Direct Potentiometric Study of Cationic and Nonionic Surfactants in Disinfectants and Personal Care Products by New Surfactant Sensor Based on 1,3-Dihexadecyl−1H-benzo[d]imidazol−3-ium
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Quaternary Alkyl Ammonium Salt 2
2.2. Sensor Characteristics
2.2.1. Response
2.2.2. Dynamic Response
2.2.3. Interferences
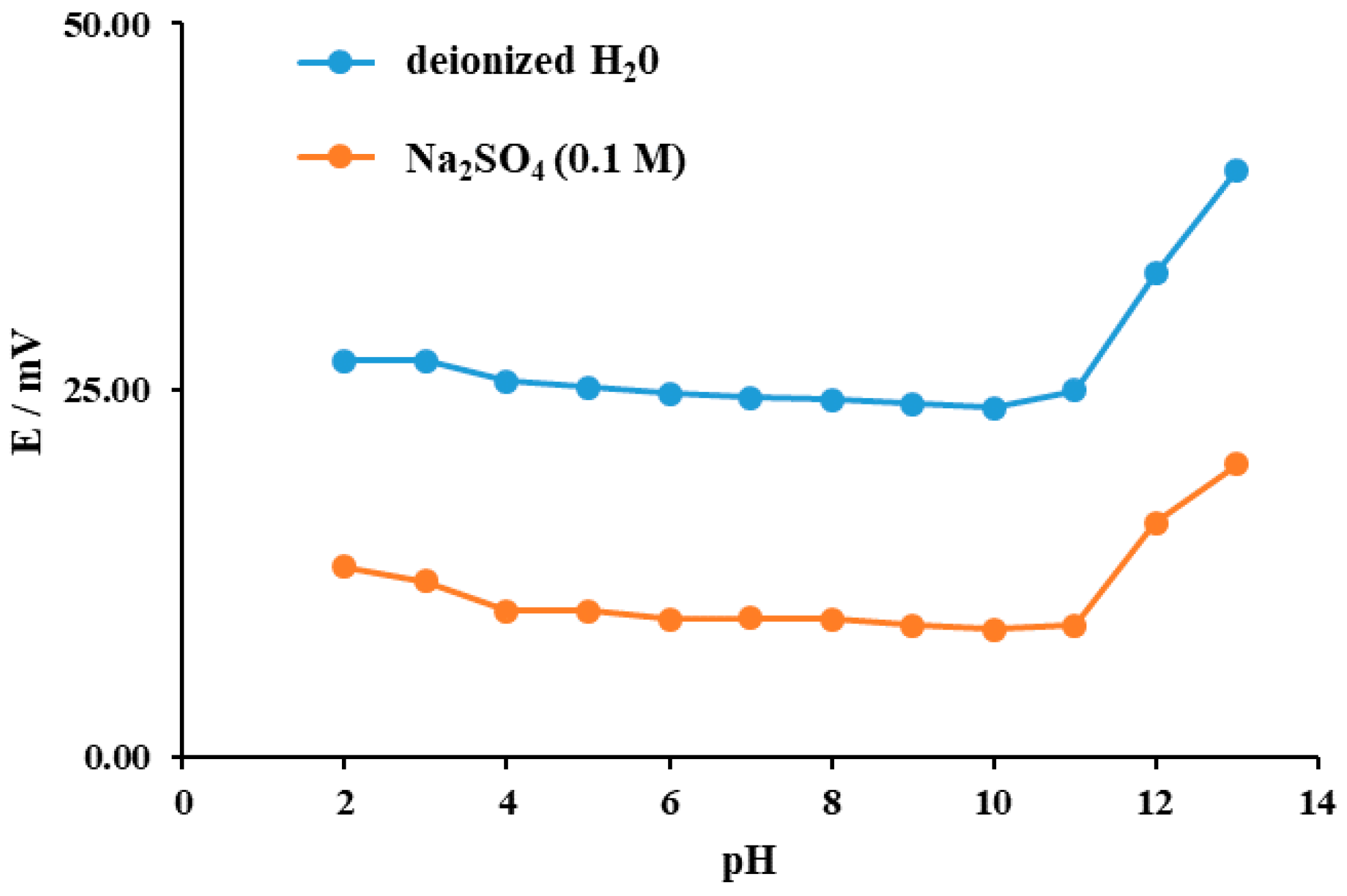
2.2.4. Influence of pH
2.3. Potentiometric Titration
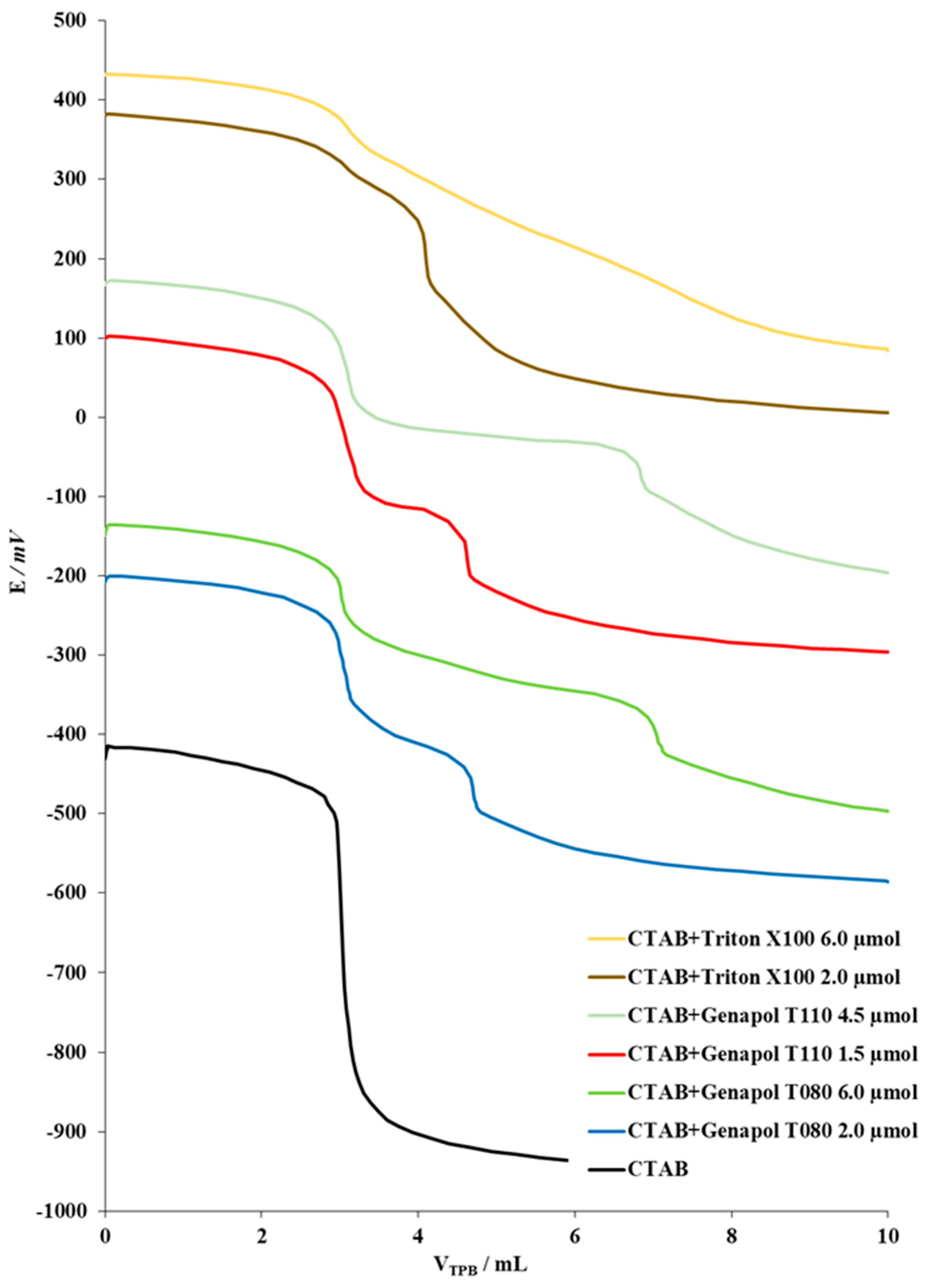
2.3.1. Titration of Model Samples Containing Cationic Surfactant and EO-Nonionic Surfactant
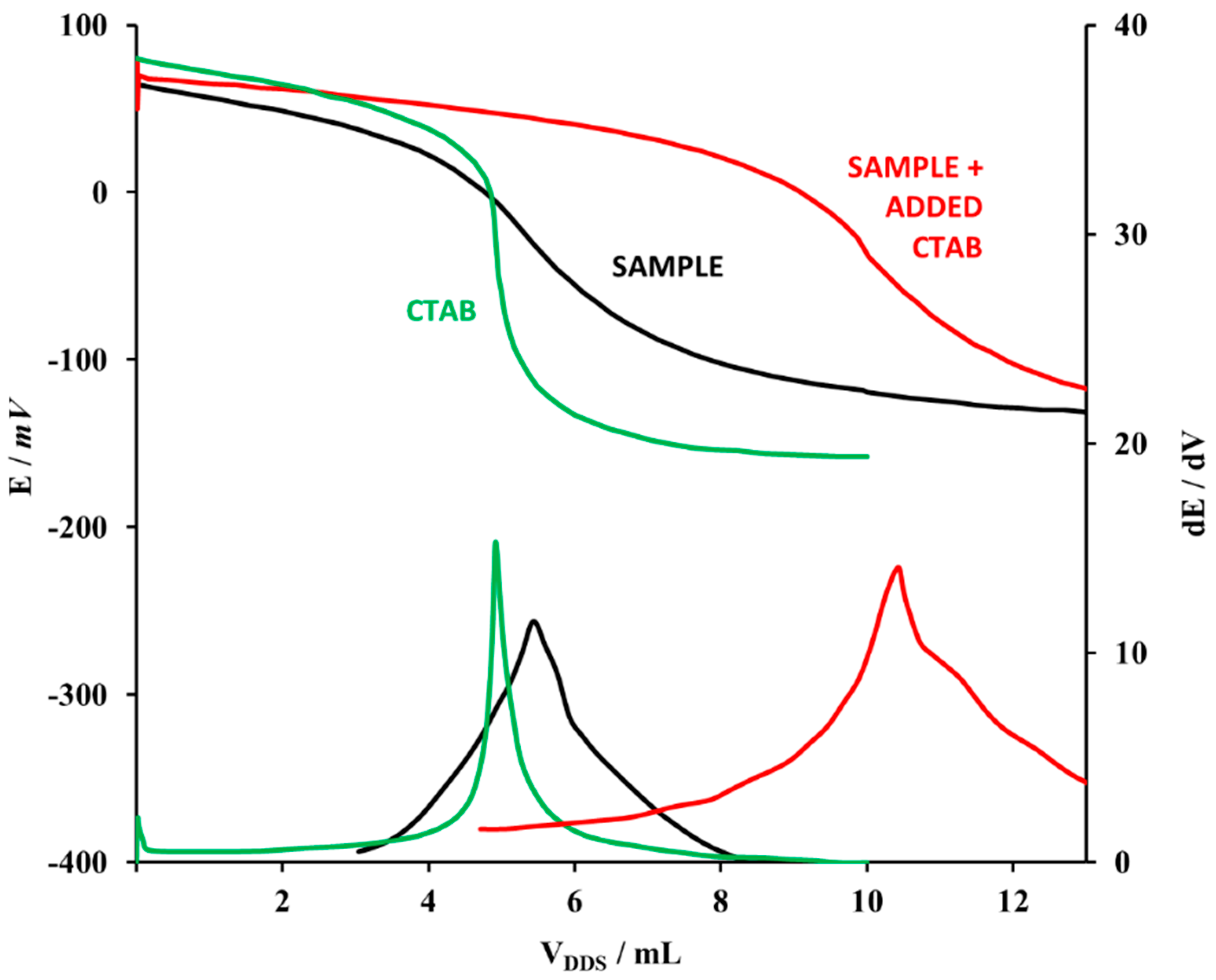
2.3.2. Titration of Commercial Samples
3. Materials and Methods
3.1. Reagents and Materials
3.2. Synthesis of Quaternary Alkyl Ammonium Salt 1
3.3. Characterization of Quaternary Alkyl Ammonium Salt
3.3.1. FTIR
3.3.2. NMR
3.3.3. LC-MS
3.3.4. Elemental Analysis
3.4. Preparation of DHBI–TPB Surfactant Sensor
3.4.1. Preparation of DHBI–TPB 2 Ion-Pair Sensing Complex
3.4.2. Sensor Membrane Preparation
3.4.3. Surfactant Sensor Preparation
3.5. Apparatus
3.6. Procedure
3.6.1. Direct Potentiometric Sensor Characterization
3.6.2. Direct Potentiometric Titrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jozanović, M.; Sakač, N.; Karnaš, M.; Medvidović-Kosanović, M. Potentiometric Sensors for the Determination of Anionic Surfactants–A Review. Crit. Rev. Anal. Chem. 2019, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets Surfactants Market by Type (Anionic, Non-Ionic, Cationic, and Amphoteric), Application (Home Care, Personal Care, Industrial & Institutional Cleaning, Textile, Elastomers & Plastics, Agrochemicals, and Food & Beverage), Region—Global Forecast to 2025; Pune, India, 2020. Available online: https://www.researchandmarkets.com/reports/3927849/surfactants-market-by-type-anionic-non-ionic (accessed on 12 December 2020).
- Sak-Bosnar, M.; Madunić-Čačić, D.; Sakač, N.; Galović, O.; Samardžić, M.; Grabarić, Z. Potentiometric Sensor for Polyethoxylated Nonionic Surfactant Determination. Electrochim. Acta 2009, 55, 528–534. [Google Scholar] [CrossRef]
- Ogino, K.; Abe, M. (Eds.) Mixed Surfactant Systems; Marcel Dekker, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Kawai, R.; Niki, M.; Yada, S.; Yoshimura, T. Surface Adsorption Properties and Layer Structures of Homogeneous Polyoxyethylene-Type Nonionic Surfactants in Quaternary-Ammonium-Salt-Type Amphiphilic Gemini Ionic Liquids with Oxygen- or Nitrogen-Containing Spacers. Molecules 2020, 25, 4881. [Google Scholar] [CrossRef] [PubMed]
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959. [Google Scholar] [CrossRef]
- Research and Markets Global Surfactants Market (2019 to 2025)—Drivers, Restraints, Opportunities and Challenges; Pune, India, 2020. Available online: https://www.prnewswire.com/news-releases/global-surfactants-market-2019-to-2025---drivers-restraints-opportunities-and-challenges-301086922.html (accessed on 12 December 2020).
- Samara, F.; Badran, R.; Dalibalta, S. Are Disinfectants for the Prevention and Control of COVID-19 Safe? Health Secur. 2020, 18, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Filippelli, G.M.; Salamova, A. Increased Indoor Exposure to Commonly Used Disinfectants during the COVID-19 Pandemic. Environ. Sci. Technol. Lett. 2020, 7, 760–765. [Google Scholar] [CrossRef]
- Fizer, M.; Fizer, O.; Sidey, V.; Mariychuk, R.; Studenyak, Y. Experimental and Theoretical Study on Cetylpyridinium Dipicrylamide—A Promising Ion-Exchanger for Cetylpyridinium Selective Electrodes. J. Mol. Struct. 2019, 1187, 77–85. [Google Scholar] [CrossRef]
- Hrenovic, J.; Ivankovic, T. Toxicity of Anionic and Cationic Surfactant to Acinetobacter Junii in Pure Culture. Open Life Sci. 2007, 2, 405–414. [Google Scholar] [CrossRef]
- Reid, V.; Longman, G.; Heinerth, E. Determination of Anionic-Active Detergents by Two-Phase Titration. Tenside Deterg. 1967, 4, 292–304. [Google Scholar]
- Miller, C.; Bageri, B.S.; Zeng, T.; Patil, S.; Mohanty, K.K. Modified Two-Phase Titration Methods to Quantify Surfactant Concentrations in Chemical-Enhanced Oil Recovery Applications. J. Surfactants Deterg. 2020, 23, 1159–1167. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Y.; Wang, Y.; Li, H. A Simple and Effective Luminescence Sensor Distinguishing Cationic Surfactants from Other Type of Surfactants. Sens. Actuators B Chem. 2016, 235, 206–212. [Google Scholar] [CrossRef]
- Olkowska, E.; Polkowska, Ż.; Namieśnik, J. A Solid Phase Extraction–Ion Chromatography with Conductivity Detection Procedure for Determining Cationic Surfactants in Surface Water Samples. Talanta 2013, 116, 210–216. [Google Scholar] [CrossRef]
- Wang, D.-E.; Zhao, L.; Yuan, M.-S.; Chen, S.-W.; Li, T.; Wang, J. Fabrication of Polydiacetylene Liposome Chemosensor with Enhanced Fluorescent Self-Amplification and Its Application for Selective Detection of Cationic Surfactants. ACS Appl. Mater. Interfaces 2016, 8, 28231–28240. [Google Scholar] [CrossRef]
- Makarova, N.M.; Kulapina, E.G. New Potentiometric Sensors Based on Ionic Associates of Sodium Dodecylsulfate and Cationic Complexes of Copper(II) with Some Organic Reagents. Electroanalysis 2015, 27, 621–628. [Google Scholar] [CrossRef]
- Matešic-Puac, R.; Sak-Bosnar, M.; Bilic, M.; Grabaric, B.S. New Ion-Pair Based All-Solid-State Surfactant Sensitive Sensor for Potentiometric Determination of Cationic Surfactants. Electroanalysis 2004, 16, 843–851. [Google Scholar] [CrossRef]
- Pechenkina, I.A.; Mikhelson, K.N. Materials for the Ionophore-Based Membranes for Ion-Selective Electrodes: Problems and Achievements (Review Paper). Russ. J. Electrochem. 2015, 51, 93–102. [Google Scholar] [CrossRef]
- Sakač, N.; Karnaš, M.; Jozanović, M.; Medvidović-Kosanović, M.; Martinez, S.; Macan, J.; Sak-Bosnar, M. Determination of Anionic Surfactants in Real Samples Using a Low-Cost and High Sensitive Solid Contact Surfactant Sensor with MWCNTs as the Ion-to-Electron Transducer. Anal. Methods 2017, 9, 2305–2314. [Google Scholar] [CrossRef]
- Najafi, M.; Maleki, L.; Rafati, A.A. Novel Surfactant Selective Electrochemical Sensors Based on Single Walled Carbon Nanotubes. J. Mol. Liq. 2011, 159, 226–229. [Google Scholar] [CrossRef]
- Sánchez Arribas, A.; Moreno, M.; González, L.; Blázquez, N.; Bermejo, E.; Zapardiel, A.; Chicharro, M. A Comparative Study of Carbon Nanotube Dispersions Assisted by Cationic Reagents as Electrode Modifiers: Preparation, Characterization and Electrochemical Performance for Gallic Acid Detection. J. Electroanal. Chem. 2020, 857, 113750. [Google Scholar] [CrossRef]
- Olkowska, E.; Polkowska, Z.; Namieśnik, J. Analytics of Surfactants in the Environment: Problems and Challenges. Chem. Rev. 2011, 111, 5667–5700. [Google Scholar] [CrossRef] [PubMed]
- Madunić-Čačić, D.; Sak-Bosnar, M. Investigation of Homologous Tallow Fatty Alcohols and Oleyl Alcohol Ethoxylates Using a Potentiometric Surfactant Sensor. Int. J. Electrochem. Sci. 2011, 6, 1630–1641. [Google Scholar]
- Madunić Čačić, D.; Sak-Bosnar, M.; Galović, O.; Sakač, N.; Matešić-Puač, R. Determination of Cationic Surfactants in Pharmaceutical Disinfectants Using a New Sensitive Potentiometric Sensor. Talanta 2008, 76, 259–264. [Google Scholar] [CrossRef]
- Samardžić, M.; Sak-Bosnar, M.; Madunić-Čačić, D. Simultaneous Potentiometric Determination of Cationic and Ethoxylated Nonionic Surfactants in Liquid Cleaners and Disinfectants. Talanta 2011, 83, 789–794. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Ali, T.A.; El-Shahat, M.F.; Al-Sabagh, A.M.; Migahed, M.A.; Khaled, E. Potentiometric Determination of Cetylpyridinium Chloride Using a New Type of Screen-Printed Ion Selective Electrodes. Anal. Chim. Acta 2010, 673, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y. Predicting the End Point Potential Break Values: A Case of Potentiometric Titration of Lipophilic Anions with Cetylpyridinium Chloride. Microchem. J. 2021, 160, 105758. [Google Scholar] [CrossRef]
- ISO7875−2:1984. Water Quality—Determination of Surfactants—Part 2: Determination of Non-Ionic Surfactants Using Dragendorff Reagent; ISO/TC 147/SC 2. Physical, Chemical and Biochemical Methods, ICS: 13.060.50. Examination of Water for Chemical Substances; International Organization for Standardization: Geneva, Switzerland, 1984.
- Lindner, E.; Tóth, K.; Pungor, E. Definition and Determination of Response Time of Ion Selective Electrodes. Pure Appl. Chem. 1986, 58, 469–479. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recomendations for Nomenclature of Ion-Selective Electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]


| Parameters | CTAB | CPC | Hyamine |
|---|---|---|---|
| Slope (mV/decade) | 59.2 ± 0.5 | 58.4 ± 0.7 | 57.1 ± 1.1 |
| Correlation coefficient (R2) | 0.9999 | 0.9992 | 0.9988 |
| Intercept (mV) | 159.1 ± 7.0 | 199.6 ± 7.2 | 174.0 ± 5.1 |
| Detection limit (M) | 0.3 × 10−6 | 0.7 × 10−6 | 1.1 × 10−6 |
| Useful linear concentration range (M) | 2.0 × 10−6 to 1.0 × 10−4 | 3.0 × 10−6 to 1.0 × 10−4 | 3.0 × 10−6 to 1.0 × 10−4 |
| Interfering Cations | |
|---|---|
| Na+ | 4.1 × 10−5 |
| K+ | 2.5 × 10−5 |
| NH4+ | 6.2 × 10−5 |
| Mg2+ | 3.6 × 10−5 |
| Ca2+ | 1.6 × 10−5 |
| Zn2+ | 2.7 × 10−5 |
| 2-aminoethanol | 0.3 × 10−5 |
| Tris(2-hydroxyethyl)amine | 1.1 × 10−5 |
| Tetraethylazanium | 2.8 × 10−5 |
| Triton B | 1.5 × 10−5 |
| Benzyltriethylammonium | 5.2 × 10−5 |
| Technical Grade Cationic Surfactant | DHBI–TPB Surfactant Sensor | Two-Phase Titration | |
|---|---|---|---|
| Found (%) | RSD (%) | Found (%) | |
| CTAB | 95.39 | 0.59 | 95.89 |
| Hyamine | 98.45 | 0.48 | 98.52 |
| CPC | 97.92 | 0.34 | 98.20 |
| BAC | 51.09 | 0.90 | 50.86 |
| DDAC | 50.97 | 0.61 | 51.43 |
| Commercial Sample | Calculated Cationic Surfactant (M) | CTAB | ||
|---|---|---|---|---|
| Added (mol) | Found (mol) a | Recovery (%) | ||
| Mouthwash 1 | 3.34 × 10−3 | 1 × 10−5 | 0.98 × 10−5 | 98.0 |
| Mouthwash 2 | 3.83 × 10−3 | 7 × 10−5 | 7.11 × 10−5 | 101.6 |
| Mouthwash 3 | 1.40 × 10−3 | 3 × 10−4 | 3.06 × 10−5 | 102.0 |
| Eye drops 1 | 6.26 × 10−4 | 5 × 10−5 | 5.03 × 10−5 | 100.6 |
| Type Of Commercial Product | DHBI–TPB Surfactant Sensor a | Commercial Surfactant Sensor b | Two Phase Titration b | |||
|---|---|---|---|---|---|---|
| Found% | RSD(%) | Confidence Interval (±) | Found% | RSD(%) | Found% | |
| Mouthwash 1 | 0.1137 | 0.74 | 0.000743 | 0.1151 | 0.76 | 0.1143 |
| Mouthwash 2 | 0.1303 | 1.06 | 0.001216 | 0.1297 | 1.12 | 0.1311 |
| Mouthwash 3 | 0.0480 | 1.49 | 0.000630 | 0.0480 | 1.39 | 0.0462 |
| Mouthwash 4 | 0.1882 | 0.34 | 0.000560 | 0.1888 | 0.39 | 0.1821 |
| Medical skin disinfectant 1 | 0.1998 | 1.27 | 0.002231 | 0.1984 | 1.32 | 0.1993 |
| Medical skin disinfectant 2 | 0.3769 | 0.91 | 0.000788 | 0.3639 | 0.89 | 0.3649 |
| Disinfectant for hospitals 1 | 4.1032 | 0.97 | 0.001862 | 4.1005 | 0.91 | 4.1025 |
| Disinfectant for hospitals 2 | 5.0964 | 0.51 | 0.000982 | 5.0979 | 0.78 | 5.1002 |
| Nose drops 1 | 0.0150 | 1.76 | 0.000232 | 0.0152 | 1.06 | 0.0142 |
| Nose drops 2 | 0.0151 | 2.07 | 0.000275 | 0.0160 | 1.90 | 0.0156 |
| Nose drops 3 | 0.0099 | 1.49 | 0.000129 | 0.0102 | 1.67 | 0.0101 |
| Nose drops 4 | 0.0178 | 1.67 | 0.000261 | 0.0185 | 1.73 | 0.0180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakač, N.; Marković, D.; Šarkanj, B.; Madunić-Čačić, D.; Hajdek, K.; Smoljan, B.; Jozanović, M. Direct Potentiometric Study of Cationic and Nonionic Surfactants in Disinfectants and Personal Care Products by New Surfactant Sensor Based on 1,3-Dihexadecyl−1H-benzo[d]imidazol−3-ium. Molecules 2021, 26, 1366. https://doi.org/10.3390/molecules26051366
Sakač N, Marković D, Šarkanj B, Madunić-Čačić D, Hajdek K, Smoljan B, Jozanović M. Direct Potentiometric Study of Cationic and Nonionic Surfactants in Disinfectants and Personal Care Products by New Surfactant Sensor Based on 1,3-Dihexadecyl−1H-benzo[d]imidazol−3-ium. Molecules. 2021; 26(5):1366. https://doi.org/10.3390/molecules26051366
Chicago/Turabian StyleSakač, Nikola, Dean Marković, Bojan Šarkanj, Dubravka Madunić-Čačić, Krunoslav Hajdek, Božo Smoljan, and Marija Jozanović. 2021. "Direct Potentiometric Study of Cationic and Nonionic Surfactants in Disinfectants and Personal Care Products by New Surfactant Sensor Based on 1,3-Dihexadecyl−1H-benzo[d]imidazol−3-ium" Molecules 26, no. 5: 1366. https://doi.org/10.3390/molecules26051366
APA StyleSakač, N., Marković, D., Šarkanj, B., Madunić-Čačić, D., Hajdek, K., Smoljan, B., & Jozanović, M. (2021). Direct Potentiometric Study of Cationic and Nonionic Surfactants in Disinfectants and Personal Care Products by New Surfactant Sensor Based on 1,3-Dihexadecyl−1H-benzo[d]imidazol−3-ium. Molecules, 26(5), 1366. https://doi.org/10.3390/molecules26051366







