Mycomedicine: A Unique Class of Natural Products with Potent Anti-tumour Bioactivities
Abstract
1. Introduction
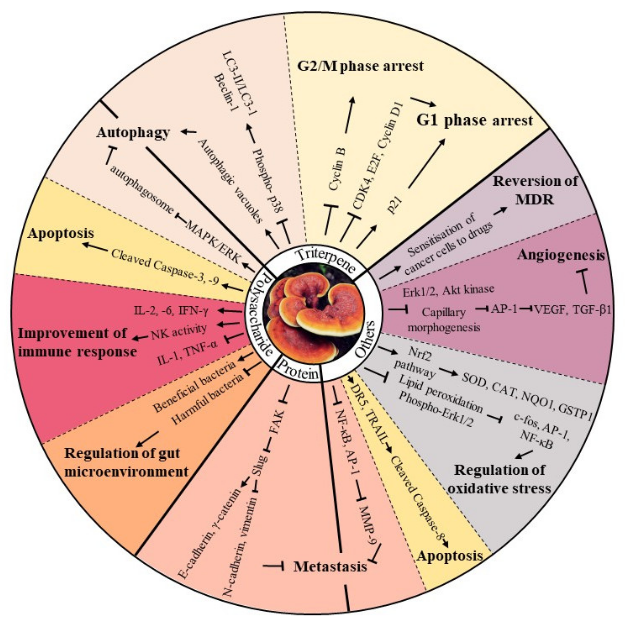
2. Bioactive Components Isolated from Mycomedicine
2.1. Polysaccharides
2.2. Terpenes and Terpenoids
2.3. Proteins and Amino Acids
2.4. Other Bioactive Compounds
3. Major Anti-tumour Bioactivities of Mycomedicine
3.1. Induction of Cell Cycle Arrest
3.2. Induction of Apoptosis
3.3. Induction of Autophagy
3.4. Suppression of Angiogenesis
3.5. Reduction in Metastatic Potential
3.6. Improvement of Immune Response
3.7. Regulation of Oxidative Stress
3.8. Regulation of Gut Microenvironment
3.9. Reversion of Multidrug Resistance
4. Clinical Evidence of Mycomedicine in Cancer Therapy
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ritchie, H. Causes of Death. Our World in Data. 2018. Available online: https://ourworldindata.org/causes-of-death (accessed on 19 February 2021).
- Da Rocha, A.B.; Lopes, R.M.; Schwartsmann, G. Natural products in anticancer therapy. Curr. Opin. Pharmacol. 2001, 1, 364–369. [Google Scholar] [CrossRef]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, S. Cancer prevention and treatment by ganoderma, a mushroom with medicinal properties. Food Rev. Int. 2003, 19, 275–325. [Google Scholar] [CrossRef]
- Li, X.; Xu, P.; Huang, W.Q.; Li, R. Review of pharmacological effects of agaricus blazei murill and its application in dietotherapy. J. Biotechnol. Res. 2019, 5, 46–49. [Google Scholar]
- He, Y.; Zhang, L.; Wang, H. The biological activities of the antitumor drug Grifola frondosa polysaccharide. Prog. Mol. Biol. Transl. Sci. 2019, 163, 221–261. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Polysaccharides in Grifola frondosa mushroom and their health promoting properties: A review. Int. J. Biol. Macromol. 2017, 101, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Fan, L. Evaluation of anticancer activities of Poria cocos ethanol extract in breast cancer: In vivo and in vitro, identification and mechanism. J. Ethnopharmacol. 2020, 257, 112851. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Endo, M.; Matsui, T.; Katsuda, I.; Emi, N.; Kawamoto, Y.; Koike, T.; Beppu, H. Agaritine from Agaricus blazei Murrill induces apoptosis in the leukemic cell line U937. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 519–525. [Google Scholar] [CrossRef]
- Wu, G.-S.; Lu, J.-J.; Guo, J.-J.; Li, Y.-B.; Tan, W.; Dang, Y.-Y.; Zhong, Z.-F.; Xu, Z.-T.; Chen, X.-P.; Wang, Y.-T. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia 2012, 83, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Li, Y.B.; Liang, X.F.; Liu, H.Z.; Xiao, J.H.; Zhong, J.J. Structurally related ganoderic acids induce apoptosis in human cervical cancer HeLa cells: Involvement of oxidative stress and antioxidant protective system. Chem. Biol. Interact. 2015, 240, 134–144. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Jaroonwitchawan, T.; Noisa, P. Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol. Vitr. 2018, 46, 113–121. [Google Scholar] [CrossRef]
- Lee, J.-D.; Jeong, J.-W.; Jin, C.-Y.; Park, C.; Han, M.H.; Kim, G.-Y.; Moon, S.-K.; Gil Kim, C.; Jeong, Y.K.; Kim, W.-J.; et al. Inhibition of migration and invasion of LNCaP human prostate carcinoma cells by cordycepin through inactivation of Akt. Int. J. Oncol. 2012, 40, 1697–1704. [Google Scholar] [CrossRef]
- Liao, Y.; Ling, J.; Zhang, G.; Liu, F.; Tao, S.; Han, Z.; Chen, S.; Chen, Z.; Le, H. Cordycepin induces cell cycle arrest and apoptosis by inducing DNA damage and up-regulation of p53 in Leukemia cells. Cell Cycle 2015, 14, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-Y.; Luo, K.-W.; Yu, Z.-M.; Co, N.-N.; Wu, S.-H.; Wu, P.; Fung, K.-P.; Kwok, T.-T. Suillin from the mushroom Suillus placidus as potent apoptosis inducer in human hepatoma HepG2 cells. Chem. Interact. 2009, 181, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Uchiyama, E.; Ukiya, M.; Tabata, K.; Kimura, Y.; Suzuki, T.; Akihisa, T. Cytotoxic and apoptosis-inducing activities of triterpene acids from Poria cocos. J. Nat. Prod. 2011, 74, 137–144. [Google Scholar] [CrossRef]
- Sun, J.; Yeung, C.A.; Tsang, T.Y.; Yau, E.; Luo, K.; Wu, P.; Wa, J.C.Y.; Fung, K.P.; Kwok, T.T.; Liu, F. Clitocine reversal of P-glycoprotein associated multi-drug resistance through down-regulation of transcription factor NF-κB in R-HepG2 cell line. PLoS ONE 2012, 7, e40720. [Google Scholar] [CrossRef] [PubMed]
- Hazama, S.; Watanabe, S.; Ohashi, M.; Yagi, M.; Suzuki, M.; Matsuda, K.; Yamamoto, T.; Suga, Y.; Suga, T.; Nakazawa, S.; et al. Efficacy of orally administered superfine dispersed lentinan (β-1, 3-glucan) for the treatment of advanced colorectal cancer. Anticancer. Res. 2009, 29, 2611–2617. [Google Scholar]
- Xu, X.; Yan, H.; Tang, J.; Chen, J.; Zhang, X. Polysaccharides in Lentinus edodes: Isolation, structure, immunomodulating activity and future prospective. Crit. Rev. Food Sci. Nutr. 2013, 54, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Zhuang, C. Maitake, Grifola frondosa : Pharmacological effects. Food Rev. Int. 1995, 11, 135–149. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Biedron, R.; Tangen, J.M.; Maresz, K.; Hetland, G. Agaricus blazei Murill—Immunomodulatory properties and health benefits. Funct. Foods Health Dis. 2012, 2, 428–447. [Google Scholar] [CrossRef]
- Lemieszek, M.; Rzeski, W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Współczesna Onkol. 2012, 4, 285–289. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Sone, Y.; Okuda, R.; Wada, N.; Kishida, E.; Misaki, A. Structures and antitumor activities of the polysaccharides isolated from fruiting body and the growing culture of mycelium of Ganoderma lucidum. Agric. Biol. Chem. 2014, 49, 2641–2653. [Google Scholar]
- Zhang, T.C.; Ouyang, P.; Kaplan, S.; Skarnes, B. Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012), Tianjin, China, 18–19 October 2012; Kaplan, S., Skarnes, B., Ouyang, P., Zang, T.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.K.S.; Bisen, P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef]
- Adachi, Y.; Ohno, N.; Ohsawa, M.; Oikawa, S.; Yadomae, T. Change of biological activities of (1.RAR.3)-.BETA.-D-glucan from Grifola frondosa upon molecular weight reduction by heat treatment. Chem. Pharm. Bull. 1990, 38, 477–481. [Google Scholar] [CrossRef]
- Dasgupta, A.; Acharya, K. Mushrooms: An emerging resource for therapeutic terpenoids. 3 Biotech 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Jesuthasan, A.C.; Bishop, K.S.; Glucina, M.P.; Ferguson, L.R. Anti-cancer activities of Ganoderma lucidum: Active ingredients and pathways. Funct. Foods Health Dis. 2013, 3, 48. [Google Scholar] [CrossRef]
- Yue, Q.-X.; Song, X.-Y.; Ma, C.; Feng, L.-X.; Guan, S.-H.; Wu, W.-Y.; Yang, M.; Jiang, B.-H.; Liu, X.; Cui, Y.-J.; et al. Effects of triterpenes from Ganoderma lucidum on protein expression profile of HeLa cells. Phytomedicine 2010, 17, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S.; Guo, J.J.; Bao, J.L.; Li, X.W.; Chen, X.P.; Lu, J.J.; Wang, Y.T. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum—A review. Expert Opin. Investig. Drugs 2013, 22, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, L.; Liu, D.; Yang, X.; Li, S.; Gao, H.; Yao, X.; Wen, H.; Liu, H. Two new sesquiterpenes and six norsesquiterpenes from the solid culture of the edible mushroom Flammulina velutipes. Tetrahedron 2012, 68, 3012–3018. [Google Scholar] [CrossRef]
- Alexandre, J.; Kahatt, C.; Bertheault-Cvitkovic, F.; Faivre, S.; Shibata, S.; Hilgers, W.; Goldwasser, F.; Lokiec, F.; Raymond, E.; Weems, G.; et al. A phase I and pharmacokinetic study of irofulven and capecitabine administered every 2 weeks in patients with advanced solid tumors. Investig. New Drugs 2007, 25, 453–462. [Google Scholar] [CrossRef]
- Bridge, P.D.; Kokubun, T.; Simmonds, M.S.J.; Cutler, P. Protein extraction from fungi. Protein Purif. Protoc. 2004, 244, 37–46. [Google Scholar] [CrossRef]
- Bernaś, E.; Jaworska, G. Effect of preservation method on amino acid content in selected species of edible mushroom. LWT 2012, 48, 242–247. [Google Scholar] [CrossRef]
- Endo, M.; Beppu, H.; Akiyama, H.; Wakamatsu, K.; Ito, S.; Kawamoto, Y.; Shimpo, K.; Sumiya, T.; Koike, T.; Matsui, T. Agaritine purified from Agaricus blazei Murrill exerts anti-tumor activity against leukemic cells. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Glasel, J.A.; Deutscher, M.P. Introduction to Biophysical Methods for Protein and Nucleic Acid Research; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Novaes, M.R.C.G.; Valadares, F.; Reis, M.C.; Gonçalves, D.R.; Menezes, M.D.C. The effects of dietary supplementation with Agaricales mushrooms and other medicinal fungi on breast cancer: Evidence-based medicine. Clinics 2011, 66, 2133–2139. [Google Scholar] [CrossRef][Green Version]
- Li, Q.-Z.; Zheng, Y.-Z.; Zhou, X.-W. Fungal immunomodulatory proteins: Characteristic, potential antitumor activities and their molecular mechanisms. Drug Discov. Today 2019, 24, 307–314. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, Q.-Z.; Bao, T.-W.; Cong, W.-R.; Song, W.-X.; Zhou, X.-W. In vitro rapid evolution of fungal immunomodulatory proteins by DNA family shuffling. Appl. Microbiol. Biotechnol. 2013, 97, 2455–2465. [Google Scholar] [CrossRef]
- Hassan, M.A.A.; Rouf, R.; Tiralongo, E.; May, T.W.; Tiralongo, J. Mushroom lectins: Specificity, structure and bioactivity relevant to human disease. Int. J. Mol. Sci. 2015, 16, 7802–7838. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K.; Kennedy, J.F. Mushroom lectins in biomedical research and development. Int. J. Biol. Macromol. 2020, 151, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, H.P.; Kanwar, J.R. Mushroom lectins as promising anticancer substances. Curr. Protein Pept. Sci. 2016, 17, 797–807. [Google Scholar] [CrossRef]
- Phan, C.-W.; Wang, J.-K.; Cheah, S.-C.; Naidu, M.; David, P.; Sabaratnam, V. A review on the nucleic acid constituents in mushrooms: Nucleobases, nucleosides and nucleotides. Crit. Rev. Biotechnol. 2018, 38, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Thomadaki, H.; Tsiapalis, C.M.; Scorilas, A. Polyadenylate polymerase modulations in human epithelioid cervix and breast cancer cell lines, treated with etoposide or cordycepin, follow cell cycle rather than apoptosis induction. Biol. Chem. 2005, 386, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Burger, P.; Vogel, M.; Friese, K.; Brüning, A. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Investig. New Drugs 2012, 30, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-G.; Ruan, F.; Zeng, X.-L.; Xiang, J.; Li, X.; Wu, P.; Fung, K.P.; Liu, F.-Y. Clitocine potentiates TRAIL-mediated apoptosis in human colon cancer cells by promoting Mcl-1 degradation. Apoptosis 2016, 21, 1144–1157. [Google Scholar] [CrossRef]
- Moss, R.J.; Petrie, C.R.; Meyer, R.B.; Nord, L.D.; Willis, R.C.; Smith, R.A.; Larson, S.B.; Kini, G.D.; Robins, R.K. Synthesis and intramolecular hydrogen bonding and biochemical studies of clitocine, a naturally occurring exocyclic amino nucleoside. J. Med. Chem. 1988, 31, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.; Russell, M. Ganoderma–A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.A.; Barros, L.; Martins, A.; Morais, J.S.; Vasconcelos, M.H.; Ferreira, I.C. Phenolic profile of seventeen portuguese wild mushrooms. LWT 2011, 44, 343–346. [Google Scholar] [CrossRef]
- Sulkowska-Ziaja, K.; Muszynska, B.; Motyl, P.; Pasko, P.; Ekiert, H. Phenolic compounds and antioxidant activity in some species of polyporoid mushrooms from Poland. Int. J. Med. Mushrooms 2012, 14, 385–393. [Google Scholar] [CrossRef]
- Triangali, C.; Piattelli, M.; Geraci, C.; Nicolosi, G. Antimicrobial tetraprenylpenols fron suillus granulatus. J. Nat. Prod. 1989, 52, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-Y.; Choi, Y.H.; Moon, N.-O.; Park, C.; Park, Y.-M.; Jeong, S.-C.; Heo, M.-S.; Lee, T.-H.; Lee, J.-D.; Kim, G.-Y. Induction of G2/M arrest and apoptosis in human gastric epithelial AGS cells by aqueous extract of Agaricus blazei. Oncol. Rep. 2006, 16, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-B.; Li, C.-H.; Lee, S.-S.; Kan, L.-S. Triterpene-enriched extracts from Ganoderma lucidum inhibit growth of hepatoma cells via suppressing protein kinase C, activating mitogen-activated protein kinases and G2-phase cell cycle arrest. Life Sci. 2003, 72, 2381–2390. [Google Scholar] [CrossRef]
- Hu, H.; Ahn, N.-S.; Yang, X.; Lee, Y.-S.; Kang, K.-S. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int. J. Cancer 2002, 102, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Qian, Z.; Guo, J.; Hu, D.; Bao, J.; Xie, J.; Xu, W.; Lu, J.; Chen, X.; Wang, Y. Ganoderma lucidum extract induces G1 Cell cycle arrest, and apoptosis in human breast cancer cells. Am. J. Chin. Med. 2012, 40, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-C.; Ou, C.-C.; Li, J.-W.; Chuang, T.-C.; Kuo, H.-P.; Liu, J.-Y.; Chen, C.-S.; Lin, S.-C.; Su, C.-H.; Kao, M.-C. Ganoderma tsugae extracts inhibit colorectal cancer cell growth via G2/M cell cycle arrest. J. Ethnopharmacol. 2008, 120, 394–401. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-C.; Wu, J.M. Regulation of cell cycle transition and induction of apoptosis in HL-60 leukemia cells by the combination of Coriolus versicolor and Ganoderma lucidum. Int. J. Mol. Med. 2013, 32, 251–257. [Google Scholar] [CrossRef]
- Prateep, A.; Sumkhemthong, S.; Suksomtip, M.; Chanvorachote, P.; Chaotham, C. Peptides extracted from edible mushroom: Lentinus squarrosulus induces apoptosis in human lung cancer cells. Pharm. Biol. 2017, 55, 1792–1799. [Google Scholar] [CrossRef]
- Jang, K.-J.; Han, M.-H.; Lee, B.-H.; Kim, B.-W.; Kim, C.-H.; Yoon, H.-M.; Choi, Y.-H. Induction of apoptosis by ethanol extracts of Ganoderma lucidum in human gastric carcinoma cells. J. Acupunct. Meridian Stud. 2010, 3, 24–31. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Shu, Y.; Wang, H.; Zheng, Z.; Wang, J.; Wang, K. Induction of apoptosis in S180 tumour bearing mice by polysaccharide from Lentinus edodes via mitochondria apoptotic pathway. J. Funct. Foods 2015, 15, 151–159. [Google Scholar] [CrossRef]
- Shang, D.; Li, Y.; Wang, C.; Wang, X.; Yu, Z.; Fu, X. A novel polysaccharide from Se-enriched Ganoderma lucidum induces apoptosis of human breast cancer cells. Oncol. Rep. 2010, 25, 267–272. [Google Scholar] [CrossRef]
- Pan, W.L.; Wong, J.H.; Fang, E.F.; Chan, Y.S.; Ye, X.J.; Ng, T.B. Differential inhibitory potencies and mechanisms of the type I ribosome inactivating protein marmorin on estrogen receptor (ER)-positive and ER-negative breast cancer cells. Biochim. Biophys. Acta Bioenerg. 2013, 1833, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Wodetzki, D.; Thorburn, A. Autophagy and cancer: Modulation of cell death pathways and cancer cell adaptations autophagy and cancer. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Padman, B.S.; Usher, J.; Oorschot, V.; Ramm, G.; Lazarou, M. Atg8 family LC3/GABARAP proteins are crucial for autophagosome–lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 2016, 215, 857–874. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Oliveira, M.; Reis, F.S.; Sousa, D.; Tavares, C.; Lima, R.T.; Ferreira, I.C.; Dos Santos, T.; Vasconcelos, M.H. A methanolic extract of Ganoderma lucidum fruiting body inhibits the growth of a gastric cancer cell line and affects cellular autophagy and cell cycle. Food Funct. 2014, 5, 1389–1394. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, C.-Y.; Lee, K.-R.; Lin, H.-J.; Lin, W.-C.; Chen, T.-H.; Wan, L. Cold-water extracts of Grifola frondosa and its purified active fraction inhibit hepatocellular carcinoma in vitro and in vivo. Exp. Biol. Med. 2016, 241, 1374–1385. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Jedinak, A.; Nguyen, H.; Terry, C.; Baldridge, L.A.; Jiang, J.; Sliva, D. Triterpenes from Ganoderma lucidum induce autophagy in colon cancer through the inhibition of p38 mitogen-activated kinase (p38 MAPK). Nutr. Cancer 2010, 62, 630–640. [Google Scholar] [CrossRef]
- Hsin, I.-L.; Ou, C.-C.; Wu, T.-C.; Jan, M.-S.; Wu, M.-F.; Chiu, L.-Y.; Lue, K.-H.; Ko, J.-L. GMI, an immunomodulatory protein from Ganoderma microsporum, induces autophagy in non-small cell lung cancer cells. Autophagy 2011, 7, 873–882. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237, 116113. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, Y.; Na, K.; Wang, Y.; Wang, L.; Li, Z.; Guo, C.; Guo, D.; Wang, X. Autophagic flux disruption contributes to Ganoderma lucidum polysaccharide-induced apoptosis in human colorectal cancer cells via MAPK/ERK activation. Cell Death Disease 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Stanley, G.; Harvey, K.; Slivova, V.; Jiang, J.; Sliva, D. Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-β1 from prostate cancer cells. Biochem. Biophys. Res. Commun. 2005, 330, 46–52. [Google Scholar] [CrossRef]
- Ruma, I.; Putranto, E.W.; Kondo, E.; Watanabe, R.; Saito, K.; Inoue, Y.; Yamamoto, K.-I.; Nakata, S.; Kaihata, M.; Murata, H.; et al. Extract of Cordyceps militaris inhibits angiogenesis and suppresses tumor growth of human malignant melanoma cells. Int. J. Oncol. 2014, 45, 209–218. [Google Scholar] [CrossRef]
- Zou, Y.; Xiong, H.; Xiong, H.; Lu, T.; Zhu, F.; Luo, Z.; Yuan, X.; Wang, Y. A polysaccharide from mushroom Huaier retards human hepatocellular carcinoma growth, angiogenesis, and metastasis in nude mice. Tumor Biol. 2015, 36, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Pantel, K.; Kang, Y. Tumor metastasis: Moving new biological insights into the clinic. Nat. Med. 2013, 19, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Hsu, H.-Y. Ling Zhi-8 reduces lung cancer mobility and metastasis through disruption of focal adhesion and induction of MDM2-mediated Slug degradation. Cancer Lett. 2016, 375, 340–348. [Google Scholar] [CrossRef]
- Wang, N.; Liu, D.; Guo, J.; Sun, Y.; Guo, T.; Zhu, X. Molecular mechanism of Poria cocos combined with oxaliplatin on the inhibition of epithelial-mesenchymal transition in gastric cancer cells. Biomed. Pharmacother. 2018, 102, 865–873. [Google Scholar] [CrossRef]
- John, A.; Tuszynski, G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001, 7, 14–23. [Google Scholar] [CrossRef]
- Weng, C.J.; Chau, C.F.; Yen, G.C.; Liao, J.W.; Chen, D.H.; Chen, K.D. Inhibitory effects of Ganoderma lucidum on tumorigenesis and metastasis of human hepatoma cells in cells and animal models. J. Agric. food Chem. 2009, 57, 5049–5057. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, M.; Furuya, S.; Fujishige, K.; Sugita, T. Highly sensitive cell-based assay system to monitor the sialyl Lewis X biosynthesis mediated by α1-3 fucosyltransferase-VII. Biochem. Biophys. Res. Commun. 2004, 324, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, L.; Zhang, C.; Fan, L.; Zhou, L.; Lin, Y.; Niu, Y.; Li, X.; Wen, X.; Sun, Y. A polysaccharide isolated from Agaricus blazei Murill inhibits sialyl Lewis X/E-selectin-mediated metastatic potential in HT-29 cells through down-regulating α-1, 3-fucosyltransferase-VII (FucT-VII). Carbohydr. Polym. 2010, 79, 921–926. [Google Scholar] [CrossRef]
- Hetland, G.; Tangen, J.-M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjønnfjord, G.E.; Johnson, E. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom extract and the related medicinal basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A Review of preclinical and clinical studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Borchers, A.T.; Krishnamurthy, A.; Keen, C.L.; Meyers, F.J.; Gershwin, M.E. The Immunobiology of mushrooms. Exp. Biol. Med. 2008, 233, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Zhang, H.; Yang, G.; Hao, M.; Sheng, S.; Sun, Y.; Long, J.; Hu, C.; Sun, X.; et al. A Huaier polysaccharide inhibits hepatocellular carcinoma growth and metastasis. Tumor Biol. 2015, 36, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, W.; Jiao, C.; Liang, H.; Yun, H.; He, C.; Chen, J.; Ma, X.; Xie, Y. Anti-tumor and immunomodulatory activity of the aqueous extract of Sarcodon imbricatus in vitro and in vivo. Food Funct. 2020, 11, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Dyck, L.; Mills, K.H. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ishikawa, S.; Matsui, Y.; Tamesada, M.; Harashima, N.; Harada, M. Oral ingestion of Lentinula edodes mycelia extract inhibits B16 melanoma growth via mitigation of regulatory T cell-mediated immunosuppression. Cancer Sci. 2011, 102, 516–521. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2019, 24, 4771–4778. [Google Scholar] [CrossRef]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Targeting cancer. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 163–175. [Google Scholar]
- Opattova, A.; Horak, J.; Vodenkova, S.; Kostovcikova, K.; Cumova, A.; Macinga, P.; Galanova, N.; Rejhova, A.; Vodickova, L.; Kozics, K.; et al. Ganoderma lucidum induces oxidative DNA damage and enhances the effect of 5-Fluorouracil in colorectal cancer in vitro and in vivo. Mutat. Res. Toxicol. Environ. Mutagen. 2019, 845, 403065. [Google Scholar] [CrossRef]
- Wu, J.M.; Hsieh, T.-C. Suppression of proliferation and oxidative stress by extracts of Ganoderma lucidum in the ovarian cancer cell line OVCAR-3. Int. J. Mol. Med. 2011, 28, 1065–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, L.-B.; Chen, B. Bioactivities of water-soluble polysaccharides from Jisongrong mushroom: Anti-breast carcinoma cell and antioxidant potential. Int. J. Biol. Macromol. 2011, 48, 1–4. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Jiang, J.; Hopf, A.; Adamec, J.; Sliva, D. Inhibition of oxidative stress-induced invasiveness of cancer cells by Ganoderma lucidum is mediated through the suppression of interleukin-8 secretion. Int. J. Mol. Med. 2006, 18, 657–664. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: A review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef]
- Cheung, M.K.; Yue, G.G.L.; Chiu, P.W.Y.; Lau, C.B.S. A Review of the effects of natural compounds, medicinal plants, and mushrooms on the gut microbiota in colitis and cancer. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Su, J.; Su, L.; Li, D.; Shuai, O.; Zhang, Y.; Liang, H.; Jiao, C.; Xu, Z.; Lai, Y.; Xie, Y. Antitumor activity of extract from the sporoderm-breaking spore of Ganoderma lucidum: Restoration on exhausted cytotoxic T cell with gut microbiota remodeling. Front. Immunol. 2018, 9, 1765. [Google Scholar] [CrossRef]
- Luo, J.; Li, T.; Xie, J.; Guo, H.; Liu, L.; Zhang, G.; Peng, X. Guar gum different from Ganoderma lucidum polysaccharide in alleviating colorectal cancer based on omics analysis. Food Funct. 2020, 11, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Lei, Y.-H.; Yao, N.; Wang, C.-R.; Hu, N.; Ye, W.-C.; Zhang, D.-M.; Chen, Z.-S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Doğan, H.H.; Kars, M.D.; Özdemir, Ö.; Gündüz, U. Fomes fomentarius and Tricholoma anatolicum (Agaricomycetes) extracts exhibit significant multiple drug-resistant modulation activity in drug-resistant breast cancer cells. Int. J. Med. Mushrooms 2020, 22, 105–114. [Google Scholar] [CrossRef]
- Sadava, D.; Still, D.W.; Mudry, R.R.; Kane, S.E. Effect of Ganoderma on drug-sensitive and multidrug-resistant small-cell lung carcinoma cells. Cancer Lett. 2009, 277, 182–189. [Google Scholar] [CrossRef]
- Chiu, L.Y.; Hu, M.E.; Yang, T.Y.; Hsin, I.L.; Ko, J.L.; Tsai, K.J.; Sheu, G.T. Immunomodulatory protein from Ganoderma microsporum induces pro-death autophagy through Akt-mTOR-p70S6K pathway inhibition in multidrug resistant lung cancer cells. PLoS ONE 2015, 10, e0125774. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-Y.; Hung, Y.-C.; Chen, Y.-H.; Chen, Y.-H.; Huang, Y.-C.; Kao, C.-W.; Su, Y.-L.; Chiu, H.-H.E.; Rau, K.-M. A preliminary randomised controlled study of short-term Antrodia cinnamomea treatment combined with chemotherapy for patients with advanced cancer. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.H.; Lin, L.Z. Effect of Jinshuibao capsule on the immunological function of 36 patients with advanced cancer. Chin. J. Integr. Tradit. West. Med. 1995, 15, 476–478. [Google Scholar]
- Gao, Y.; Zhou, S.; Jiang, W.; Huang, M.; Dai, X. Effects of Ganopoly®(A ganoderma lucidum polysaccharide extract) on the immune functions in Advanced-Stage cancer patients. Immunol. Investig. 2003, 32, 201–215. [Google Scholar] [CrossRef]
- Kodama, N.; Komuta, K.; Nanba, H. Effect of maitake (Grifola frondosa) D-Fraction on the activation of NK cells in cancer pa-tients. J. Med. Food 2003, 6, 371–377. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Miyahara, E.; Hihara, J. Efficacy and safety of orally administered Lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: A pilot study. Am. J. Chin. Med. 2011, 39, 451–459. [Google Scholar] [CrossRef]
- Suzuki, N.; Takimoto, Y.; Suzuki, R.; Arai, T.; Uebaba, K.; Nakai, M.; Strong, J.M.; Tokuda, H. Efficacy of oral administration of Lentinula eododes mycelia extract for breast cancer patients undergoing postoperative hormone therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3469–3472. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom polysaccharide lentinan for treating different types of cancers: A review of 12 years clinical studies in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 297–328. [Google Scholar] [CrossRef] [PubMed]
- LY Eliza, W.; K Fai, C.; P Chung, L. Efficacy of Yun Zhi (Coriolus versicolor) on survival in cancer patients: Systematic review and meta-analysis. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-D.; Cheng, K.-C. From nutraceutical to clinical trial: Frontiers in Ganoderma development. Appl. Microbiol. Biotechnol. 2018, 102, 9037–9051. [Google Scholar] [CrossRef] [PubMed]

| Species | Genus | Family | Order | Class | Phylum | Kingdom | Synonyms | Common Names |
|---|---|---|---|---|---|---|---|---|
| Ganoderma lucidum (Curtis) P. Karst. | Ganoderma | Ganodermataceae | Polyporales | Agaricomycetes | Basidiomycota | Fungi | Boletus lucidus Curtis Polyporus lucidus (Curtis) Fr. | Lingzhi (In Chinese) Reshi mushroom (In Japanese) |
| Ganoderma tsugae Murrill | Fomes tsugae (Murrill) Sacc. and D. Sacc. Polyporus tsugae (Murrill) Overh. | Hemlock varnish shelf | ||||||
| Ganoderma microsporum R.S. Hseu | N.A. | N.A. | ||||||
| Grifola frondosa (Dicks.) Gray | Grifola | Grifolaceae | Boletus frondosus Dicks. Polyporus frondosus (Dicks.) Fr. | Huishuhua (In Chinese) Maitake (In Japanese) | ||||
| Trametes versicolor (L.) Lloyd | Trametes | Polyporaceae | Boletus versicolor L. Coriolus versicolor (L.) Quél. | Yunzhi (In Chinese) Turkey tail (Common name) | ||||
| Trametes robiniophila Murrill | Perenniporia robiniophila (Murrill) Ryvarden Poria robiniophila (Murrill) Ginns | Huaier (In Chinese) | ||||||
| Fomes fomentarius (L.) Fr. | Fomes | Agaricus fomentarius (L.) Lam. Boletus fomentarius L. | Tinder fungus (Common name) Hoof fungus (Common name) Ice man fungus (Common name) | |||||
| Macrohyporia cocos (Schwein.) I. Johans. and Ryvarden | Macrohyporia | Poria cocos (Schwein.) F.A. Wolf Sclerotium cocos Schwein. | Fuling (In Chinese) Hoelen (Common name) | |||||
| Lentinus squarrosulus Mont. | Lentinus | Pleurotus squarrosulus (Mont.) Singer Lentinus crenulatus Massee | N.A. | |||||
| Agaricus blazei Murrill | Agaricus | Agaricaceae | Agaricales | N.A. | Jisongrong (In Chinese) Himematsutake (In Japanese) God’s mushroom (Common name) | |||
| Lentinula edodes (Berk.) Pegler | Lentinula | Omphalotaceae | Collybia shiitake J.Schröt. Agaricus edodes Berk. | Xianggu (In Chinese) Shiitake (In Japanese) Black mushroom (Common name) | ||||
| Hypholoma lateritium (Schaeff.) P. Kumm. | Hypholoma | Strophariaceae | Deconica squamosa Cooke Geophila sublateritia (Fr.) Quél. | Brick cap (Common name) Red woodlover (Common name) | ||||
| Schizophyllum commune Fr. | Schizophyllum | Schizophyllaceae | Agaricus alneus L. Agaricus multifidus Batsch | Split gill mushroom | ||||
| Hypsizygus marmoreus (Peck) H.E. Bigelow | Hypsizygus | Lyophyllaceae | Clitocybe marmorea (Peck) Sacc. Agaricus marmoreus Peck | Beech mushroom (Common name) | ||||
| Leucopaxillus giganteus (Sowerby) Singer | Leucopaxillus | Tricholomataceae | Agaricus giganteus Sowerby Paxillus giganteus (Sowerby) Fr. | Giant leucopax (Common name) | ||||
| Flammulina velutipes (Curtis) Singer | Flammulina | Physalacriaceae | Collybia eriocephala Rea Agaricus atropes Schumach. | Velvet shank (Common name) Golden needle mushroom (In Chinese) Enokitake (In Japanese) | ||||
| Omphalotus illudens (Schwein.) Bresinsky and Besl | Omphalotus | Omphalotaceae | Clitocybe illudens (Schwein.) Sacc. Panus illudens (Schwein.) Fr. | Eastern jack-o’lantern mushroom (Common name) | ||||
| Tricholoma anatolicum H.H. Doğan and Intini | Tricholoma | Tricholomataceae | N.A. | Katran Mantari (In Turkish) | ||||
| Suillus placidus (Bonord.) Singer | Suillus | Suillaceae | Boletales | Gyrodon fusipes (Fr.) Sacc. Gyrodon placidus (Bonord.) Fr. | Slippery white bolete | |||
| Sarcodon imbricatus (L.) P. Karst. | Sarcodon | Bankeraceae | Thelephorales | Hydnum imbricatum L. Sarcodon gracilis (Fr.) Quél. | Shingled hedgehog (Common name) Scaly hedgehog (Common name) | |||
| Cordyceps militaris (L.) Fr. | Cordyceps | Cordycipitaceae | Hypocreales | Sordariomycetes | Ascomycota | Clavaria granulosa Bull. Hypoxylon militare (L.) Mérat | N.A. | |
| Ophiocordyceps sinensis (Berk.) G.H. Sung, J.M. Sung, Hywel-Jones and Spatafora | Ophiocordyceps | Ophiocordycipitaceae | Cordyceps sinensis (Berk.) Sacc. Sphaeria sinensis Berk | Dongchongxiacao (In Chinese) Yartsa gunbu (Common name) | ||||
| N.A. represents not available | ||||||||
| Species | Compound Name | Structure | Anti-tumour Bioactivity | Reference |
|---|---|---|---|---|
| Agaricus blazei | Agaritine |  | Induction of intrinsic apoptosis | [9] |
| Ganoderma lucidum | Ganoderic acid DM |  | Induction of G1-phase cell cycle arrest; induction of apoptosis | [10] |
| Ganoderic acid T |  | Induction of apoptosis; regulation of oxidative stress | [11] | |
| Cordyceps militaris | Cordycepin |  | Induction of S-phase cell cycle arrest; inhibition of autophagy; reduction in cancer metastasis | [12,13,14] |
| Suillus placidus | Suillin |  | Induction of extrinsic apoptosis | [15] |
| Poria cocos | Poricotriol A |  | Induction of intrinsic apoptosis through caspase-independent pathway | [16] |
| Leucopaxillus giganteus | Clitocine |  | Reversion of multidrug resistance (MDR) | [17] |
| Lentinula edodes | Lentinan |  | Improvement in quality of life of cancer patients | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, R.; Liu, M.; Nik Nabil, W.N.; Xi, Z.; Xu, H. Mycomedicine: A Unique Class of Natural Products with Potent Anti-tumour Bioactivities. Molecules 2021, 26, 1113. https://doi.org/10.3390/molecules26041113
Dai R, Liu M, Nik Nabil WN, Xi Z, Xu H. Mycomedicine: A Unique Class of Natural Products with Potent Anti-tumour Bioactivities. Molecules. 2021; 26(4):1113. https://doi.org/10.3390/molecules26041113
Chicago/Turabian StyleDai, Rongchen, Mengfan Liu, Wan Najbah Nik Nabil, Zhichao Xi, and Hongxi Xu. 2021. "Mycomedicine: A Unique Class of Natural Products with Potent Anti-tumour Bioactivities" Molecules 26, no. 4: 1113. https://doi.org/10.3390/molecules26041113
APA StyleDai, R., Liu, M., Nik Nabil, W. N., Xi, Z., & Xu, H. (2021). Mycomedicine: A Unique Class of Natural Products with Potent Anti-tumour Bioactivities. Molecules, 26(4), 1113. https://doi.org/10.3390/molecules26041113







