Recovery of Metals from Electronic Waste-Printed Circuit Boards by Ionic Liquids, DESs and Organophosphorous-Based Acid Extraction
Abstract
:1. Introduction
2. Results and Discussion
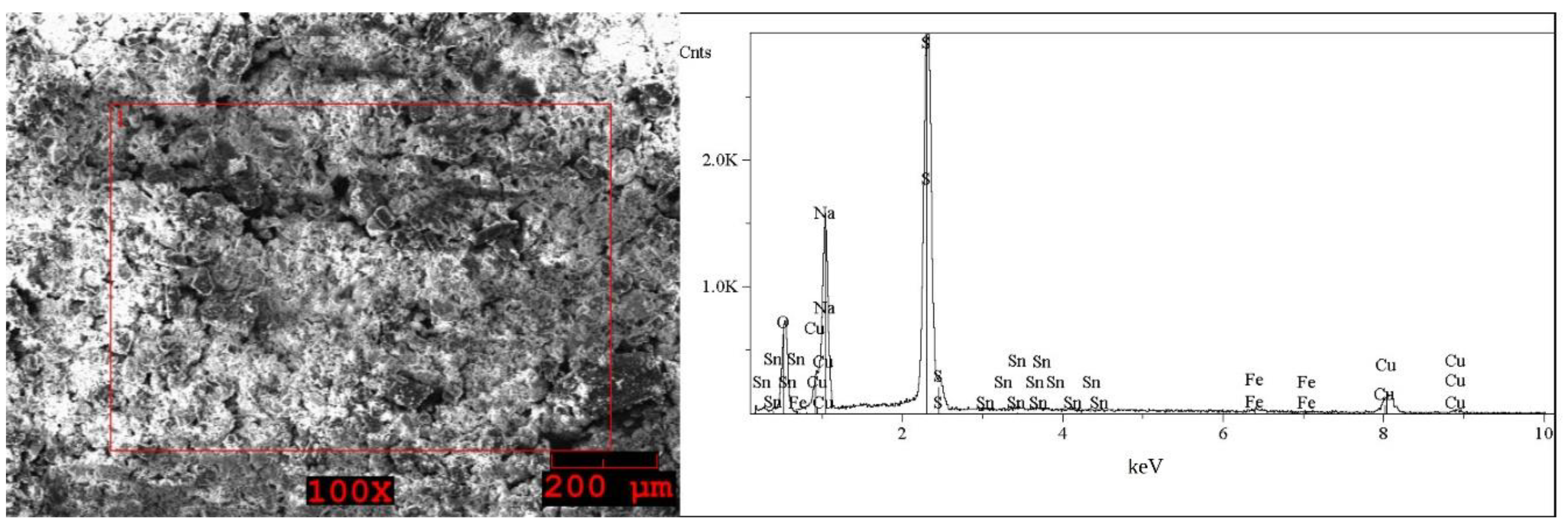
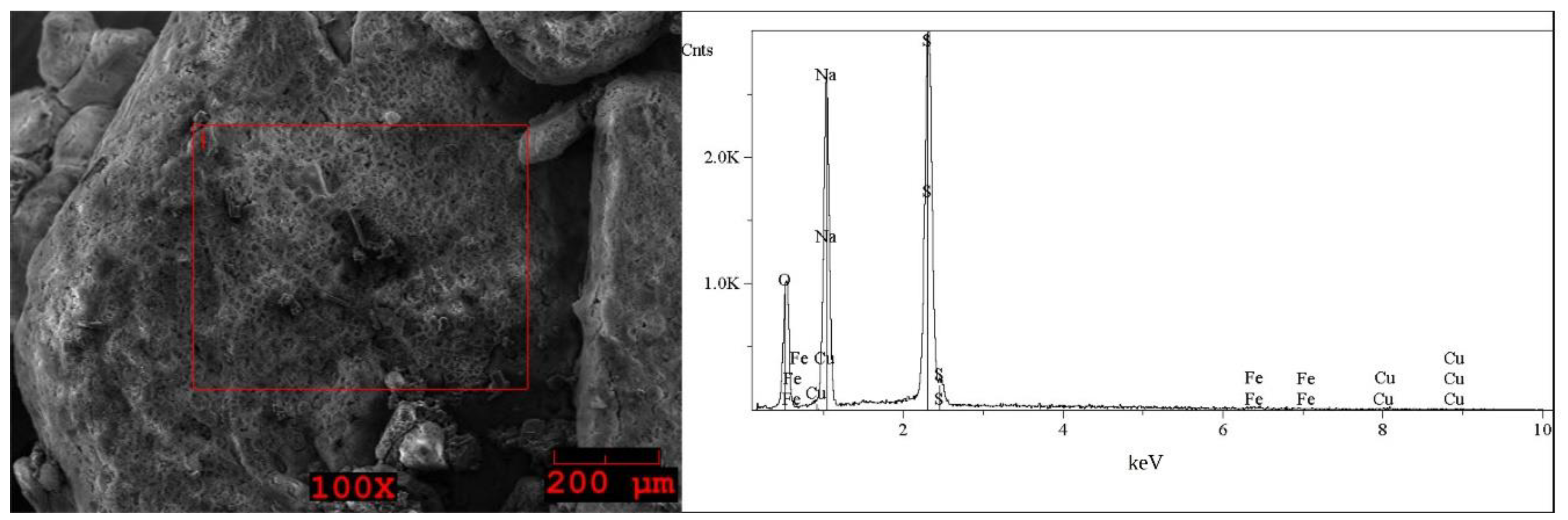
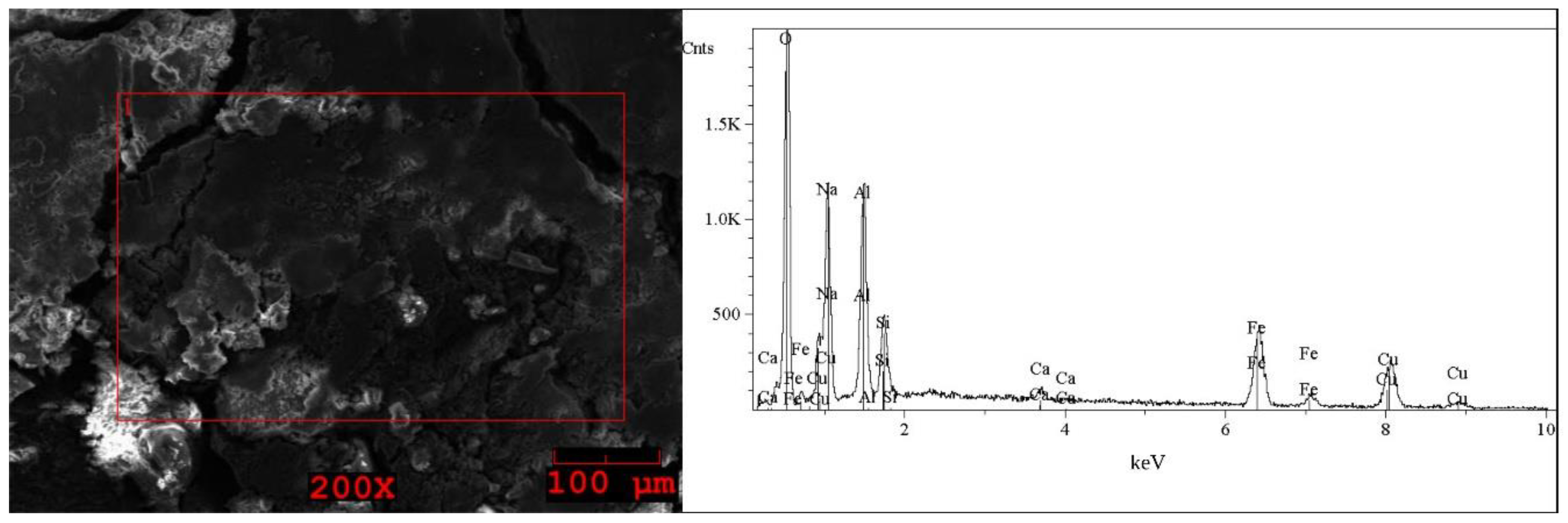
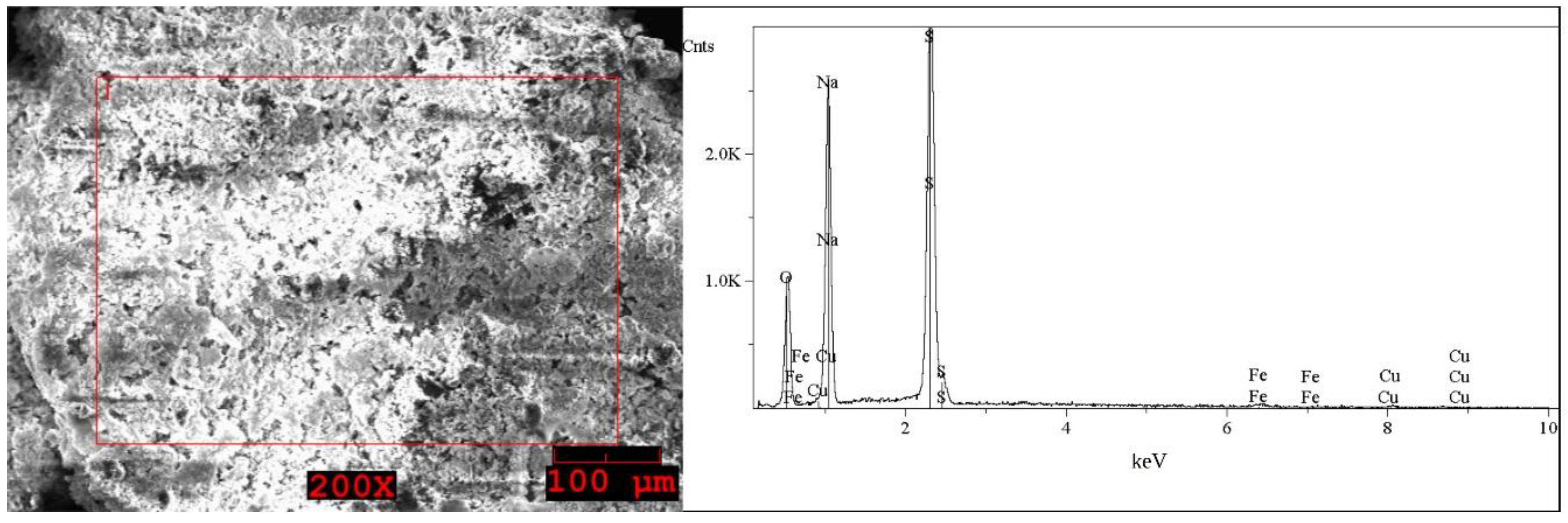
2.1. Analysis of the Solid WPCBs Samples and Post-Leaching Solutions
2.2. Extraction with Ionic Liquids and Organophosphorus Based Acid from the Post-Leaching Solutions
2.3. Extraction with ILs from the Post-Leaching Solutions Using ABS Method
2.4. Extraction with DESs and bi-Functional ILs from the Solid WPCB Samples
3. Materials and Methods
3.1. Materials
3.2. Reagents and Chemicals
3.3. Synthesis of DESs
3.4. Extraction Procedure
3.5. Extraction with ILs or Organophosphorous Based Acid from the Post-Leaching Solutions
3.6. ABS Method of Extraction from the Post-Leaching Solutions
3.7. Extraction with DESs from the Solid Material
3.8. Extraction with bi-Functional Ionic Liquids from the Solid Material
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamberovic, Z.; Korac, M.; Ranitovic, M. Hydrometallurgical process for extraction of metals from electronic waste-Part II: Development for the processes of the recovery for copper from Printed Circuit Boards (PCB). Metalurgija-MJoM 2011, 17, 139–149. [Google Scholar]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometalurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Rai, V.; Liu, D.; Xia, D.; Jayaraman, Y.; Gabriel, J.-C.P. Electrochemical approaches for the recovery of metals from electronic waste: A critical review. Recycling 2021, 6, 53. [Google Scholar] [CrossRef]
- Aliakbari, R.; Marfavi, Y.; Kowsari, E.; Ramakrishna, S. Recent studies on ionic liquids in metal recovery from e-waste and secondary sources by liquid liquid extraction and electrodeposition: A Review. Mater. Circ. Econ. 2020, 2, 10–18. [Google Scholar] [CrossRef]
- Tran, T.T.; Liu, Y.; Lee, M.S. Recovery of pure molybdenum and vanadium compounds from spent petroleum catalysts by treatment with ionic liquid solution in the presence of oxidizing agent. Sep. Purif. Technol. 2021, 255, 117734. [Google Scholar] [CrossRef]
- Atanossova, M. Solvent extraction of metallic species in ionic liquids: An overview of s-, p- and d-element. J. Chem. Technol. Metall. 2021, 56, 443–466. [Google Scholar]
- Schaeffer, N.; Passos, H.; Billard, I.; Papaiconomou, N.; Coutinho, J.A.P. Recovery of metals from waste electrical and electronic equipment (WEEE) using unconventional solvents based on ionic liquids. Environ. Sci. Technol. Taylor & Francis 2018, 48, 859–922. [Google Scholar]
- Hsu, E.; Barmak, K.; Westa, A.C.; Park, A.-H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Domańska, U. Separation of cobalt, lithium and nickel from the “black mass” of waste Li-ion batteries by ionic liquids, DESs and organophosphorous-based acids extraction. J. Mol. Liq. 2021, 343, 117694. [Google Scholar] [CrossRef]
- Wstawski, S.; Emmons-Burzyńska, M.; Rzelewska-Piekut, M.; Skrzypczak, A.; Regel-Rosocka, M. Studies on copper(II) leaching from e-waste with hydrogen sulfate ionic liquids: Effect of hydrogen peroxide. Hydrometallurgy 2021, 205, 105730. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Dong, L.; Li, Y.-T.; Wu, Y.; Ma, Y.-X.; Yang, B. Copper leaching from waste printed circuit boards using typical acidic ionic liquids recovery of e-wastes’ surplus value. Waste Menag. 2018, 78, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Masilela, M.; Ndlovu, S. Extraction of Ag and Au from chloride electronic easte leach solutions using ionic liquids. J. Environ. Chem. Eng. 2019, 7, 102810. [Google Scholar] [CrossRef]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 164, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Kilicarslan, A.; Nezihi Saridede, M.; Stopic, S.; Friedrich, B. Use of ionic liquid in leaching process of brass wastes for copper and zinc recovery. Int. J. Miner. Metal. Mater. 2014, 21, 138–143. [Google Scholar] [CrossRef]
- Whitehead, J.A.; Lawrance, G.A.; McCluskey, A. Green leaching: Recyclable and selective leaching of gold-bearing ore in an ionic liquid. Green Chem. 2004, 6, 313–315. [Google Scholar] [CrossRef]
- Whitehead, J.A.; Zhang, J.; Pereira, N.; McCluskey, A.; Lawrance, G.A. Application of 1-alkyl-3-methyl-imidazolium ionic liquids in the oxidative leaching of sulphidic copper, gold and silver ores. Hydrometallurgy 2007, 88, 109–120. [Google Scholar] [CrossRef]
- Wellens, S.; Vander Hoogerstraete, T.; Möller, C.; Thijs, B.; Luyten, J.; Binnemans, K. Dissolution of metal oxides in an acid-saturated ionic liquid solution and investigation of the back-extraction behavior to the aqueous phase. Hydrometallurgy 2014, 144–145, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Niknam, K.; Damya, M. 1-Butyl-3-methylimidazolium hydrogen sulfate [Bmim][HSO4]: An efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes. J. Chin. Chem. Soc. 2009, 56, 659–665. [Google Scholar] [CrossRef]
- Poulimenou, N.I.; Giannopoulou, I.; Panias, D. Use of ionic liquids as innovative solvents in primary aluminum production. Mater. Manuf. Processes 2015, 30, 1403–1407. [Google Scholar] [CrossRef]
- Hu, J.; Tian, G.; Zi, F.; Hu, X. Leaching of chalcopyrite with hydrogen peroxide in 1-hexyl-3-methyl-imidazolium hydrogen sulfate ionic liquid aqueous solution. Hydrometallurgy 2017, 169, 1–8. [Google Scholar] [CrossRef]
- Fang, X.; Tong, X.; Zhong, M.; Chen, W. Intensification of silver leaching process by [Bmim]HSO4] from silver powder and silver sulfide with thiourea. Chin. J. Rare Metals 2014, 38, 464–470. [Google Scholar]
- Davris, P.; Balomenos, E.; Panias, D. Leaching of rare earths from bauxite residues using imidazolium based ionic liquids. In Proceedings of the ERES2014 1st International Conference on European Rare Earth Resources, Milos Island, Greece, 1–3 September 2014; pp. 241–252. [Google Scholar] [CrossRef]
- Alguacil, F.J. Non-dispersive extraction of gold(III) with ionic liquid Cyphos IL101. Sep. Purif. Technol. 2017, 179, 72–76. [Google Scholar] [CrossRef]
- Shen, L.; Chen, J.; Chen, L.; Liu, C.; Zhang, D.; Zhang, Y.; Su, W.; Deng, Y. Extraction of mid-heavy rare earth metal ions from sulphuric acid media by ionic liquid [A336][P507]. Hydrometallurgy 2016, 161, 152–159. [Google Scholar] [CrossRef]
- Fischer, L.; Falta, T.; Koellensperger, G.; Stojanovic, A.; Kogelnig, D.; Galanski, M.; Krachler, R.; Keppler, B.K.; Hann, S. Ionic liquids for extraction of metals and metal containing compounds from communal and industrial waste water. Water Res. 2011, 45, 4601–4614. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, S.; Watanabe, Y.; Araki, Y.; Kudo, Y. Extraction of gold(III) from hydrochloric acid into various ionic liquids: Relationship between extraction efficiency and aqueous solubility of ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 564–571. [Google Scholar] [CrossRef]
- Navarro, R.; Saucedo, I.; Gonzalez, C.; Guibal, E. Amberlite XAD-7 impregnated with Cyphos IL-101 (tetraalkylphosphonium ionic liquid) for Pd(II) recovery from HCl solutions. Chem. Eng. J. 2012, 185–186, 226–235. [Google Scholar] [CrossRef]
- Campos, K.; Vincent, T.; Bunio, P.; Trochimczuk, A.; Guibal, E. Gold recovery from HCl solutions using Cyphos IL-101 (a quaternary phosphonium ionic liquid) immobilized in biopolymer capsules. Solvent Extr. Ion Exch. 2008, 26, 570–601. [Google Scholar] [CrossRef]
- Feng, F.; Sun, Y.; Rui, J.; Yu, L.; Liu, J.; Zhang, N.; Zhao, M.; Wei, L.; Lu, C.; Zhao, J.; et al. Study of the “Oxidation-Complexation” Coordination Composite Ionic Liquid System for Dissolving Precious Metals. Appl. Sci. 2020, 10, 3625. [Google Scholar] [CrossRef]
- Huang, J.; Chen, M.; Chen, H.; Chen, S.; Sun, Q. Leaching behavior of copper from waste printed circuit boards with Brønsted acidic ionic liquid. Waste Manag. 2014, 34, 483–488. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of precious metals from waste printed circuit boards using cyanide-free alkaline glycine solution in the presence of an oxidant. Miner. Eng. 2022, 181, 107501. [Google Scholar] [CrossRef]
- Han, Y.; Yi, X.; Wang, R.; Huang, J.; Chen, M.; Sun, Z.; Sun, S.; Shu, J. Copper extraction from waste printed circuit boards by glicyne. Sep. Purif. Technol. 2020, 253, 117463. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Dupont, L.; Martinez, A.; Dechamps, I. Use of dicyanamide ionic liquids for extraction of metal ions. RSC Adv. 2016, 6, 107894. [Google Scholar] [CrossRef]
- Wang, F.; He, F.; Zhao, J.; Siu, N.; Xu, L.; Liu, H. Extraction and separation of cobalt(II), copper(II) and manganese(II) by Cyanex272, PC-88A and their mixtures. Sep. Purif. Technol. 2012, 93, 8–14. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Domańska, U. Liquid-liquid extraction of cobalt(II) and zinc(II) from aqueous solutions using novel ionic liquids as extractants. J. Mol. Liq. 2020, 307, 112955. [Google Scholar] [CrossRef]
- Wellens, S.; Thijs, B.; Binnemans, K. An environmentally friendlier approach to hydrometallurgy: Highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids. Green Chem. 2012, 14, 1657–1665. [Google Scholar] [CrossRef] [Green Version]
- Vander Hoogerstraete, T.; Binnemans, K. Highly efficient separation of rare earths from nickel and cobalt by solvent extraction with the ionic liquid trihexyl(tetradecyl)phosphonium nitrate: A process relevant to the recycling of rare earths from permanent magnets and nickel metal hydride batteries. Green Chem. 2014, 16, 1594–1606. [Google Scholar]
- Dong, T.; Hua, Y.; Zhang, Q.; Zhou, D. Leaching of chalcopyrite with Brønsted acidic ionic liquid. Hydrometallurgy 2009, 99, 33–38. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Chen, M.-N.; Hao, Y.; Jiang, X.; Zhou, X.-L.; Zhang, Z.-H. Choline chloride and lactic acid: A natural deep eutectic solvent for one-pot rapid construction of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines]. J. Mol. Liq. 2019, 278, 124–129. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Lach, J.; Wróbel, K.; Domańska, U. Recovery of zinc and manganese from “black mass” of waste Zn-MnO2 alkaline batteries by solvent extraction technique with ionic liquids, DES and organophosphorous-based acids. J. Mol. Liq. 2021, 338, 116590. [Google Scholar] [CrossRef]




| Metal Content | Mass of Sample | ||||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Ni | Cu | Zn | Ag | Pb | kg |
| g/kg | mg/kg | g/kg | |||||
| Starting material | |||||||
| 81.9 | 79.4 | 1.47 | 224 | 18.1 | 483 | 7.8 | 1.000 |
| Solid material after thermal pre-treatment | |||||||
| 122 | 119 | 2.20 | 335 | 27.0 | 721 | 11.6 | 0.670 |
| Solid material after the leaching I | |||||||
| 130 | 106 | 1.69 | 235 | 20.6 | 766 | 12.7 | 0.575 |
| Solid material after the leaching II (second step) | |||||||
| 96.9 | 11.5 | < * | < | < | < | < | 0.328 |
| Metal Content | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| mg/kg | |||||||||
| Al | Fe | Ni | Cu | Zn | Pd | Ag | Sn | Au | Pb |
| After the I leaching | |||||||||
| 864 | 5059 | 59.2 | 10,505 | 733 | 0.57 | 5.0 | 533 | 3.3 | 53.0 |
| After the II leaching (first step) | |||||||||
| 6110 | 6985 | 213 | 29,544 | 2205 | <0.5 | 0.5 | 59.9 | <1 | 1194 |
| After the II leaching (second step) | |||||||||
| 254 | 7008 | 51.4 | 904 | 257 | 1.25 | 100 | 834 | 2.7 | 14.8 |
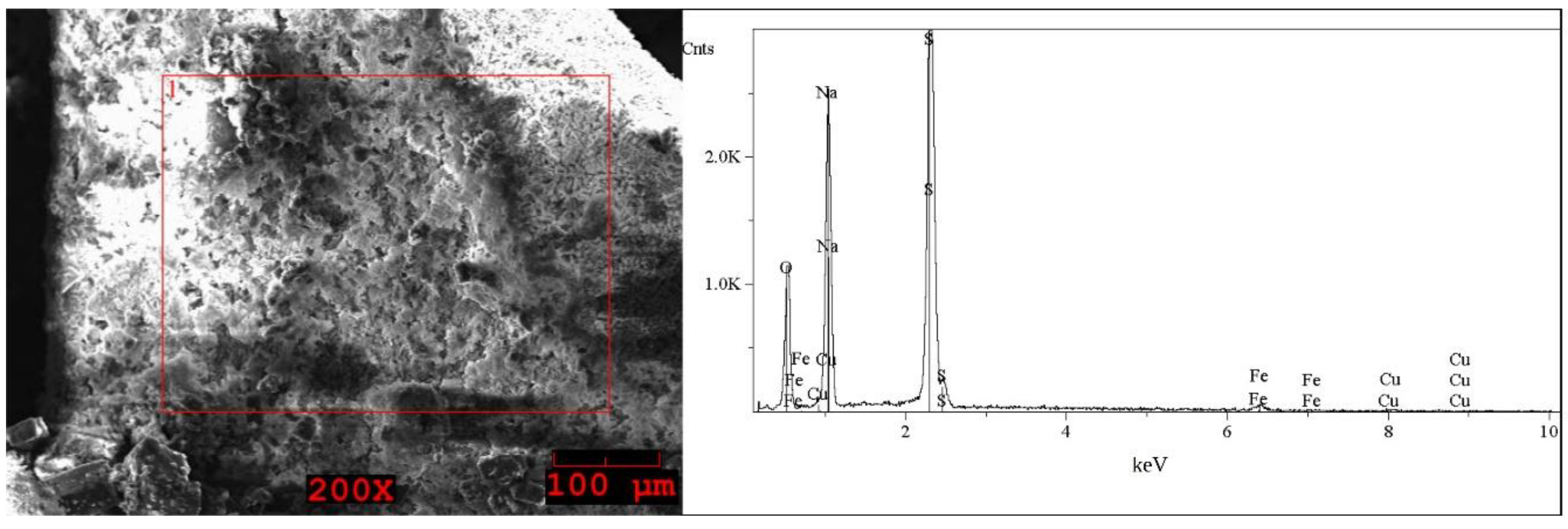
| Element | I (Grey) wt% | Element | I (White) wt% | Element | II First Step (Brown) wt% | Element | II Second Step (Grey) wt% | Element | II Second Step (White) wt% |
|---|---|---|---|---|---|---|---|---|---|
| O | 33–34 | O | 39–41 | O | 48–49 | O | 40 | O | 38–42 |
| Na | 22 | Na | 29–30 | Na | 13–17 | Na | 28–29 | Na | 23–30 |
| S | 35–36 | S | 29–31 | Al | 11–14 | S | 30–31 | S | 30–37 |
| Fe | 0.5 | Fe | 0.2–0.4 | Si | 3–8 | Fe | 0.5–1 | Fe | 0.5–2 |
| Cu | 7–8 | Cu | 0.3–0.4 | Ca | < * | Cu | 0.5–1 | Cu | 0.5 |
| Sn | 0.8–1 | Fe | 9–11 | ||||||
| Cu | 7–11 |
| Metal content in the liquid leachate phase I | ||||||
| g/kg | g/kg | g/kg | g/kg | g/kg | mg/kg | g/kg |
| Al | Fe | Ni | Cu | Zn | Ag | Pb |
| 0.808 | 5.556 | 0.0764 | 0.776 | 0.8055 | 0.7069 | 0.0402 |
| Metal content in the liquid leachate phase II (first step) | ||||||
| g/kg | g/kg | g/kg | g/kg | g/kg | mg/kg | g/kg |
| Al | Fe | Ni | Cu | Zn | Ag | Pb |
| 3.206 | 3.596 | - | 21.146 | 1.806 | 0.1383 | - |
| Metal content in the liquid leachate phase II (second step) | ||||||
| g/kg | g/kg | g/kg | g/kg | g/kg | mg/kg | g/kg |
| Al | Fe | Ni | Cu | Zn | Ag | Pb |
| 0.0965 | 9.7897 | - | 1.2833 | 0.4305 | 146.804 | - |
| Chemical Structure | Name, Abbreviation, Supplier, CAS Number | M/(g mol−1) | Purity in Mass Percent (%) |
|---|---|---|---|
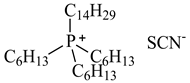 | Trihexyltetradecylphosphonium thiocyanate, [P6,6,6,14][SCN], synthesized [9] | 542.06 | >95 |
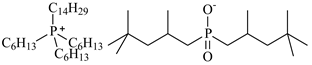 | Trihexyltetradecylphosphonium bis(2,4,4-trimethylpentyl)phosphinate, [P6,6,6,14][Cyanex272], ([P6,6,6,14][BTMPP]), IoLiTec, CAS: 465527-59-7 | 773.27 | >90 |
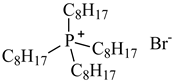 | Tetraoctylphosphonium bromide, [P8,8,8,8][Br], IoLiTec, CAS: 23906-97-0 | 563.76 | >95 |
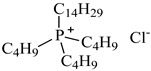 | Tributyltetradecylphosphonium chloride, [P4,4,4,14][Cl], IoLiTec, CAS: 81741-28-8 | 435.24 | >95 |
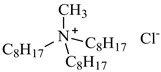 | Methyltrioctylammonium chloride, Aliquat 336, [N1,8,8,8][Cl], Alfa Aesar, CAS: 63393-96-4 | 404.17 | >97 |
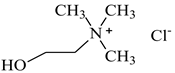 | Choline chloride, [N2OH,1,1,1][Cl], Sigma-Aldrich, CAS: 67-48-1 | 139.62 | >98 |
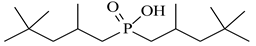 | Bis(2,4,4-trimethylpentyl)phosphinic acid, Cyanex 272, Chem Scene LLC, CAS: 83411-71-6 | 290.42 | 90 |
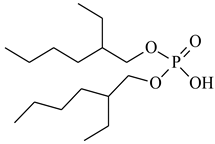 | Bis(2-ethylhexyl) phosphate, D2EHPA, Heavy Water, CAS: 298-07-7 | 322.40 | >95 |
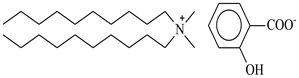 | Didecyldimethylammonium salicylate, [N10,10,1,1][Sal], C29H53NO3, synthesized | 463.83 | >95 |
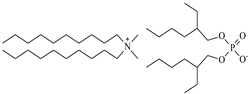 | Didecyldimethylammonium bis(2-ethylhexyl) phosphate, [N10,10,1,1][DEHPA], C38H82NO4P, synthesized | 648.13 | >95 |
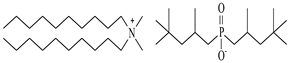 | Didecyldimethylammonium bis(2,4,4-trimethylpentyl)phosphinate, [N10,10,1,1][Cyanex272], synthesized | 616.12 | >95 |
| Name, Supplier, CAS Number | M/(g mol−1) | Purity in Mass Percent (%) |
|---|---|---|
| Lactic acid, C2H4OHCOOH, Sigma-Aldrich, CAS 50-21-5 | 90.08 | purified, 333 K, 13 hPa, 85 wt% aq. solution |
| Malonic acid, C3H4O4, Reachim, CAS 141-82-2 | 104.06 | 99.0 |
| Diethyl phosphite, C4H11PO3 Sigma-Aldrich, CAS: 762-04-9 | 138.10 | 98.0 |
| Didecyldimethylammonium chloride, [N10,10,1,1][Cl], DDACl, Alpinus Sp. z o.o., CAS 7173-51-5 | 362.16 | 50 wt% aq. solution |
| Hydrogen peroxide, H2O2, Chempur, CAS 7722-84-1 | 34.01 | 30 wt% aq. solution |
| Sulfuric acid, H2SO4, Riedel-de Haën, CAS 7664-93-9 | 98.08 | 96.0 |
| Sodium sulfate, Na2SO4, Chempur, CAS 7757-82-6 | 142.04 | 99.0 |
| Sodium hydroxide, NaOH, POCh, CAS 1310-73-2 | 40.0 | 98.8 |
| Toluene, C6H5CH3, Chempur, CAS 108-88-3 | 92.14 | 98.8 |
| IL | Ion | m0/mg | mE,O/mg | E/wt% | D | pH |
|---|---|---|---|---|---|---|
| [P6,6,6,14][Cyanex272]/toluene | Cu(II) | 3.78 | 2.24 | 59.3 | 0.59 | 7 |
| Ag(I) | 0.003 | 0.002 | 66.7 | 0.67 | ||
| Al(III) | 5.93 | 5.37 | 90.5 | 0.91 | ||
| Fe(II) | 27.04 | 4.67 | 17.3 | 0.17 | ||
| Zn(II) | 4.92 | 4.72 | 95.9 | 0.96 | ||
| Cyanex 272 + ester | Cu(II) | 5.66 | 0.55 | 9.7 | 0.01 | 3 |
| Ag(I) | 0.005 | 0.005 | 100.0 | 1.00 | ||
| Al(III) | 5.90 | 0.72 | 12.2 | 0.12 | ||
| Fe(II) | 40.56 | 4.79 | 11.8 | 0.12 | ||
| Zn(II) | 5.88 | 3.04 | 51.7 | 0.52 |
| Mixture | Ion | m0/mg | mE,A/mg | mE,O/mg | E/wt% | pH |
|---|---|---|---|---|---|---|
| Metal content in the liquid leachate phase I | ||||||
| {[P8,8,8,8][Br] + NaCl + H2O2 + leachate I} | Cu(II) | 9.44 | 0.08 | 9.36 | 99.1 | 3 |
| Ag(I) | 0.009 | <0.0004 | >0.008 | 88.9 | ||
| Al(III) | 14.00 | 13.86 | 0 | 99.0 * | ||
| Fe(II) | 67.60 | 4.58 | 63.02 | 93.2 | ||
| Zn(II) | 9.80 | 0.011 | 9.79 | 99.9 | ||
| {[P4,4,4,14][Cl] + NaCl + H2O2 + leachate I} | Cu(II) | 9.44 | 0.003 | 9.44 | 100.0 | 3 |
| Ag(I) | 0.009 | <0.0004 | >0.008 | 93.3 | ||
| Al(III) | 13.02 | 12.98 | 0 | 99.7 * | ||
| Fe(II) | 67.60 | 3.96 | 63.64 | 94.1 | ||
| Zn(II) | 9.80 | 0.011 | 9.79 | 99.9 | ||
| Metal content in the liquid leachate phase II (first step) | ||||||
| {[P8,8,8,8][Br] + NaCl + H2O2 + leachate II (first step)} | Cu(II) | 259.90 | 1.00 | 258.90 ** | 99.6 | 3 |
| Ag(I) | 0.0017 | 0.0001 | 0.0016 ** | 94.1 | ||
| Al(III) | 39.40 | 5.17 | 34.23 ** | 86.9 | ||
| Fe(II) | 44.20 | 2.85 | 41.35 ** | 93.5 | ||
| Zn(II) | 22.20 | 0.0115 | 22.19 ** | 99.9 | ||
| {[P8,8,8,8][Br] + NaCl + leachate II (first step)} | Cu(II) | 259.90 | 77.20 | 182.7 ** | 70.3 | 3 |
| Ag(I) | 0.0017 | 0.001 | 0.0007 ** | 41.2 | ||
| Al(III) | 39.40 | 18.25 | 21.15 ** | 39.4 | ||
| Fe(II) | 44.20 | 17.38 | 26.82 ** | 60.7 | ||
| Zn(II) | 22.20 | 12.30 | 9.90 ** | 44.6 | ||
| {[P4,4,4,14][Cl] + NaCl + H2O2 + leachate II (first step)} | Cu(II) | 259.90 | 9.24 | 250.66 | 96.4 | 3 |
| Ag(I) | 0.002 | 0 | 0.002 | 100.0 | ||
| Al(III) | 39.40 | 30.80 | 8.60 | 78.2 * | ||
| Fe(II) | 44.20 | 24.09 | 20.11 | 45.5 | ||
| Zn(II) | 22.20 | 0.10 | 22.10 | 99.5 | ||
| Metal content in liquid leachate phase II (second step) | ||||||
| {[P4,4,4,14][Cl] + NaCl + H2O2 + leachate II (second step)} | Cu(II) | 15.56 | 0.30 | 15.26 | 98.1 | 3 |
| Ag(I) | 1.78 | 0.0004 | 1.78 | 100.0 | ||
| Al(III) | 1.17 | 1.04 | 0.132 | 88.9 * | ||
| Fe(II) | 118.7 | 8.02 | 110.7 | 93.3 | ||
| Zn(II) | 5.22 | 0.003 | 5.22 | 100.0 | ||
| {[P8,8,8,8][Br] + NaCl + H2O2 + leachate II (second step)} | Cu(II) | 15.56 | 0.46 | 15.10 | 97.0 | 3 |
| Ag(I) | 1.78 | 0.0025 | 1.78 | 100.0 | ||
| Al(III) | 1.85 | 1.80 | 0 | 97.3 * | ||
| Fe(II) | 118.7 | 20.70 | 98.00 | 82.6 | ||
| Zn(II) | 5.22 | 0.004 | 5.22 | 100.0 | ||
| Mixture | Ion | m0/mg | mE,O/mg | E/wt% | D | pH |
|---|---|---|---|---|---|---|
| DES 1 | Cu(II) | 352.5 | 15.07 | 4.3 | 0.04 | 2.5 |
| Ag(I) | 1.149 | 0.06 | 5.2 | 0.05 | ||
| Al(III) | 195.0 | 99.94 | 51.2 | 0.51 | ||
| Fe(II) | 159.0 | 44.01 | 27.7 | 0.28 | ||
| Zn(II) | 30.90 | 1.092 | 3.5 | 0.03 | ||
| DES 2 | Cu(II) | 352.5 | 31.08 | 8.8 | 0.09 | 2.5 |
| Ag(I) | 1.149 | 0.303 | 26.4 | 0.26 | ||
| Al(III) | 195.0 | 178.4 | 91.5 | 0.91 | ||
| Fe(II) | 159.0 | 31.29 | 19.7 | 0.20 | ||
| Zn(II) | 30.90 | 0.72 | 2.3 | 0.02 | ||
| DES 1 | Cu(II) | 352.5 | 12.31 | 3.5 | 0.03 | 5 |
| Ag(I) | 1.149 | 0.15 | 13.0 | 0.13 | ||
| Al(III) | 195.0 | 102.8 | 52.7 | 0.53 | ||
| Fe(II) | 159.0 | 38.19 | 24.0 | 0.24 | ||
| Zn(II) | 30.90 | 1.05 | 3.40 | 0.03 | ||
| DES 2 | Cu(II) | 352.5 | 55.66 | 15.8 | 0.16 | 5 |
| Ag(I) | 1.149 | 0.231 | 20.1 | 0.20 | ||
| Al(III) | 195.0 | 95.32 | 48.9 | 0.49 | ||
| Fe(II) | 159.0 | 39.36 | 24.7 | 0.25 | ||
| Zn(II) | 30.90 | 0.623 | 2.0 | 0.02 | ||
| [N10,10,1,1][Sal] | Cu(II) | 352.5 | 32.37 | 9.2 | 0.09 | 6 |
| Ag(I) | 1.149 | 0.0022 | 0.2 | 0 | ||
| Al(III) | 195.0 | 49.68 | 25.5 | 0.25 | ||
| Fe(II) | 159.0 | 12.43 | 7.8 | 0.08 | ||
| Zn(II) | 30.90 | 0.50 | 1.6 | 0.02 | ||
| [N10,10,1,1][D2EHPA] | Cu(II) | 352.5 | 8.96 | 2.5 | 0.02 | 6 |
| Ag(I) | 1.149 | 0.00056 | 0.05 | 0 | ||
| Al(III) | 195.0 | 7.78 | 4.0 | 0.04 | ||
| Fe(II) | 159.0 | 1.64 | 1.0 | 0.01 | ||
| Zn(II) | 30.90 | 0.133 | 0.4 | 0 | ||
| [N10,10,1,1][Cyanex272] | Cu(II) | 352.5 | 13.33 | 3.8 | 0.04 | 6 |
| Ag(I) | 1.149 | 0 | 0 | 0 | ||
| Al(III) | 195.0 | 16.77 | 8.6 | 0.09 | ||
| Fe(II) | 159.0 | 2.34 | 1.5 | 0.01 | ||
| Zn(II) | 30.90 | 0.19 | 0.6 | 0 |
| Mixture | Ion | m0/mg | mE,O/mg | E/wt% | D | pH |
|---|---|---|---|---|---|---|
| DES 2 | Cu(II) | 502.50 | 48.45 | 9.6 | 0.09 | 5 |
| Ag(I) | 1.082 | 0.154 | 14.2 | 0.14 | ||
| Al(III) | 183.00 | 123.13 | 67.3 | 0.67 | ||
| Fe(II) | 178.50 | 18.06 | 10.1 | 0.10 | ||
| Zn(II) | 40.50 | 2.63 | 6.5 | 0.06 | ||
| DES 2 + Na2SO4 | Cu(II) | 502.50 | 27.35 | 5.4 | 0.05 | 5 |
| Ag(I) | 1.082 | 0.115 | 10.6 | 0.11 | ||
| Al(III) | 183.00 | 86.33 | 47.2 | 0.47 | ||
| Fe(II) | 178.50 | 12.81 | 7.2 | 0.07 | ||
| Zn(II) | 40.50 | 2.15 | 5.3 | 0.05 | ||
| [N10,10,1,1][Sal] | Cu(II) | 502.50 | 30.16 | 6.0 | 0.06 | 6 |
| Ag(I) | 1.082 | 0.0044 | 0.4 | 0 | ||
| Al(III) | 183.00 | 62.30 | 34.0 | 0.34 | ||
| Fe(II) | 178.50 | 6.65 | 3.7 | 0.03 | ||
| Zn(II) | 40.50 | 2.61 | 6.4 | 0.06 | ||
| [N10,10,1,1][D2EHPA] | Cu(II) | 502.50 | 5.77 | 1.1 | 0.01 | 6 |
| Ag(I) | 1.082 | 0.0007 | 0.06 | 0 | ||
| Al(III) | 183.00 | 18.48 | 10.1 | 0.10 | ||
| Fe(II) | 178.50 | 1.37 | 0.8 | 0.01 | ||
| Zn(II) | 40.50 | 0.97 | 2.4 | 0.02 | ||
| [N10,10,1,1][Cyanex272] | Cu(II) | 502.50 | 35.30 | 7.0 | 0.07 | 6 |
| Ag(I) | 1.082 | 0.0015 | 0.1 | 0 | ||
| Al(III) | 183.00 | 33.86 | 18.5 | 0.18 | ||
| Fe(II) | 178.50 | 1.40 | 0.8 | 0.01 | ||
| Zn(II) | 40.50 | 0.79 | 1.9 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Lach, J.; Wróbel, K.; Kolasa, D.; Domańska, U. Recovery of Metals from Electronic Waste-Printed Circuit Boards by Ionic Liquids, DESs and Organophosphorous-Based Acid Extraction. Molecules 2022, 27, 4984. https://doi.org/10.3390/molecules27154984
Łukomska A, Wiśniewska A, Dąbrowski Z, Lach J, Wróbel K, Kolasa D, Domańska U. Recovery of Metals from Electronic Waste-Printed Circuit Boards by Ionic Liquids, DESs and Organophosphorous-Based Acid Extraction. Molecules. 2022; 27(15):4984. https://doi.org/10.3390/molecules27154984
Chicago/Turabian StyleŁukomska, Aneta, Anna Wiśniewska, Zbigniew Dąbrowski, Jakub Lach, Kamil Wróbel, Dorota Kolasa, and Urszula Domańska. 2022. "Recovery of Metals from Electronic Waste-Printed Circuit Boards by Ionic Liquids, DESs and Organophosphorous-Based Acid Extraction" Molecules 27, no. 15: 4984. https://doi.org/10.3390/molecules27154984
APA StyleŁukomska, A., Wiśniewska, A., Dąbrowski, Z., Lach, J., Wróbel, K., Kolasa, D., & Domańska, U. (2022). Recovery of Metals from Electronic Waste-Printed Circuit Boards by Ionic Liquids, DESs and Organophosphorous-Based Acid Extraction. Molecules, 27(15), 4984. https://doi.org/10.3390/molecules27154984







