Poly(Ethylene Glycol)-b-Poly(D,L-Lactide) Nanoparticles as Potential Carriers for Anticancer Drug Oxaliplatin
Abstract
1. Introduction
2. Results and Discussion
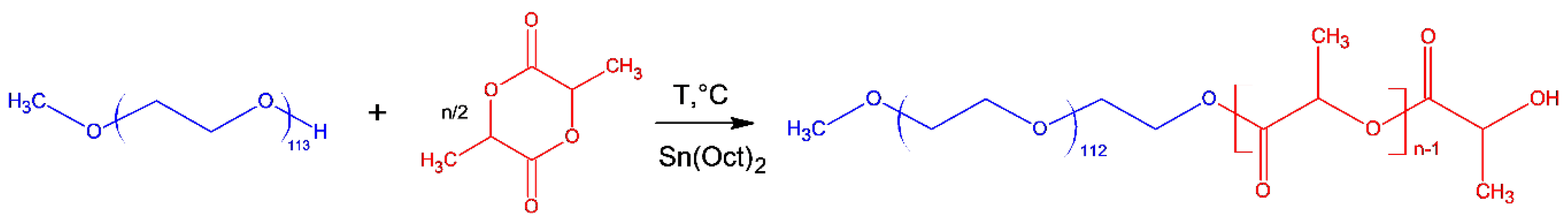
2.1. Synthesis of mPEG-b-P(D,L)LA Copolymers
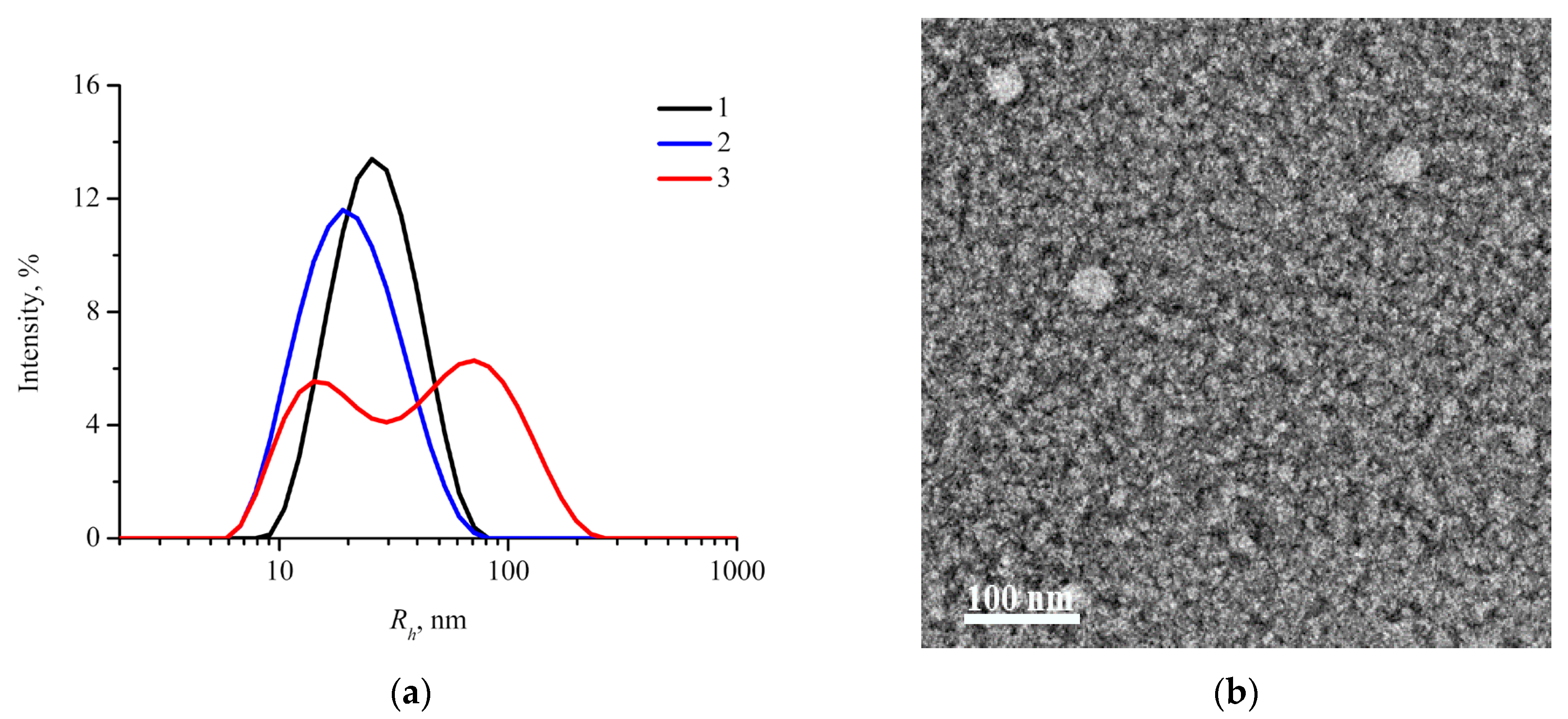
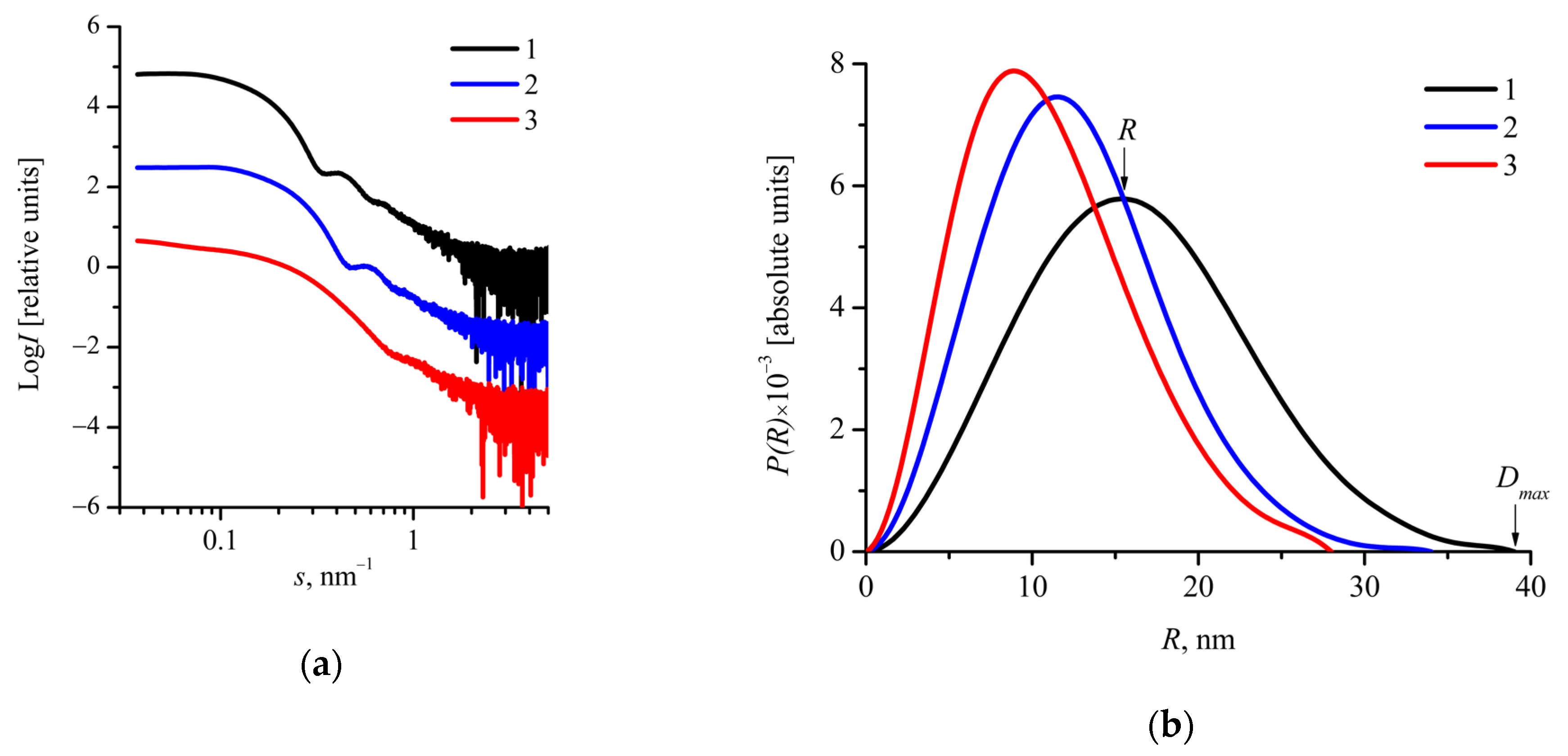
2.2. Characterization of Drug-Free mPEG-b-(D,L)LA Nanoparticles
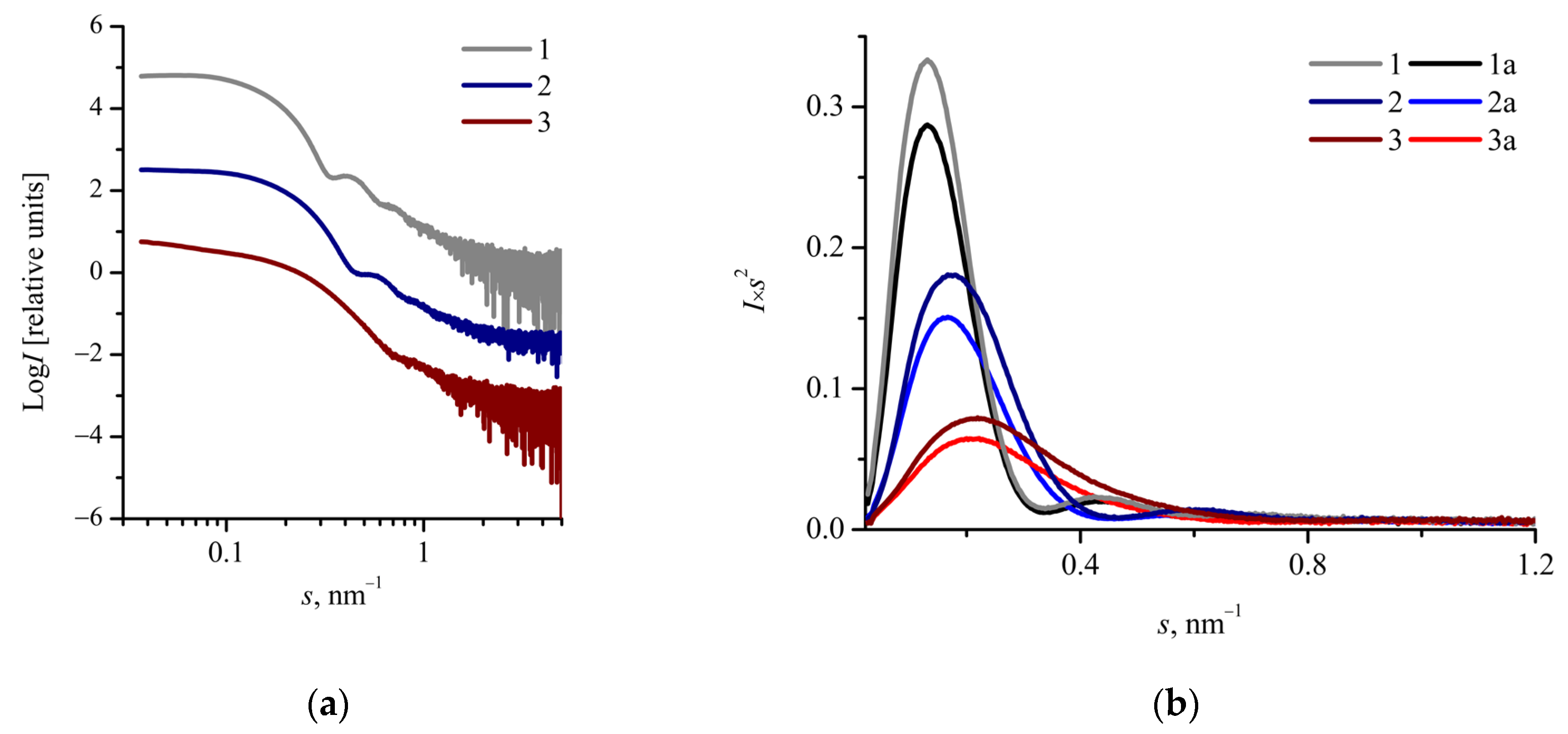
2.3. Characterization of Oxaliplatin-Loaded mPEG-b-(D,L)LA Nanoparticles
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Block Copolymers
3.3. Characterization of mPEG-b-P(D,L)LA Copolymers
3.4. Preparation of mPEG-b-P(D,L)LA Nanoparticles
3.5. Characterization of mPEG-b-P(D,L)LA Nanoparticles
3.6. Evaluation of Drug Loading
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Majumder, N.; Das, G.N. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Froiio, F.; Lammari, N. Chapter 16—Polymer-based nanocontainers for drug delivery. In Smart Nanocontainers, 1st ed.; Nguyen-Tri, P., Do, T., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 271–285. [Google Scholar]
- Li, Z.; Tan, S. Cancer drug delivery in the nano era: An overview and perspectives (Review). Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Khoury, A.; Deo, K.M. Recent advances in platinum-based chemotherapeutics that exhibit inhibitory and targeted mechanisms of action. J. Inorg. Biochem. 2020, 207, 111070. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Aquib, M. Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: An overview. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1674–1692. [Google Scholar] [CrossRef] [PubMed]
- Hang, Z.; Cooper, M.A. Platinum-based anticancer drugs encapsulated liposome and polymeric micelle formulation in clinical trials. Biochem. Compd. 2016, 4. [Google Scholar] [CrossRef]
- Browning, R.J.; Reardon, P.J.T. Drug delivery strategies for platinum-based chemotherapy. ACS Nano 2017, 11, 8560–8578. [Google Scholar] [CrossRef]
- Brown, S.D.; Nativo, P. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef]
- Ho, M.N.; Bach, L.G. PEGylated PAMAM dendrimers loading oxaliplatin with prolonged release and high payload without burst effect. Biopolymers 2019, 110, e23272. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Nukolova, N.V. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv. Rev. 2013, 65, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Sun, Y. Preparation and evaluations in vitro of oxaliplatin polylactic acid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2013, 41, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, H.S.; Nukolova, N.V. Preparation and in vivo evaluation of dichloro(1,2-Diaminocyclohexane)platinum(II)-loaded core cross-linked polymer micelles. Chemother. Res. Pract. 2012, 1–10. [Google Scholar] [CrossRef]
- Wei, H.; Xu, S. Preliminary pharmacokinetics of PEGylated oxaliplatin polylactic acid nanoparticles in rabbits and tumor-bearing mice. Artif. Cells Nanomed. Biotechnol. 2015, 43, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, X. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur. J. Pharm. Sci. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Stathopoulos, G.P.; Boulikas, T. Liposomal oxaliplatin in the treatment of advanced cancer: A phase I study. Anticancer Res. 2006, 26, 1489–1893. [Google Scholar]
- Senzer, N.N.; Matsuno, K. Abstract C36: MBP-426, a novel liposome-encapsulated oxaliplatin, in combination with 5-FU/leucovorin (LV): Phase I results of a Phase I/II study in gastro-esophageal adenocarcinoma, with pharmacokinetics. Mol. Cancer Ther. 2009, 8, C36. [Google Scholar] [CrossRef]
- Perez-Soler, R. Liposomes as carriers of antitumor agents: Toward a clinical reality. Cancer Treat. Rev. 1989, 16, 67–82. [Google Scholar] [CrossRef]
- Cabral, H.; Nishiyama, N. Preparation and biological properties of dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt)-loaded polymeric micelles. J. Control. Release 2005, 101, 223–232. [Google Scholar] [CrossRef]
- Cabral, H.; Nishiyama, N. Optimization of (1,2-diamino-cyclohexane)platinum(II)-loaded polymeric micelles directed to improved tumor targeting and enhanced antitumor activity. J. Control. Release. 2007, 121, 146–155. [Google Scholar] [CrossRef]
- Liu, G.; Gao, H. DACHPt-loaded unimolecular micelles based on hydrophilic dendritic block copolymers for enhanced therapy of lung cancer. ACS Appl. Mater. Interfaces 2017, 9, 112–119. [Google Scholar] [CrossRef]
- Xiao, H.; Zhou, D. A complex of cyclohaxane-1,2-diaminoplatinum with an amphiphilic biodegradable polymer with pendant carboxyl groups. Acta Biomater. 2012, 8, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, X. Biological characterization of folate-decorated biodegradable polymer-platinum(II) complex micelles. Mol. Pharm. 2012, 9, 3200–3208. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pramanick, S. Polymeric biomaterials for the delivery of platinum-based anticancer drugs. Biomater. Sci. 2015, 3, 1002–1017. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Du, Y.-Z. Improved cytotoxicity and multidrug resistance reversal of chitosan based polymeric micelles encapsulating oxaliplatin. J. Drug Target. 2011, 19, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Capuano, G. Different insight into amphiphilic PEG-PLA copolymers: Influence of macromolecular architecture on the micelle formation and cellular uptake. Biomacromolecules 2014, 15, 403–415. [Google Scholar] [CrossRef]
- Jelonek, K.; Li, S. Self-assembled filomicelles prepared from polylactide/poly(ethylene glycol) block copolymers for anticancer drug delivery. Int. J. Pharm. 2015, 485, 357–364. [Google Scholar] [CrossRef]
- Kapse, A.; Anup, N. Chapter 6—Polymeric micelles: A ray of hope among new drug delivery systems. In Drug Delivery Systems, 1st ed.; Tekade, R.K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 235–289. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Govender, T.; Riley, T. Defining the drug incorporation properties of PLA-PEG nanoparticles. Int. J. Pharm. 2000, 199, 95–110. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, S.-S. Methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) nanoparticles for controlled delivery of anticancer drugs. Biomaterials 2004, 25, 2843–2849. [Google Scholar] [CrossRef]
- Dionzou, M.; Morère, A. Comparison of methods for the fabrication and the characterization of polymer self-assemblies: What are the important parameters? Soft Matter. 2016, 12, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, M.; Kalkowski, J. Core-shell structure and aggregation number of micelles composed of amphiphilic block copolymers and amphiphilic heterografted polymer brushes determined by small-angle X-ray scattering. ACS Macro Lett. 2017, 6, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Kelley, E.G.; Murphy, R.P. Size evolution of highly amphiphilic macromolecular solution assemblies via a distinct bimodal pathway. Nat. Commun. 2014, 5, 3599. [Google Scholar] [CrossRef] [PubMed]
- Riley, T.; Heald, C.R. Core-shell structure of PLA-PEG nanoparticles used for drug delivery. Langmuir 2003, 19, 8428–8435. [Google Scholar] [CrossRef]
- Ma, C.; Pan, P. Core-shell structure, biodegradation, and drug release behavior of poly(lactic acid)/poly(ethylene glycol) block copolymer micelles tuned by macromolecular stereostructure. Langmuir 2015, 31, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Razuvaeva, E.V.; Kulebyakina, A.I. Effect of composition and molecular structure of poly(L-lactic acid)/poly(ethylene oxide) block copolymers on micellar morphology in aqueous solution. Langmuir 2018, 34, 15470–15482. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, Z.L.; Shen, Y. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y. Polymer-drug compatibility: A guide to the development of delivery systems for the anticancer agent, ellipticine. J. Pharm. Sci. 2004, 93, 132–143. [Google Scholar] [CrossRef]
- Lin, W.-J.; Juang, L.-W. Stability and release performance of a series of pegylated copolymeric micelles. Pharm. Res. 2003, 20, 668–673. [Google Scholar] [CrossRef]
- Abyaneh, H.S.; Vakili, M.R. Rational design of block copolymer micelles to control burst drug release at a nanoscale dimension. Acta Biomater. 2015, 24, 127–139. [Google Scholar] [CrossRef]
- Zhang, X.; Jackson, J.K. Development of amphiphilic diblock copolymers as micellar carriers of taxol. Int. J. Pharm. 1996, 132, 195–206. [Google Scholar] [CrossRef]
- Shuai, X.; Merdan, T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjug. Chem. 2004, 15, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Hua, A. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J. Control. Rel. 2004, 98, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, A.; Duda, A. Kinetics and mechanism of cyclic esters polymerization initiated with tin(II) octoate. 3. Polymerization of L,L-dilactide. Macromolecules 2000, 33, 7359–7370. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Akiba, I.; Terada, N. Encapsulation of a hydrophobic drug into a polymer-micelle core explored with synchrotron SAXS. Langmuir 2010, 26, 7544–7551. [Google Scholar] [CrossRef]
- Kikhney, A.G.; Svergun, D.I. A practical guide to small angle X-ray scattering (SAXS) of flexible and intrinsically disordered proteins. FEBS Lett. 2015, 589, 2570–2577. [Google Scholar] [CrossRef]
- Svergun, D.I.; Koch, M.H.J. Small-angle scattering studies of biological macromolecules in solution. Rep. Prog. Phys. 2003, 66, 1735–1782. [Google Scholar] [CrossRef]
- Hussain, H.; Tan, B.H. Synthesis, micelle formation, and bulk properties of poly(ethylene glycol)-b-poly(pentafluorostyrene)-g-polyhedral oligomeric silsesquioxane amphiphilic hybrid copolymers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 152–163. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.-Q. Micellization phenomena of amphiphilic block copolymers based on methoxy poly(ethylene glycol) and either crystalline or amorphous poly(caprolactone-b-lactide). Biomacromolecules 2006, 7, 2492–2500. [Google Scholar] [CrossRef]
- Theerasilp, M.; Nasongkla, N. Comparative studies of poly(ε-caprolactone) and poly(D,L-Lactide) as core materials of polymeric micelles. J. Microencapsul. 2013, 30, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Matsumoto, Y. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumors depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, N.; Savino, S. Encapsulation of lipophilic kiteplatin Pt(IV) prodrugs in PLGA-PEG nanoparticles. Dalton Trans. 2016, 45, 13070–13081. [Google Scholar] [CrossRef] [PubMed]
- Al-Allaf, T.A.; Rashan, L.J. Palladium(II) and platinum(II) complexes of (1R,2R)-(−)-1,2-diaminocyclohexane (DACH) with various carboxylato ligands and their cytotoxicity evaluation. Appl. Organometal. Chem. 2009, 23, 173–178. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, 1–4. [Google Scholar] [CrossRef]
- Konarev, P.V.; Volkov, V.V. PRIMUS: A Windows PC-based system for small-angle scattering data analysis. J. Appl. Cryst. 2003, 36, 1277–1282. [Google Scholar] [CrossRef]
- Franke, D.; Petoukhov, M.V. ATSAS 2.8: A comprehensive data analysis suite for small-angle scattering from macromolecular solutions. J. Appl. Cryst. 2017, 50, 1212–1225. [Google Scholar] [CrossRef]
- Svergun, D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Cryst. 1992, 25, 495–503. [Google Scholar] [CrossRef]
| Sample | Mn1, g/mol | Mn2, g/mol | Mw2, g/mol | PDI 2 |
|---|---|---|---|---|
| mPEG113-b-P(D,L)LA62 | 9500 | 7300 | 10,500 | 1.4 |
| mPEG113-b-P(D,L)LA135 | 14,700 | 9000 | 14,600 | 1.6 |
| mPEG113-b-P(D,L)LA173 | 17,500 | 10,400 | 18,000 | 1.7 |
| Sample | Rh1, nm | Rg2, nm | Rg3, nm | Dmax/24, nm | R5, nm | 2Rg/Dmax | Rg/R | ζ 6, mV |
|---|---|---|---|---|---|---|---|---|
| mPEG113-b- P(D,L)LA173 | 28 ± 10 | 11.4 ± 0.1 | 12.5 ± 0.1 | 19.5 ± 1 | 15.4 ± 1 | 0.64 ± 0.05 | 0.81 ± 0.06 | −14 ± 8 |
| mPEG113-b- P(D,L)LA135 | 22 ± 10 | 10.1 ± 0.1 | 9.7 ± 0.1 | 17 ± 1 | 11.5 ± 1 | 0.57 ± 0.06 | 0.84 ± 0.09 | −15 ± 4 |
| mPEG113-b- P(D,L)LA62 | 16 ± 6 | 9.1 ± 0.1 | 8.6 ± 0.1 | 14 ± 1 | 9 ± 1 | 0.61 ± 0.07 | 0.96 ± 0.11 | −20 ± 4 |
| Sample | Rh1, nm | Rg2, nm | Rg3, nm | Dmax/24, nm | R5, nm | 2Rg/Dmax | Rg/R | ζ 6, mV | DLC 7, wt.% |
|---|---|---|---|---|---|---|---|---|---|
| mPEG113-b- P(D,L)LA173 | 27 ± 10 | 11.1 ± 0.1 | 12.3 ± 0.1 | 19.5 ± 1.0 | 15.3 ± 1.0 | 0.63 ± 0.05 | 0.80 ± 0.07 | −16 ± 9 | 1.5 |
| mPEG113-b- P(D,L)LA135 | 25 ± 12 | 9.4 ± 0.1 | 10.4 ± 0.1 | 17.0 ± 1.0 | 12.2 ± 1.0 | 0.61 ± 0.06 | 0.85 ± 0.08 | −17 ± 4 | 2.3 |
| mPEG113-b- P(D,L)LA62 | 19 ± 8 | 9.3 ± 0.1 | 8.7 ± 0.1 | 14.0 ± 1.0 | 9.2 ± 1.0 | 0.62 ± 0.07 | 0.95 ± 0.11 | −24 ± 8 | 3.8 |
| Sample | Rc1, nm | sint2, nm2/g | σ3,nm−2 | DLC 4, wt.% | EE 5, % |
|---|---|---|---|---|---|
| mPEG113-b-P(D,L)LA173 | 15.3 | 1.5 × 1020 | 1.0 | 1.5 | 30 |
| mPEG113-b-P(D,L)LA135 | 12.2 | 2.0 × 1020 | 0.9 | 2.3 | 46 |
| mPEG113-b-P(D,L)LA62 | 9.2 | 2.7 × 1020 | 1.6 | 3.8 | 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadina, Y.A.; Razuvaeva, E.V.; Streltsov, D.R.; Sedush, N.G.; Shtykova, E.V.; Kulebyakina, A.I.; Puchkov, A.A.; Volkov, D.S.; Nazarov, A.A.; Chvalun, S.N. Poly(Ethylene Glycol)-b-Poly(D,L-Lactide) Nanoparticles as Potential Carriers for Anticancer Drug Oxaliplatin. Molecules 2021, 26, 602. https://doi.org/10.3390/molecules26030602
Kadina YA, Razuvaeva EV, Streltsov DR, Sedush NG, Shtykova EV, Kulebyakina AI, Puchkov AA, Volkov DS, Nazarov AA, Chvalun SN. Poly(Ethylene Glycol)-b-Poly(D,L-Lactide) Nanoparticles as Potential Carriers for Anticancer Drug Oxaliplatin. Molecules. 2021; 26(3):602. https://doi.org/10.3390/molecules26030602
Chicago/Turabian StyleKadina, Yulia A., Ekaterina V. Razuvaeva, Dmitry R. Streltsov, Nikita G. Sedush, Eleonora V. Shtykova, Alevtina I. Kulebyakina, Alexander A. Puchkov, Dmitry S. Volkov, Alexey A. Nazarov, and Sergei N. Chvalun. 2021. "Poly(Ethylene Glycol)-b-Poly(D,L-Lactide) Nanoparticles as Potential Carriers for Anticancer Drug Oxaliplatin" Molecules 26, no. 3: 602. https://doi.org/10.3390/molecules26030602
APA StyleKadina, Y. A., Razuvaeva, E. V., Streltsov, D. R., Sedush, N. G., Shtykova, E. V., Kulebyakina, A. I., Puchkov, A. A., Volkov, D. S., Nazarov, A. A., & Chvalun, S. N. (2021). Poly(Ethylene Glycol)-b-Poly(D,L-Lactide) Nanoparticles as Potential Carriers for Anticancer Drug Oxaliplatin. Molecules, 26(3), 602. https://doi.org/10.3390/molecules26030602







