-Bauer Disc Diffusion Method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar]
- Bujor, O.-C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- Ciulca, S.; Roma, G.; Alexa, E.; Radulov, I.; Cocan, I.; Madosa, E.; Ciulca, A. Variation of Polyphenol Content and Antioxidant Activity in Some Bilberry (Vaccinium myrtillus L.) Populations from Romania. Agronomy 2021, 11, 2557. [Google Scholar] [CrossRef]
- Åkerström, A.; Jaakola, L.; Bång, U.; Jäderlund, A. Effects of Latitude-Related Factors and Geographical Origin on Anthocyanidin Concentrations in Fruits of Vaccinium myrtillus L. (Bilberries). J. Agric. Food Chem. 2010, 58, 11939–11945. [Google Scholar] [CrossRef]
- Rieger, G.; Müller, M.; Guttenberger, H.; Bucar, F. Influence of Altitudinal Variation on the Content of Phenolic Compounds in Wild Populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Filipiak-Szok, A.; Kurzawa, M.; Szłyk, E. Determination of anti-oxidant capacity and content of phenols, phenolic acids, and flavonols in Indian and European gooseberry. Chem. Pap. 2012, 66, 259–268. [Google Scholar] [CrossRef]
- Pantelidis, G.; Vasilakakis, M.; Manganaris, G.; Diamantidis, G. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Liu, W.; Li, C. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hydamaka, A.; Lowry, L.; Beta, T. Comparison of antioxidant capacity and phenolic compounds of berries, chokecherry and seabuckthorn. Open Life Sci. 2009, 4, 499–506. [Google Scholar] [CrossRef]
- Hajazimi, E.; Landberg, R.; Zamaratskaia, G. Simultaneous determination of flavonols and phenolic acids by HPLC-CoulArray in berries common in the Nordic diet. LWT 2016, 74, 128–134. [Google Scholar] [CrossRef]
- Berk, S.; Gundogdu, M.; Tuna, S.; Tas, A. Role of Maturity Stages on Phenolic Compounds and Organic Acids Contents in Red Currant Fruits. Int. J. Fruit Sci. 2020, 20, S1054–S1071. [Google Scholar] [CrossRef]
- LaPierre, L.; Cornejo, J.; Asun, A.; Vergara, C.; Varela, D. M02–A12 Laboratory Guide: Methodologies for Antimicrobial Susceptibility Testing, APEC Sub-Committee on Standards and Conformance, May 2020. Available online: https://www.apec.org/docs/default-source/Publications/2020/5/Laboratory-Guide---Methodologies-for-Antimicrobial-Susceptibility-Testing/220_CTI_SCSC_Laboratory-Guide-Methodologies-for-Antimicrobial-Susceptibility-Testing.pdf (accessed on 5 May 2022).
- CLSI standard M02; Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Upadhyay, R.K. GC-MS Analysis and In Vitro Antimicrobial Susceptibility of Foeniculum Vulgare Seed Essential Oil. AJPS 2015, 6, 1058–1068. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Mosoni, P.; Leroy, S.; Kaewkod, T.; Desvaux, M.; Tragoolpua, Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants 2022, 11, 602. [Google Scholar] [CrossRef]
- Hisano, M.; Bruschini, H.; Nicodemo, A.C.; Srougi, M. Cranberries and lower urinary tract infection prevention. Clinics 2012, 67, 661–667. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products Assessment Report on Vaccinium myrtillus L., Fructus Recens and Vaccinium myrtillus L., Fructus Siccus, Report, 29 September 2015, EMA/HMPC/555161/2013. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-vaccinium-myrtillus-l-fructus-recens-vaccinium-myrtillus-l-fructus-siccus_en.pdf (accessed on 5 May 2022).
- Mosquito, S.; Pons, M.J.; Riveros, M.; Ruiz, J.; Ochoa, T.J. Diarrheagenic Escherichia coli Phylogroups Are Associated with Antibiotic Resistance and Duration of Diarrheal Episode. Sci. World J. 2015, 2015, 610403. [Google Scholar] [CrossRef] [PubMed]
- Vučić, D.M.; Petković, M.R.; Rodić-Grabovac, B.B.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. Antibacterial and antioxidant activities of bilberry (Vaccinium myrtillus L.) In Vitro. Afr. J. Microbiol. Res. 2013, 7, 5130–5136. [Google Scholar] [CrossRef]
- Burdulis, D.; Sarkinas, A.; Jasutiené, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar] [PubMed]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kahkonen, M.; Heinonen, M.; Maatta-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef]
- Paunović, S.M.; Mašković, P.; Nikolić, M.; Miletić, R. Bioactive compounds and antimicrobial activity of black currant (Ribes nigrum L.) berries and leaves extract obtained by different soil management system. Sci. Hortic. 2017, 222, 69–75. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Bendokas, V.; Šarkinas, A.; Jasinauskienë, D.; Anisimovienë, N.; Morkûnaitë-Haimi, Š.; Stanys, V.; Šikšnianas, T. Antimicrobial activity of berries extracts of four Ribes species, their phenolic content and anthocyanin composition. Folia Hortic. 2018, 30, 249–257. [Google Scholar] [CrossRef]
- Aly, A.A.; Ali, H.G.M.; Eliwa, N.E.R. Phytochemical screening, anthocyanins and antimicrobial activities in some berries fruits. Food Meas. 2019, 13, 911–920. [Google Scholar] [CrossRef]
- Sandulachi, E.; Macari, A.; Cojocari, D.; Balan, G.; Popa, S.; Turculet, N.; Ghendov-Moşanu, A.; Sturza, R. Antimicrobial properties of sea buckthorn grownin the Republic of Moldova. J. Eng. Sci. 2022, 29, 164–175. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- İduğ, T.; Hızlı, H.; Şen, A.; Koç, F. In Vitro antimicrobial and antioxidant activity of some berry species. ACTA Pharm. Sci. 2018, 56, 51. [Google Scholar] [CrossRef][Green Version]
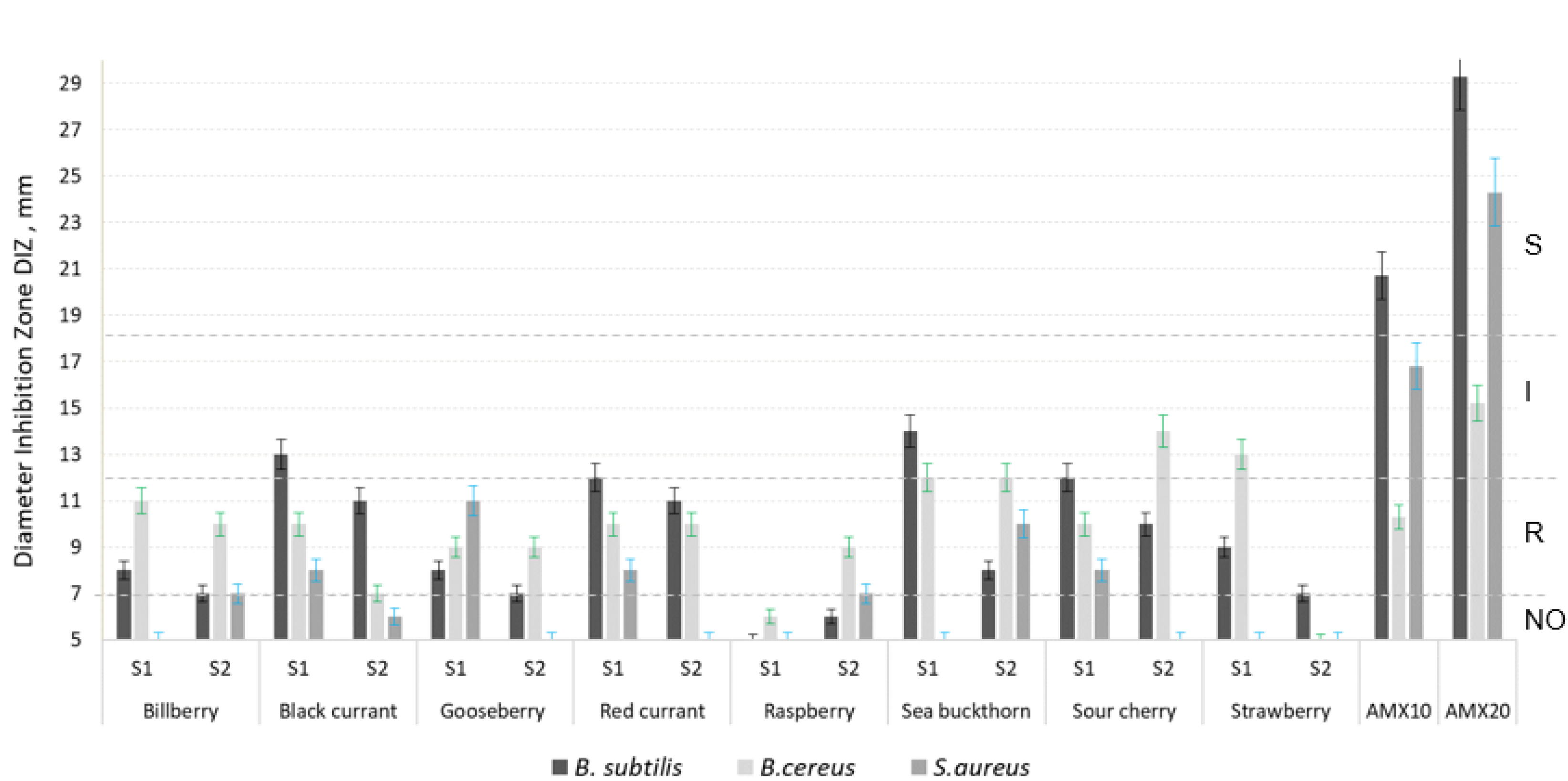

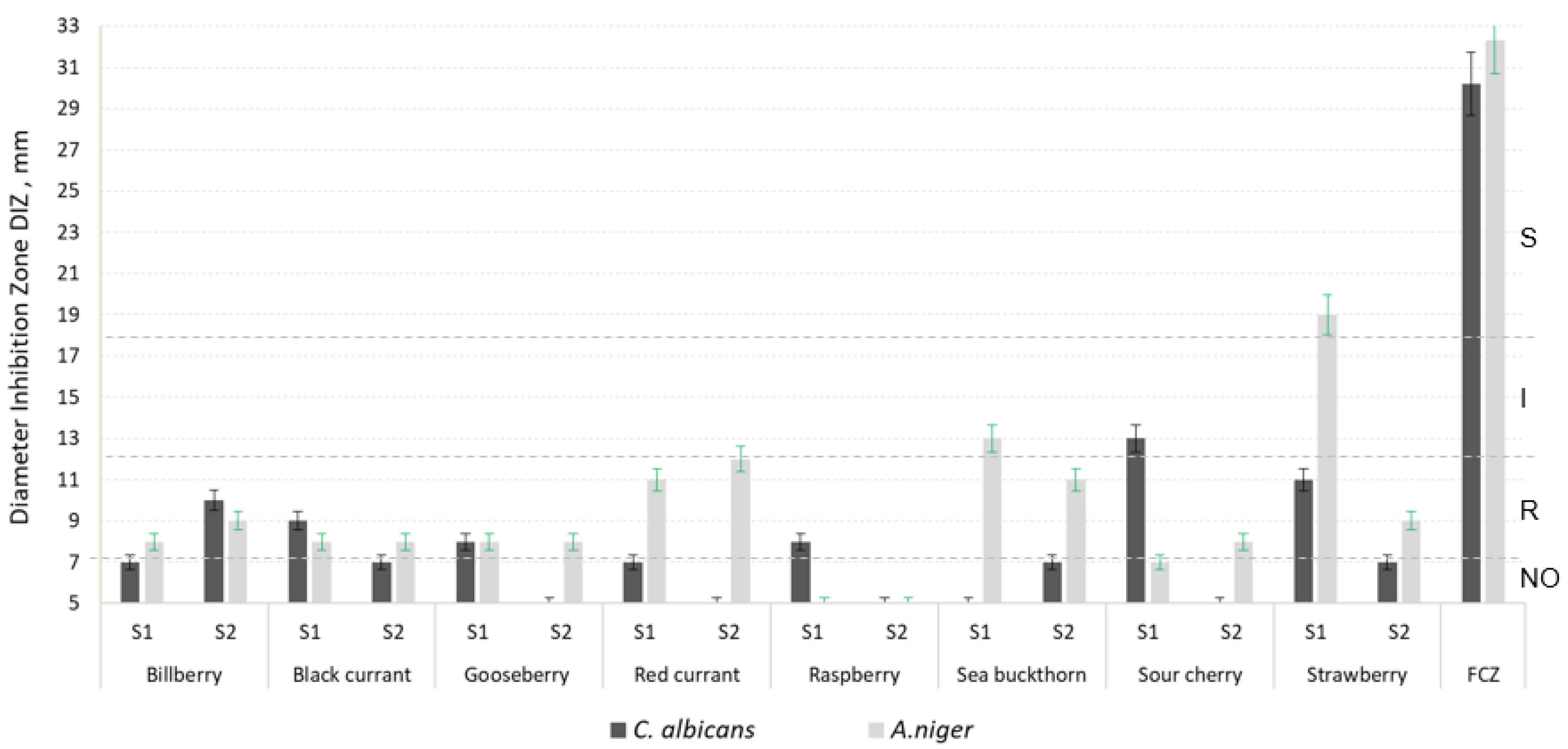
| Sample | TPC mg GAE/g d.w. | TFC mg QE/g d.w. | TAC mg/g d.w. | FRAP µmols TE/g d.w. | RSA % |
|---|---|---|---|---|---|
| S1 | |||||
| Bilberry | 22.20 ± 0.33 a | 8.87 ± 0.23 a | 3.58 ± 0.37 a,b | 57.23 ± 0.22 a | 83.81 ± 0.64 a |
| Black currant | 10.23 ± 0.72 c | 4.72 ± 0.35 d,f | 2.89 ± 0.63 b,c | 36.51 ± 0.69 d | 77.38 ± 0.48c |
| Gooseberry | 4.82 ± 0.75 g | 3.33 ± 0.20 h,i | 0.25 ± 0.16 d | 6.02 ± 0.42 j | 21.28 ± 0.70 j |
| Red currant | 4.39 ± 0.50 g | 3.55 ± 0.42 e,f,g,h,i | 0.42 ± 0.31 d | 6.39 ± 0.41 j | 16.25 ± 0.25 l |
| Raspberry | 12.72 ± 0.74 b | 4.61 ± 0.37 d,h | 0.88 ± 0.35 d | 40.15 ± 0.39 b | 70.65 ± 0.48 d |
| Sea buckthorn | 7.40 ± 0.16 e,f | 4.36 ± 0.45 e,f,g,h,i | 0.17 ± 0.07 d | 18.38 ± 0.33 g,h | 34.62 ± 0.38 h |
| Sour cherry | 8.92 ± 0.15 d | 7.40 ± 0.18b | 0.20 ± 0.10 d | 20.36 ± 0.18 f | 46.22 ± 0.38 f |
| Strawberry | 7.79 ± 0.22 d,e | 4.05 ± 0.51 e,f,g,h,i | 0.18 ± 0.10 d | 12.83 ± 0.78 i | 33.84 ± 0.62 h |
| S2 | |||||
| Bilberry | 21.85 ± 0.56 a | 6.86 ± 0.81 b,c | 3.94 ± 0.32 a | 56.31 ± 0.68 a | 79.40 ± 0.91 b |
| Black currant | 8.94 ± 0.12 c,d | 5.86 ± 0.65 c,d | 2.49 ± 0.24 c | 33.30 ± 0.25 e | 69.55 ± 0.73 d |
| Gooseberry | 4.13 ± 0.39 g | 3.63 ± 0.19 e,f,g,h,i | 0.17 ± 0.08 d | 6.14 ± 0.39 j | 20.91 ± 0.95 j |
| Red currant | 4.26 ± 0.15 g | 3.54 ± 0.19 e,f,g,h,i | 0.26 ± 0.12 d | 6.50 ± 0.19 j | 18.44 ± 0.75 k |
| Raspberry | 12.42 ± 0.43 b | 4.36 ± 0.46 e,f,g,h,i | 0.70 ± 0.14 d | 37.90 ± 0.68 c | 63.99 ± 0.39 e |
| Sea buckthorn | 7.16 ± 0.38 e,f | 4.73 ± 0.39 d,e | 0.28 ± 0.17 d | 17.59 ± 0.39 h | 32.60 ± 0.90 h |
| Sour cherry | 8.74 ± 0.11 d | 6.25 ± 0.57 b,c | 0.35 ± 0.08 d | 19.65 ± 0.12 f,g | 43.16 ± 0.31 g |
| Strawberry | 7.75 ± 0.12 d,f | 4.72 ± 0.25 d,g | 0.13 ± 0.03 d | 12.61 ± 0.28 i | 30.23 ± 0.81 i |
| Concentration of Phenolic Compound (µg/g d.w.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid | (+)-Catechin | Syringic Acid | Cinnamic Acid | Resveratrol | Caffeic Acid | Ferulic Acid | Rutin | Quercetin | |
| S1 | |||||||||
| Bilberry | 92.48 ± 0.97 d | 139.34 ± 1.17 e | 46.92 ± 1.48 e | 141.87 ± 1.74 b | 9.86 ± 0.76 d | 19.33 ± 0.71 f | 4.47 ± 0.48 g,i | 378.60 ± 0.93 a | 483.74 ± 1.55 c |
| Black currant | 139.47 ± 0.65 a | 275.31 ± 1.67 b | 41.56 ± 1.10 f | 50.97 ± 1.25 e | 4.85 ± 0.11 e,g | 33.50 ± 1.13 e | 13.43 ± 1.00 e | 64.88 ± 0.93 f | 1022.67 ± 1.27 b |
| Gooseberry | 41.63 ± 0.68 h | 201.64 ± 0.73 d | 6.71 ± 0.38 i,j | 16.84 ± 0.42 g | 18.34 ± 0.34 b | 10.69 ± 0.95 h | 14.63 ± 1.15 e | 15.87 ± 0.50 i | 59.32 ± 1.37 j |
| Red currant | 72.03 ± 0.47 f | 65.48 ± 1.30 i | 5.45 ± 0.57 j,l | 5.27 ± 0.25 i,k | 2.25 ± 0.30 h,i | 10.24 ± 1.02 h | 1.51 ± 0.53 k | 11.55 ± 0.85 j | 129.89 ± 1.70 g |
| Raspberry | 18.87 ± 1.27 k | 62.56 ± 0.84 i | 3.67 ± 0.38 k,l | 1.58 ± 0.43 l | 5.62 ± 0.27 e,f | 14.55 ± 0.60 g | 4.14 ± 0.41 g,j | 45.62 ± 1.25 g | 53.83 ± 1.54 k |
| Sea buckthorn | 79.36 ± 1.29 e | 230.67 ± 0.98 c | 24.67 ± 0.83 h | 20.37 ± 0.97 f | 17.76 ± 0.57 b | 33.17 ± 0.27 e | 18.20 ± 0.44 d | 45.26 ± 0.42 g | 86.53 ± 0.57 i |
| Sour cherry | 27.80 ± 1.16 j | n.d. | 27.37 ± 1.62 h | 7.71 ± 0.35 h,i | 13.41 ± 0.58 c | 62.29 ± 1.00 c | 44.74 ± 0.56 a | 43.65 ± 0.71 g | 266.22 ± 1.03 e |
| Strawberry | 27.20 ± 1.30 j | 36.45 ± 1.21 l | 135.85 ± 1.50 b | 52.50 ± 1.46 e | 18.89 ± 0.27 b | 170.51 ± 1.11 b | 4.52 ± 0.21 g,h | 119.44 ± 0.61 c | 6.87 ± 0.23 m |
| S2 | |||||||||
| Bilberry | 119.15 ± 1.58 b | 87.16 ± 1.76 g | 34.84 ± 0.67 g | 187.38 ± 1.04 a | 6.08 ± 0.34 e | 11.08 ± 0.16 h | 2.00 ± 0.17 k | 322.61 ± 1.72 b | 345.57 ± 0.84 d |
| Black currant | 93.43 ± 1.25 c,d | 203.52 ± 1.96 d | 63.30 ± 1.09 d | 67.33 ± 1.10 d | 3.43 ± 0.52 g,i | 64.62 ± 0.67 c | 14.57 ± 1.13 e | 45.25 ± 0.45 g | 1160.97 ± 1.03 a |
| Gooseberry | 31.59 ± 1.35 i | 115.60 ± 0.83 f | 6.02 ± 0.04 j,k | 15.68 ± 0.57 g | 14.17 ± 1.06 c | 14.01 ± 0.26 g | 10.44 ± 0.79 f | 9.74 ± 0.54 j | 46.28 ± 0.80 l |
| Red currant | 63.28 ± 1.17 g | 41.91 ± 0.69 k | 9.01 ± 0.26 i | 6.40 ± 0.37 i,j | 1.79 ± 0.55 i | 9.09 ± 0.54 h | 1.94 ± 0.65 k | 10.91 ± 0.28 j | 126.71 ± 1.12 g |
| Raspberry | 6.23 ± 0.36 l | 77.89 ± 0.67 h | 8.91 ± 0.27 i | 4.85 ± 0.25 j,k | 3.51 ± 0.43 g,h,i | 10.43 ± 0.99 h | 2.43 ± 0.23 h,i,j,k | 37.83 ± 0.32 h | 48.31 ± 1.15 l |
| Sea buckthorn | 96.72 ± 1.07 c | 309.62 ± 0.71 a | 36.94 ± 1.01 g | 15.50 ± 1.03 g | 21.90 ± 0.77 a | 46.55 ± 1.18 d | 20.97 ± 0.66 c | 66.73 ± 1.23 e,f | 122.39 ± 1.30 h |
| Sour cherry | 9.06 ± 0.50 l | n.d. | 71.64 ± 0.93 c | 9.91 ± 0.28 h | 4.09 ± 0.16 f,g,i | 21.24 ± 0.74 f | 24.34 ± 1.14 b | 68.93 ± 1.37 e | 142.51 ± 1.53 f |
| Strawberry | 17.73 ± 1.31 k | 49.02 ± 1.31 j | 209.55 ± 0.93 a | 87.62 ± 1.01 c | 14.69 ± 0.90 c | 269.31 ± 1.52 a | 4.78 ± 0.37 g | 88.59 ± 1.16 d | 8.65 ± 1.03 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgescu, C.; Frum, A.; Virchea, L.-I.; Sumacheva, A.; Shamtsyan, M.; Gligor, F.-G.; Olah, N.K.; Mathe, E.; Mironescu, M. Geographic Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties. Molecules 2022, 27, 4986. https://doi.org/10.3390/molecules27154986
Georgescu C, Frum A, Virchea L-I, Sumacheva A, Shamtsyan M, Gligor F-G, Olah NK, Mathe E, Mironescu M. Geographic Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties. Molecules. 2022; 27(15):4986. https://doi.org/10.3390/molecules27154986
Chicago/Turabian StyleGeorgescu, Cecilia, Adina Frum, Lidia-Ioana Virchea, Anastasiia Sumacheva, Mark Shamtsyan, Felicia-Gabriela Gligor, Neli Kinga Olah, Endre Mathe, and Monica Mironescu. 2022. "Geographic Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties" Molecules 27, no. 15: 4986. https://doi.org/10.3390/molecules27154986
APA StyleGeorgescu, C., Frum, A., Virchea, L.-I., Sumacheva, A., Shamtsyan, M., Gligor, F.-G., Olah, N. K., Mathe, E., & Mironescu, M. (2022). Geographic Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties. Molecules, 27(15), 4986. https://doi.org/10.3390/molecules27154986






