Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation
Abstract
1. Introduction
2. Results and Discussion
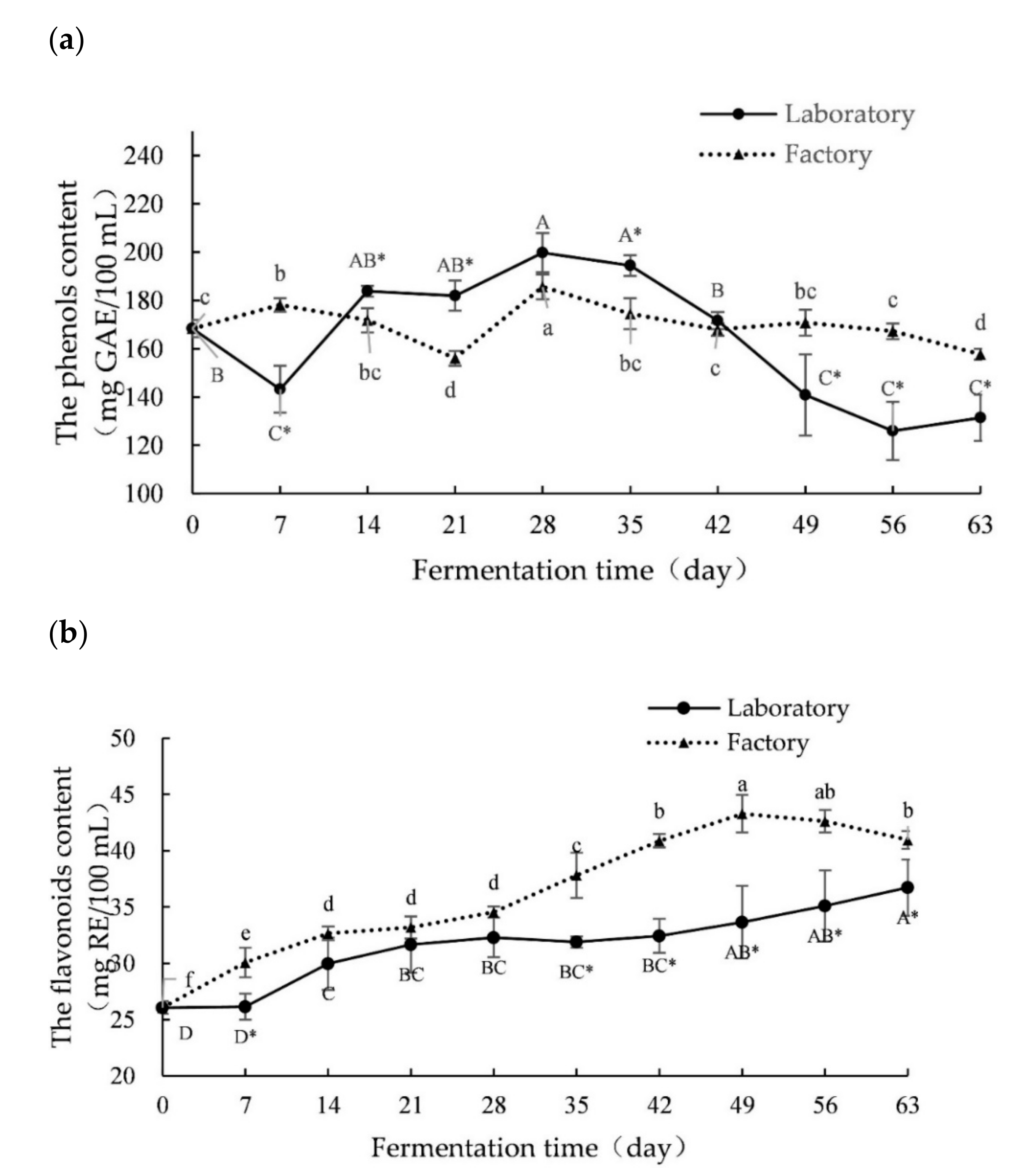
2.1. Changes in Polyphenols, Flavonoids and Polysaccharides during Fermentation
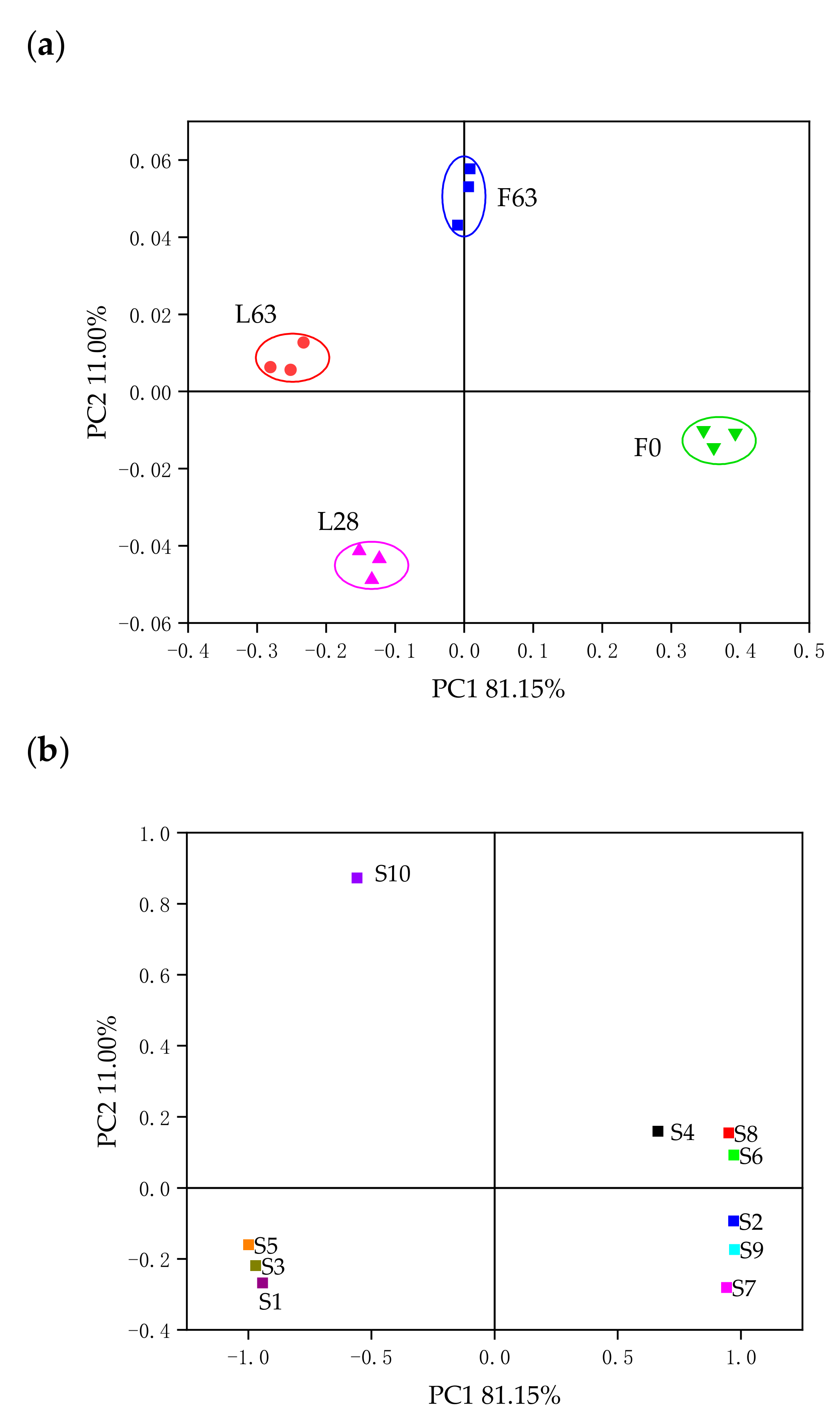
2.2. Characterization of Volatile Compounds
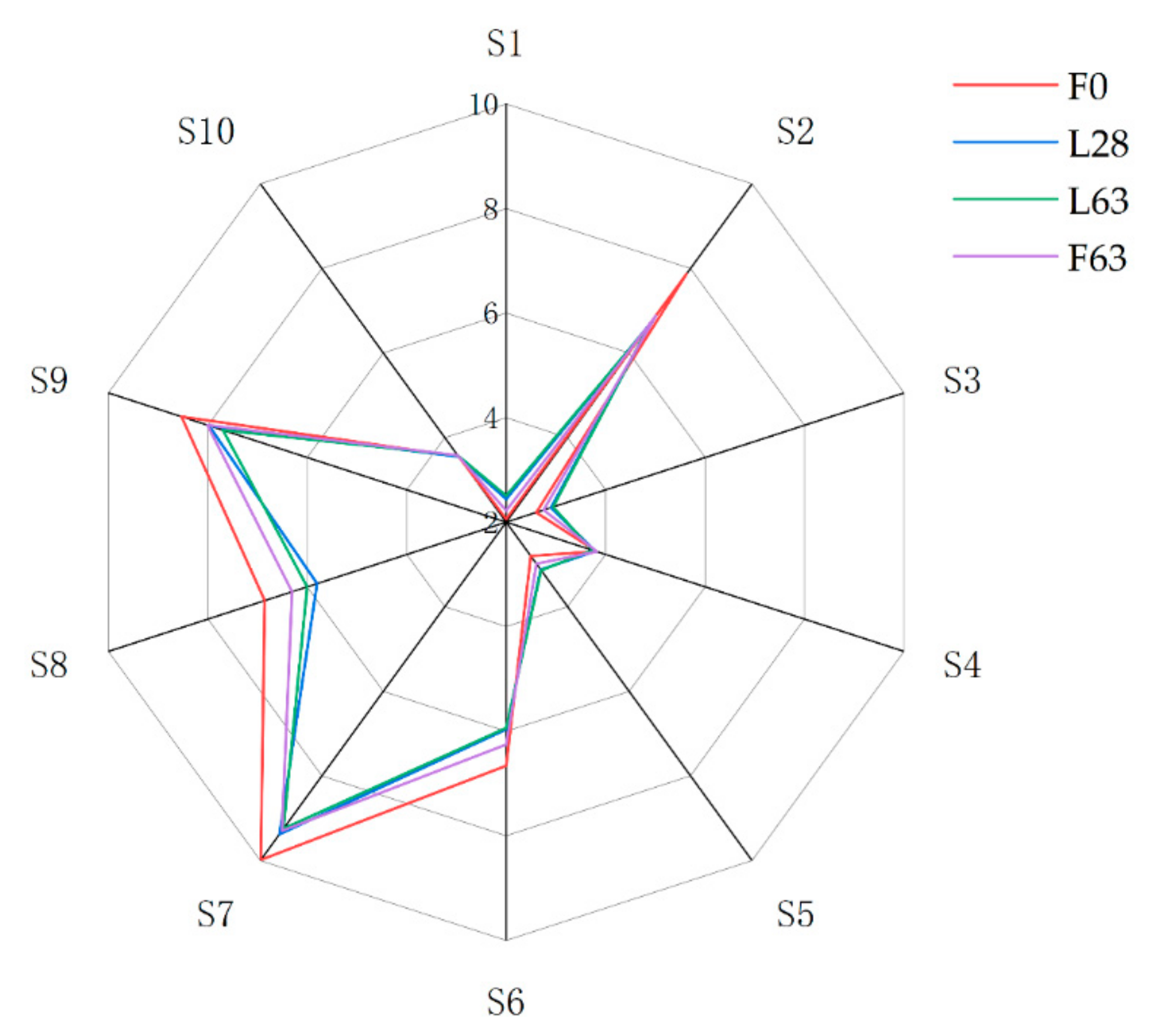
2.3. Electronic Nose Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Noni Fruit Fermentation
3.2.1. Factory-Scale Fermentation
3.2.2. Laboratory-Scale Fermentation
3.3. Determination of Polyphenols and Flavonoids
3.4. Determination of Polysaccharides
3.5. GC-MS Analysis
3.6. Electronic Nose Analysis
3.7. Statistical Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Almeida, E.S.; de Oliveira, D.; Hotza, D. Properties and applications of Morinda citrifolia (noni): A review. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef]
- Chan, B.Y.; Vaillant, F.; Mercedes Perez, A.; Reynes, M.; Brillouet, J.M.; Brat, P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J. Agric. Food Chem. 2006, 19, 645–654. [Google Scholar]
- Ali, M.; Kenganora, M.; Manjula, S.N. Health benefits of Morinda citrifolia (Noni): A review. Phcog. J. 2016, 8, 321–334. [Google Scholar] [CrossRef]
- Dixon, A.R.; Mcmillen, H.; Etkin, N.L. Ferment this: The transformation of noni, a traditional polynesian medicine (Morinda Citrifolia, Rubiaceae). Econ. Bot. 1999, 53, 51–68. [Google Scholar] [CrossRef]
- Jin, M.; Wang, Y.; Yang, X.; Yin, H.; Nie, S.; Wu, X. Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice. Food Hydrocoll. 2019, 90, 189–197. [Google Scholar] [CrossRef]
- Bui, A.K.T.; Bacic, A.; Pettolino, F. Polysaccharide composition of the fruit juice of Morinda citrifolia (noni). Phytochemistry 2006, 67, 1271–1275. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef]
- Potterat, O.; Hamburger, M. Morinda citrifolia (noni) fruit--phytochemistry, pharmacology, safety. Planta Med. 2007, 73, 191–199. [Google Scholar] [CrossRef]
- Dussossoy, E.; Bichon, F.; Bony, E.; Portet, K.; Brat, P.; Vaillant, F.; Michel, A.; Poucheret, P. Pulmonary anti-inflammatory effects and spasmolytic properties of Costa Rican noni juice (Morinda citrifolia L.). J. Ethnopharmacol. 2016, 192, 264–272. [Google Scholar] [CrossRef]
- Gilani, A.H.; Mandukhail, S.U.R.; Iqbal, J.; Yasinzai, M.; Aziz, N.; Khan, A.; Najeeb ur, R. Antispasmodic and vasodilator activities of Morinda citrifolia root extract are mediated through blockade of voltage dependent calcium channels. BMC Complement. Altern. Med. 2010, 10, 2. [Google Scholar] [CrossRef]
- Nayak, B.S.; Marshall, J.R.; Isitor, G.; Adogwa, A. Hypoglycemic and hepatoprotective activity of fermented fruit juice of Morinda citrifolia (Noni) in diabetic rats. Evid. Based Complementary Altern. Med. 2011, 2011, 1–5. [Google Scholar]
- Yang, S.C.; Chen, T.I.; Li, K.Y.; Tsai, T.C. Change in phenolic compound content, reductive capacity and ACE inhibitory activity in noni juice during traditional fermentation. J. Food. Drug Anal. 2007, 15, 290–298. [Google Scholar]
- Guo, M.; Mao, B.; Ahmed Sadiq, F.; Hao, Y.; Cui, S.; Yi, M.; Hong, Q.e.; Lee, Y.K.; Zhao, J. Effects of noni fruit and fermented noni juice against acute alcohol induced liver injury in mice. J. Funct. Food. 2020, 70, 103995. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chang, Y.Y.; Yang, D.J.; Tzang, B.S.; Chen, Y.C. Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chem. 2013, 140, 31–38. [Google Scholar] [CrossRef]
- Sousa, A.; Neto, M.A.S.; Garruti, D.D.; Sousa, J.; de Brito, E.S. Evaluation of noni (Morinda citrifolia) volatile profile by dynamic headspace and gas chromatography-mass spectrometry. Ciencia. Tecnol. Alime. 2010, 30, 641–644. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Chen, X.; Zhang, J.; Kilmartin, P.A.; Quek, S.Y. Exploring the effects of microencapsulation on odour retention of fermented noni juice. J. Food Eng. 2019, 273, 109892. [Google Scholar] [CrossRef]
- Pino, J.A.; Marquez, E.; Quijano, C.E.; Castro, D. Volatile compounds in noni (Morinda citrifolia L.) at two ripening stages. Ciencia. Tecnol. Alime. 2010, 30, 183–187. [Google Scholar] [CrossRef]
- Nelson, S.C.; Elevitch, C.R. Noni: The Complete Guide for Consumers and Growers, 1st ed.; Permanent Agriculture Resources: Holualoa, HI, USA, 2006; pp. 67–80. [Google Scholar]
- Bergh, A.J.; Muller, M.; van der Rijst, M.; Joubert, E. Optimisation and validation of high-temperature oxidation of Cyclopia intermedia (honeybush)—From laboratory to factory. S. Afr. J. Bot. 2017, 110, 152–160. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of lactic acid fermentation on color, phenolic compounds and antioxidant activity in African nightshade. Microorganisms 2020, 8, 1324. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of foods and beverages as a tool for increasing availability of bioactive compounds. Focus on short-chain fatty acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Kelanne, N.; Yang, B.R.; Liljenback, L.; Laaksonen, O. Phenolic compound profiles in alcoholic black currant beverages produced by fermentation with Saccharomyces and non-Saccharomyces yeasts. J. Agric. Food Chem. 2020, 68, 10128–10141. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.N.; Zeng, L.P.; Xiao, N.; Wang, C.; Liang, Z.H.; Wu, Q.J.; Zhang, Y.J.; Du, B.; Li, P. Quality enhancement of dendrobium officinale and banana juice through probiotic fermentation using beneficial lactic acid-producing bacteria. Int. J. Food Eng. 2020, 16, 20190370. [Google Scholar] [CrossRef]
- Landete, J.M.; Curiel, J.A.; Rodriguez, H.; de las Rivas, B.; Munoz, R. Aryl glycosidases from Lactobacillus plantarum increase antioxidant activity of phenolic compounds. J. Funct. Food. 2014, 7, 322–329. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.K.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.L.; Tahir, H.E. Effect of Lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Septembre Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, X.; Zhuang, X.; Han, W.; Guo, W.; Xiong, J.; Zhang, X. Rice bran polysaccharides and oligosaccharides modified by grifola frondosa fermentation: Antioxidant activities and effects on the production of NO. Food Chem. 2017, 223, 49–53. [Google Scholar] [CrossRef]
- Xu, Y.; Hlaing, M.M.; Glagovskaia, O.; Augustin, M.A.; Terefe, N.S. Fermentation by probiotic Lactobacillus gasseri strains en hances the carotenoid and fibre contents of carrot juice. Foods 2020, 9, 1803. [Google Scholar] [CrossRef]
- Zeng, H.W.; Shuai, Y.Y.; Zeng, X.; Xin, B.Y.; Huang, M.Q.; Li, B.; Wang, C.G. Evaluation of health-related composition and bioactivity of five fruit juices following Lactobacillus plantarum fermentation and simulated digestion. Int. J. Food. Sci. Tech. 2020, 56, 648–660. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Cell wall polysaccharides released during the alcoholic fermentation by schizosac charomyces pombe and s. japonicus: Quantification and characterization. Food Microbiol. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.R.; Ong, Y.L.; Yu, B.; Curran, P.; Liu, S.Q. Evolution of volatile compounds in papaya wine fermented with three Williopsis saturnus yeasts. Int. J. Food Sci. Tech. 2010, 45, 2032–2041. [Google Scholar] [CrossRef]
- Djojoputro, H.; Ismadji, S. Density and viscosity correlation for several common fragrance and flavor esters. J. Chem. Eng. Data. 2005, 50, 727–731. [Google Scholar] [CrossRef]
- Campillo, N.; Penalver, R.; Lopez-Garcia, I.; Hernandez, C.M. Headspace solid-phase microextraction for the determination of volatile organic sulphur and selenium compounds in beers, wines and spirits using gas chromatography and atomic emission detection. J. Chromatogr. A. 2009, 1216, 6735–6740. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma characterization of Chinese rice wine by gas chromatography-Olfactometry, chemical quantitative analysis, and aroma reconstitution. J. Agric. Food Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, W.; Qian, M.C. Characterization of aroma compounds in apple cider using solvent-assisted flavor evaporation and headspace solid-phase microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar] [CrossRef]
- Fan, W.; Qian, M.C. Headspace solid phase microextraction and gas chromatography-olfactometry dilution analysis of young and aged Chinese “Yanghe Daqu” liquors. J. Agric. Food Chem. 2005, 53, 7931–7938. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gambacorta, G.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Volatilome and bioaccessible phenolics profiles in lab-scale fermented bee pollen. Foods 2021, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in Oolong tea infusions using GC-olfactometry, GC-FPD, and GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L-plantarum, L-rhamnosus and L-casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef]
- Nogueira, J.P.; Pires de Siqueira, A.C.; Dutra Sandes, R.D.; de Sousa Galvão, M.; Santos Leite Neta, M.T.; Narain, N. An insight into key volatile compounds in acerola (Malpighia emarginata DC.) pulp based on their odour activity values and chemometric evaluation. Anal. Methods 2018, 10, 5851–5866. [Google Scholar]
- Zhang, L.; Liu, S.X.; Chen, Y.; Guo, J.B.; Li, C.F.; Zhang, J.C. Metatranscriptomic approach reveals the functional and enzyme dynamics of core microbes during noni fruit fermentation. Food Res. Int. 2020, 141, 109999. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, M.; Chen, M.; Li, M.; Gao, X. Influence of Eurotium cristatum and Aspergillus niger individual and collaborative inoculation on volatile profile in liquid-state fermentation of instant dark teas. Food Chem. 2021, 350, 129234. [Google Scholar] [CrossRef] [PubMed]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biot. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, H.; Ding, S.; Zhou, H.; Wang, R. Changes in volatile compounds of fermented minced pepper during natural and inoculated fermentation process based on headspace–gas chromatography–ion mobility spectrometry. Food Sci. Nutr. 2020, 8, 3362–3379. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Sang, M.L.; Yong, H.C.; Hurh, B.S.; Kim, Y.S. Comparative volatile profiles in soy sauce according to inoculated microorganisms. Biosci. Biotech. Bioch. 2013, 77, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Ding, W.; Ma, L.J.; Jia, R. Discrimination and characterization of different intensities of goaty flavor in goat milk by means of an electronic nose. J. Dairy Sci. 2015, 98, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Ruengdech, A.; Siripatrawan, U. Visualization of mulberry tea quality using an electronic sensor array, SPME-GC/MS, and sen sory evaluation. Food Biosci. 2020, 36, 100593. [Google Scholar]
- Cui, S.; Wang, J.; Yang, L.; Wu, J.; Wang, X. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using e-nose and gc–ms combined with chemometrics. J. Pharmaceut. Biomed. 2015, 102, 64–77. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Dubois, M.K.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.A.J.N. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar]

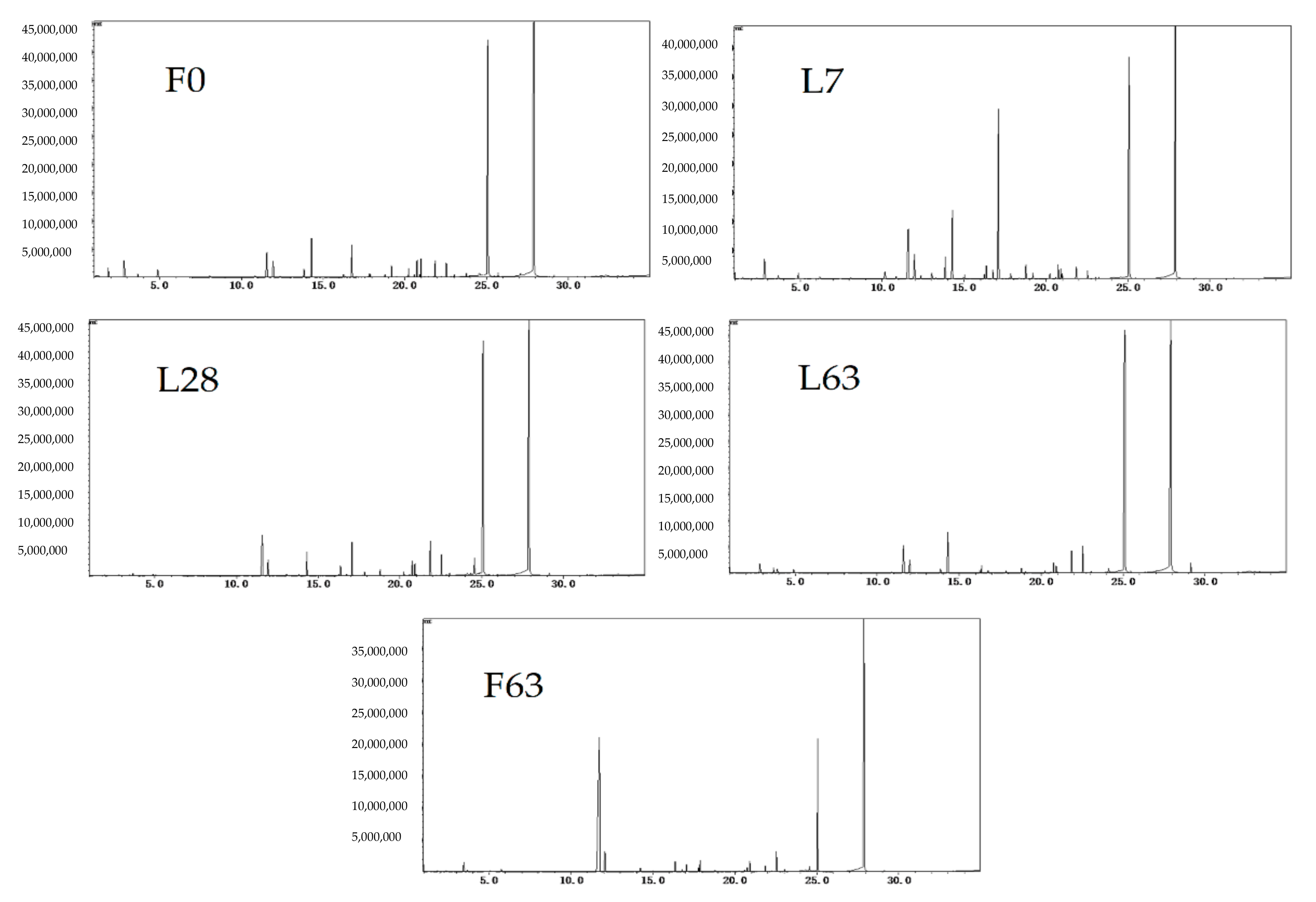
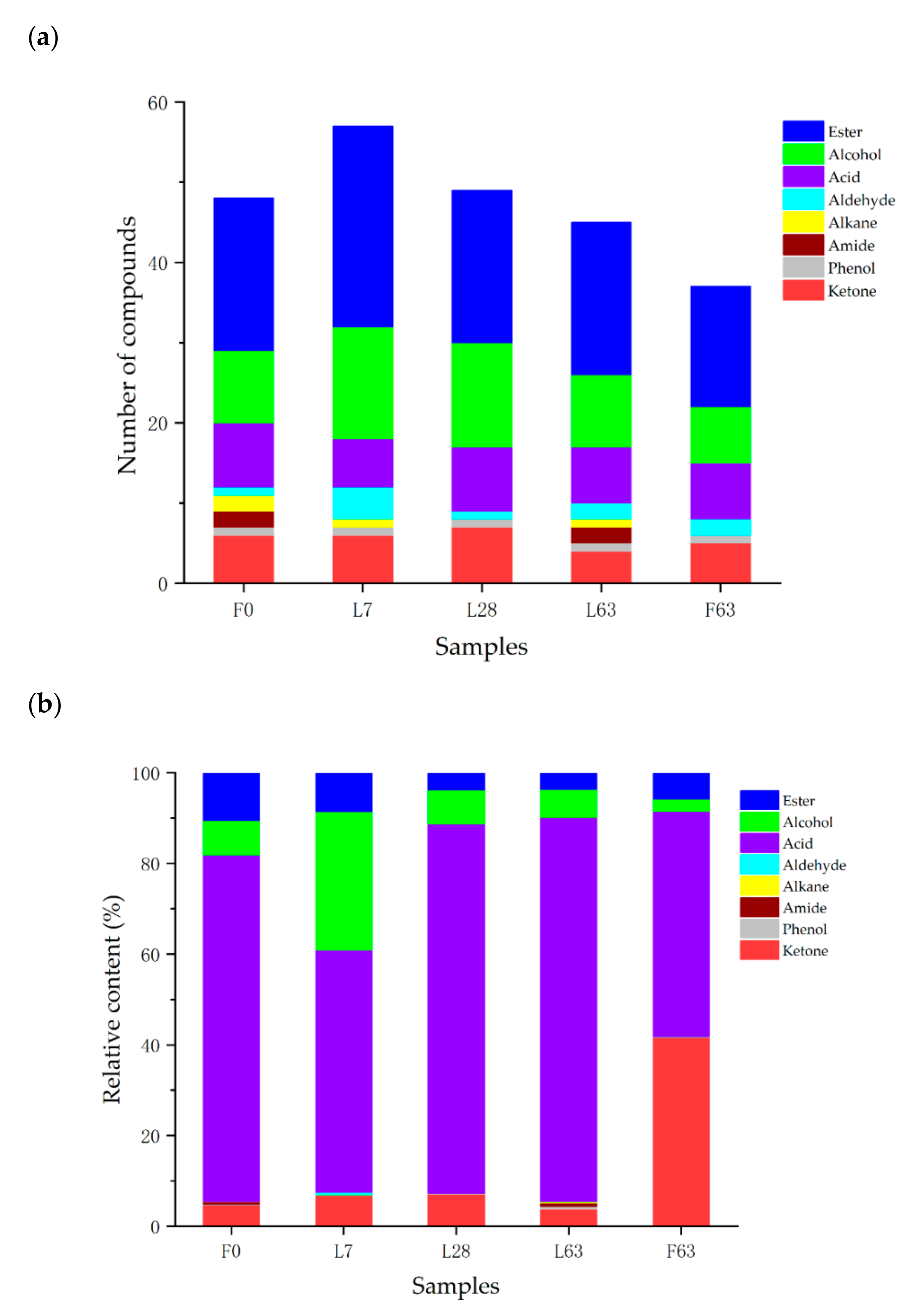
| Compounds | Rention Time/min | Relative Content (%) | ||||
|---|---|---|---|---|---|---|
| F0 | L7 | L28 | L63 | F63 | ||
| Esters (28) | ||||||
| methyl acetate | 1.93 | nd | 0.03 | 0.07 | 0.03 | 0.06 |
| ethyl acetate | 2.35 | 0.02 | 0.08 | 0.05 | 0.06 | 0.12 |
| Methyl butanoate | 3.66 | 0.34 | 0.29 | 0.22 | 0.44 | 0.21 |
| methyl 2-methylbutyrate | 4.18 | nd | nd | nd | 0.01 | 0.03 |
| ethyl butyrate | 4.88 | 0.70 | 0.48 | 0.12 | 0.28 | 0.04 |
| vinyl butyrate | 5.07 | nd | nd | nd | nd | 0.24 |
| ethyl 2-methylbutyrate | 5.36 | nd | 0.02 | 0.01 | 0.02 | nd |
| 3-butenoic acid-3-methyl-methyl ester | 8.07 | 0.21 | 0.22 | 0.05 | 0.06 | 0.10 |
| methyl hexanoate | 11.93 | 1.97 | 2.54 | 2.05 | 1.39 | 3.33 |
| 3-methyl-3-buten-1-ol acetate | 12.36 | 0.13 | 0.33 | 0.03 | 0.05 | nd |
| butyl butyrate | 13.28 | nd | 0.10 | nd | nd | nd |
| ethyl hexanoate | 13.82 | 0.77 | 1.71 | 0.12 | 0.36 | 0.03 |
| 3-methyl-2-buten-1-ol acetate | 14.42 | 0.02 | 0.04 | nd | nd | nd |
| 4-pentenyl butyrate | 14.94 | 2.40 | 0.66 | 0.20 | 0.19 | 0.31 |
| hexyl acetate | 15.03 | nd | 0.28 | nd | nd | nd |
| methyl octanoate | 17.84 | 0.25 | 0.32 | 0.27 | 0.15 | 1.03 |
| 3-methylbutyl-2-alkenyl butyrate | 17.9 | 0.17 | 0.04 | 0.01 | 0.01 | nd |
| ethyl octanoate | 18.79 | 0.20 | nd | nd | nd | nd |
| 3-methyl-3-butenoate | 19.18 | 0.77 | 0.34 | 0.04 | 0.02 | 0.05 |
| ethyl-2-(5-methyl-5-vinyltetrahydrofuran-2-yl)-2-propyl-carbonate | 19.33 | 0.02 | 0.01 | 0.01 | 0.03 | 0.02 |
| methyl 3-(methylthio)propionate | 20.23 | 0.60 | 0.33 | 0.26 | 0.15 | 0.11 |
| ethyl 3-(methylthio)propionate | 20.98 | 1.25 | 0.29 | 0.09 | 0.09 | nd |
| 3-methylbutyl-3-alkenyl isobutylate | 23.75 | 0.25 | 0.01 | nd | nd | nd |
| methyl phenylacetate | 23.90 | nd | 0.02 | 0.02 | 0.03 | nd |
| methyl salicylate | 24.10 | 0.04 | 0.09 | 0.16 | 0.27 | 0.09 |
| hexyl octanoate | 24.90 | nd | 0.23 | nd | nd | nd |
| butyric acid, di(tert-butyl)silyl ester | 24.98 | nd | 0.09 | nd | nd | nd |
| amyl 3-(methylthio)propionate | 25.7 | 0.37 | 0.02 | nd | nd | nd |
| Alcohols (15) | ||||||
| ethanol | 2.82 | 2.44 | 2.17 | 0.12 | 1.20 | nd |
| 2-methyl-3-buten-2-ol | 5.05 | nd | nd | 0.12 | 0.07 | nd |
| 2-methyl-1-propanol | 7.46 | nd | 0.10 | nd | nd | nd |
| 1-butanol | 10.17 | nd | 1.05 | 0.06 | nd | nd |
| 2-methyl-1-butanol | 13.02 | nd | 0.63 | 0.20 | nd | nd |
| 3-methyl-3-buten-1-ol | 14.24 | 3.52 | 5.57 | 2.17 | 3.30 | 0.47 |
| 3-methyl-2-buten-1-ol | 16.24 | 0.21 | 0.34 | 0.12 | 0.20 | 0.07 |
| 2-heptanol | 16.35 | 0.08 | 0.82 | 0.74 | 0.46 | 0.93 |
| 1-hexanol | 17.04 | 0.05 | 18.31 | 2.67 | 0.04 | 0.68 |
| 1-octene-3-ol | 19.04 | 0.01 | 0.01 | 0.04 | 0.14 | 0.04 |
| 3,7-dimethyl-1,6-octanethiol-3-ol | 20.74 | 1.08 | 0.73 | 0.97 | 0.61 | 0.38 |
| 1-octanol | 20.9 | nd | 0.58 | nd | nd | nd |
| alpha-terpineol | 23.03 | 0.18 | 0.09 | 0.22 | nd | 0.20 |
| 3-(methylthio)-1-propanol | 23.22 | nd | 0.10 | 0.01 | nd | nd |
| benzyl alcohol | 25.45 | 0.02 | 0.04 | 0.06 | 0.10 | nd |
| Acids (10) | ||||||
| cyclopentadecandecanoic acid | 1.02 | 0.04 | nd | nd | nd | nd |
| cyclohexanecarboxylic acid | 10.82 | 0.23 | 0.35 | 0.08 | 0.13 | nd |
| acetic acid | 18.76 | nd | 1.21 | 0.53 | 0.35 | 0.19 |
| sarnic acid | 20.10 | 0.07 | nd | nd | nd | nd |
| 2-methylpropionic acid | 20.90 | 0.17 | nd | 0.89 | 0.46 | 0.95 |
| butyric acid | 21.85 | 1.14 | 0.85 | 2.57 | 1.41 | 0.53 |
| 2-methylbutyric acid | 22.53 | 1.03 | 0.50 | 1.59 | 1.68 | 1.88 |
| 2-pentenoic acid | 24.55 | nd | nd | 1.45 | nd | 0.48 |
| hexanoic acid | 25.05 | 34.08 | 21.6 | 34.17 | 36.93 | 12.20 |
| octanoic acid | 27.87 | 39.69 | 28.88 | 40.17 | 43.75 | 33.41 |
| Ketones (7) | ||||||
| acetone | 1.85 | 0.88 | nd | 0.02 | nd | 0.13 |
| 2-butanone | 2.44 | nd | 0.01 | 0.05 | 0.05 | 0.12 |
| 2-pentanone | 3.45 | 0.02 | 0.04 | 0.12 | 0.03 | 1.15 |
| 2-heptanone | 11.56 | 3.64 | 6.59 | 6.71 | 3.59 | 39.82 |
| 2-nonanone | 17.74 | 0.07 | 0.08 | 0.08 | 0.03 | 0.38 |
| 2-octene-4-one | 19.49 | 0.03 | 0.03 | 0.01 | nd | nd |
| 5-butyldihydro-2(3H)-furanone | 25.98 | 0.01 | 0.01 | 0.06 | nd | nd |
| Aldehydes (5) | ||||||
| acetaldehyde | 1.50 | nd | 0.11 | nd | nd | nd |
| hexanal | 6.20 | nd | 0.20 | nd | nd | nd |
| Octanal | 15.33 | 0.01 | 0.03 | nd | nd | 0.05 |
| benzaldehyde | 20.10 | nd | nd | 0.02 | 0.01 | 0.03 |
| 2,4-dimethylbenzaldehyde | 24.57 | nd | 0.16 | nd | 0.07 | nd |
| Alkanes (4) | ||||||
| cyclotriazane | 2.16 | 0.03 | nd | nd | nd | nd |
| 6-methyltridecane | 22.25 | 0.04 | nd | nd | nd | nd |
| 8-hexyl-pentadecane | 24.84 | nd | 0.14 | ns | nd | nd |
| 2,6,11-trimethyl-dodecane | 24.91 | nd | nd | nd | 0.26 | nd |
| Amides (2) | ||||||
| erucamide | 32.04 | 0.30 | nd | nd | 0.68 | nd |
| oleic acid amide | 32.3 | 0.29 | nd | nd | 0.15 | nd |
| Phenols (2) | ||||||
| 2-methoxy-3-(2-ene)-phenol | 29.12 | 0.11 | 0.05 | nd | nd | 0.12 |
| eugenol | 29.13 | nd | nd | 0.17 | 0.63 | nd |
| total | 99.95 | 99.95 | 99.97 | 99.97 | 99.98 | |
| Sensor Number in Array | Sensor Name | General Description |
|---|---|---|
| S1 | W1C | Aromatic compounds |
| S2 | W5S | Reacts to nitrogen oxide |
| S3 | W3C | Ammonia, aromatic compounds |
| S4 | W6S | Mainly hydride |
| S5 | W5C | Alkanes, aromatic compounds, less polar compounds |
| S6 | W1S | Methane |
| S7 | W1W | Sulfur compounds, otherwise sensitive to many terpenes and organic sulfur compounds, which are important for smell, limonene, pyrazine |
| S8 | W2S | Alcohol, partially aromatic compounds |
| S9 | W2W | Aromatic compounds, organic sulfur compounds |
| S10 | W3S | Reacts to high concentrations, selective methane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Dou, R.; Yang, R.; Cai, K.; Li, C.; Li, W. Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation. Molecules 2021, 26, 2604. https://doi.org/10.3390/molecules26092604
Wang Z, Dou R, Yang R, Cai K, Li C, Li W. Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation. Molecules. 2021; 26(9):2604. https://doi.org/10.3390/molecules26092604
Chicago/Turabian StyleWang, Zhulin, Rong Dou, Ruili Yang, Kun Cai, Congfa Li, and Wu Li. 2021. "Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation" Molecules 26, no. 9: 2604. https://doi.org/10.3390/molecules26092604
APA StyleWang, Z., Dou, R., Yang, R., Cai, K., Li, C., & Li, W. (2021). Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation. Molecules, 26(9), 2604. https://doi.org/10.3390/molecules26092604






