High-Toughness Poly(lactic Acid)/Starch Blends Prepared through Reactive Blending Plasticization and Compatibilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the PLA/PSt/PEG/CA Sample
2.3. Characterization
2.3.1. Mechanical Property Measurements
2.3.2. Rheological Behavior Analysis
2.3.3. Differential Scanning Calorimeter (DSC)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. X-Ray Diffraction (XRD)
2.3.6. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.7. Hydrophilicity Test
3. Results and Discussions
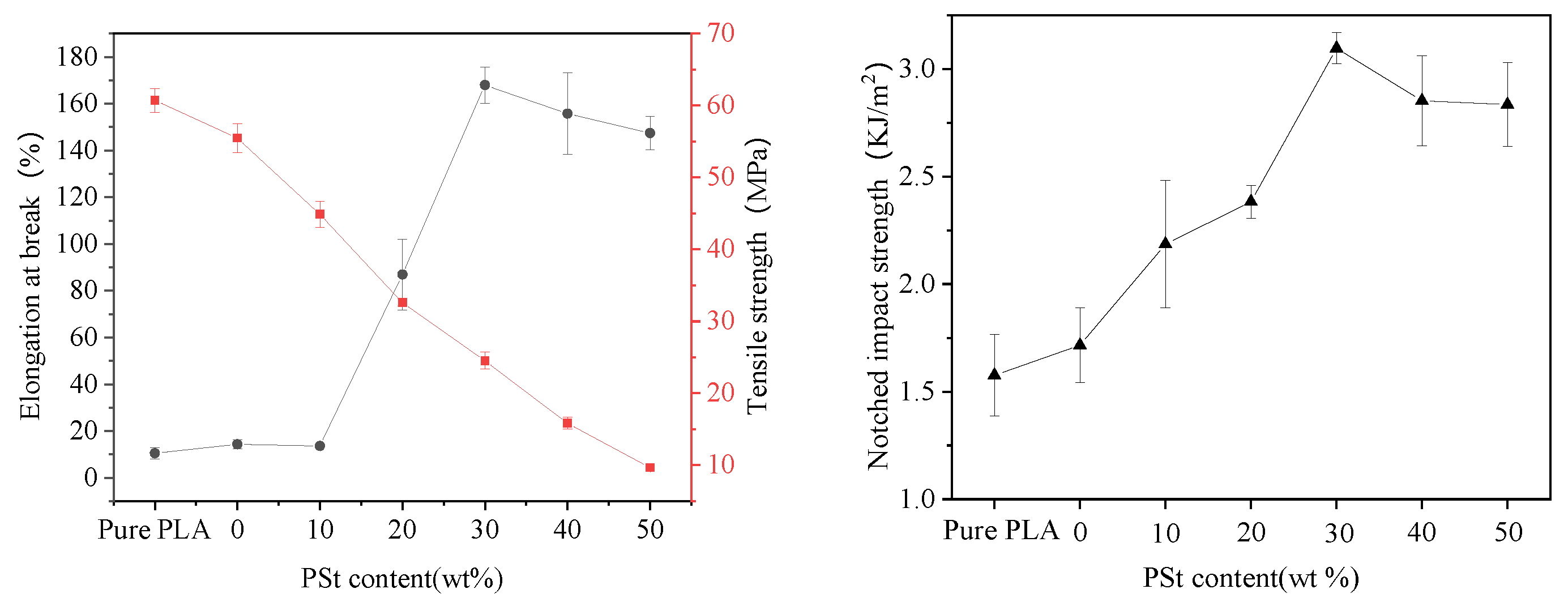
3.1. Mechanical Properties of PLA/PSt/PEG/CA Blends
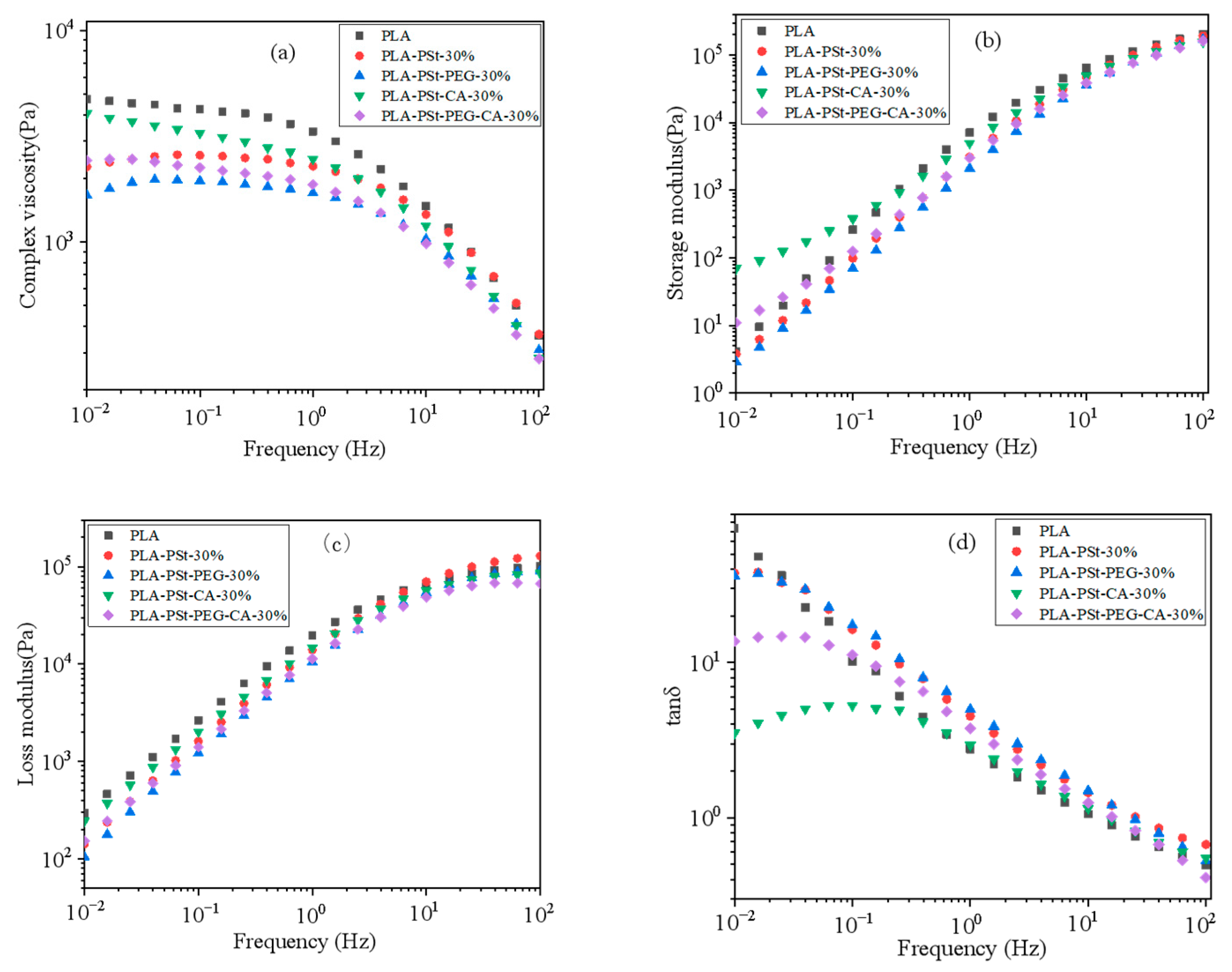
3.2. Rheological Behavior of PLA/PSt/PEG/CA Blends
3.3. Crystallization Behavior of PLA/PSt/PEG/CA Blends
3.4. Morphology of the Dispersed Phase
3.5. Crystallization of the Dispersed Phase
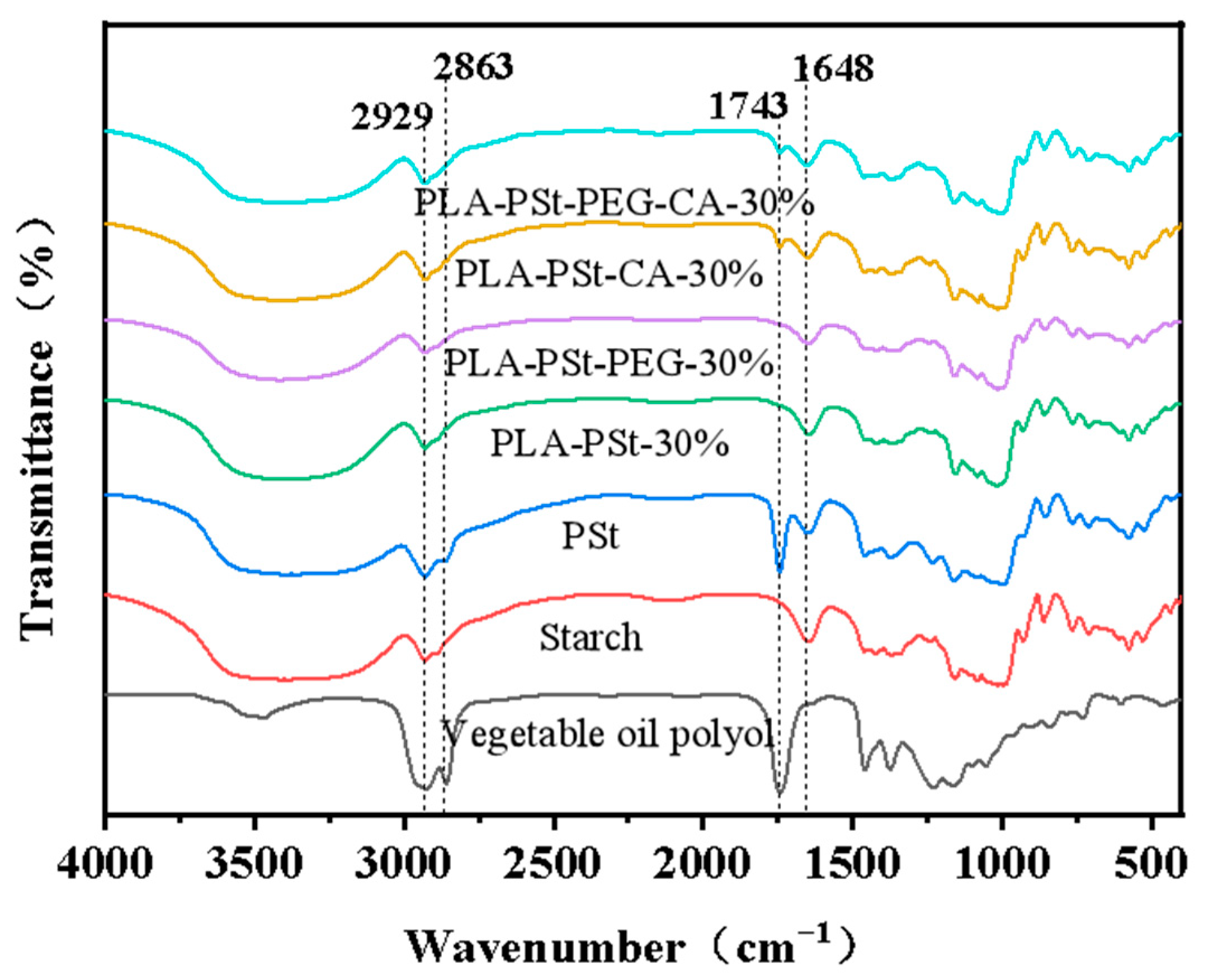
3.6. FTIR Analysis of the Dispersed Phase
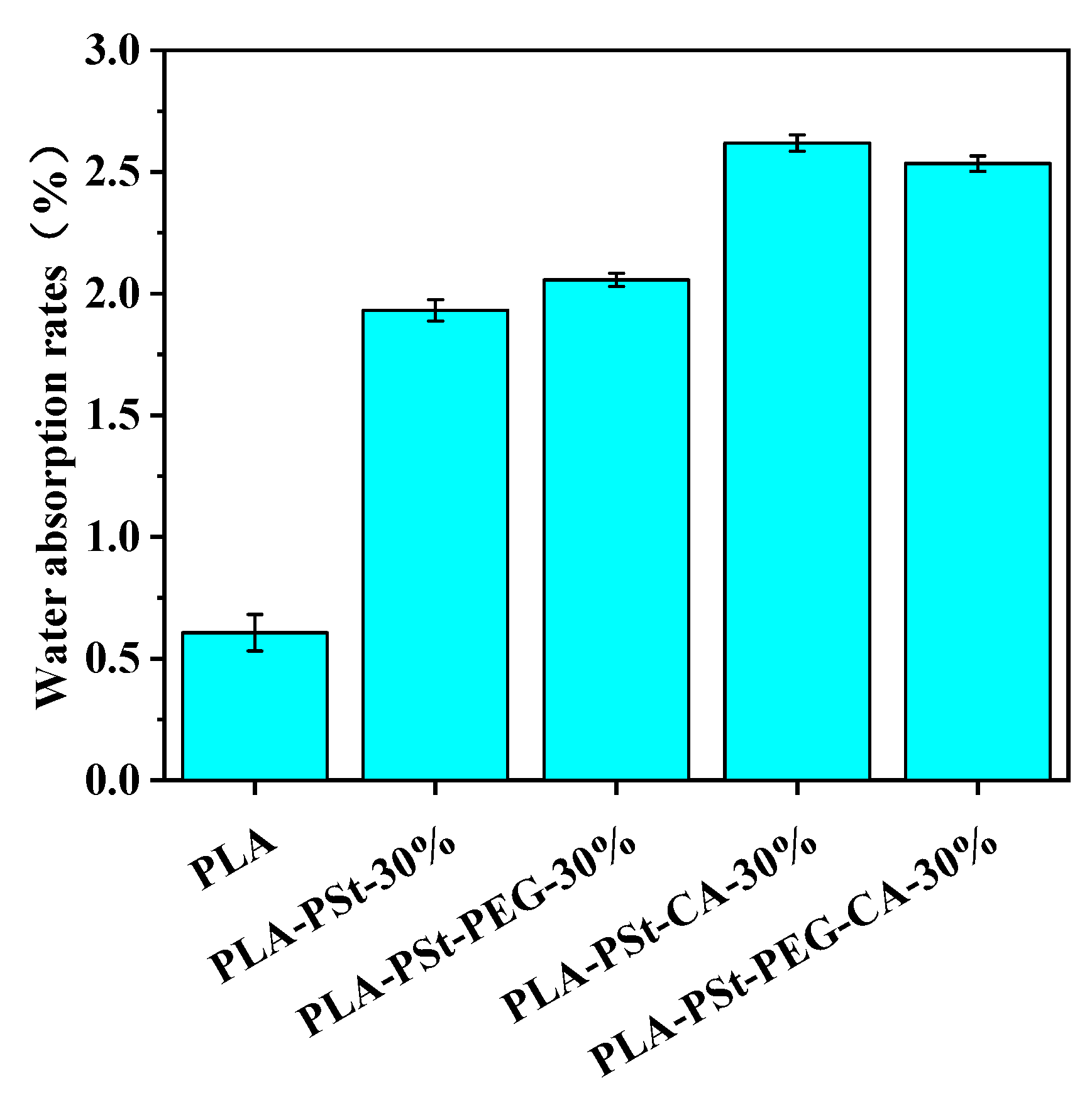
3.7. Hydrophilicity Test of PLA/PSt/PEG/CA Blends
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakdaronnarong, C.; Srimarut, N.; Lucknakhul, N.; Na-songkla, N.; Jonglertjunya, W. Two-step acid and alkaline ethanolysis/alkaline peroxide fractionation of sugarcane bagasse and rice straw for production of polylactic acid precursor. Biochem. Eng. J. 2014, 85, 49–62. [Google Scholar] [CrossRef]
- Tajitsu, Y.; Kawase, Y.; Katsuya, K.; Tamura, M.; Sakamoto, K.; Kawahara, K.; Harada, Y.; Kondo, T.; Imada, Y. New wearable sensor in the shape of a braided cord (Kumihimo). IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 772–777. [Google Scholar] [CrossRef]
- Asmawi, N.N.M.; Sapuan, S.M.; Yusoff, M.Z.M.; Ilyas, R.A.; Sherwani, s.f. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 1–12. [Google Scholar]
- Davachi, S.M.; Kaffashi, B. Polylactic acid in medicine. Polym. Plast. Technol. Eng. 2015, 54, 944–967. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, L.; Ni, Q.; Ruan, F.; Wang, H. Fabrication of high-performance green hemp/polylactic acid fibre composites. J. Eng. Fibers Fabr. 2019, 14, 1558925019834497. [Google Scholar] [CrossRef]
- Zhao, X.P.; Hu, H.; Wang, X.; Yu, X.L.; Zhou, W.Y.; Peng, S.X. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. A review on tensile and morphological properties of poly (lactic acid) (PLA)/thermoplastic starch (TPS) blends. Polym. Plast. Technol. Mater. 2019, 58, 1945–1964. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, X.; Zhu, S.; Huan, Q.; Han, K.; Ma, Y.; Yu, M. Novel toughening mechanism for polylactic acid (PLA)/starch blends with layer-like microstructure via pressure-induced flow (PIF) processing. Mater. Lett. 2013, 98, 238–241. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Cheng, F.; Lin, Y.; Zhou, M.; Zhu, P. Poly(citrate glyceride): A hyperbranched polyester for starch plasticization. Polym. Int. 2018, 67, 399–404. [Google Scholar] [CrossRef]
- Volpe, V.; De Feo, G.; De Marco, I.; Pantani, R. Use of sunflower seed fried oil as an ecofriendly plasticizer for starch and application of this thermoplastic starch as a filler for PLA. Ind. Crop. Prod. 2018, 122, 545–552. [Google Scholar] [CrossRef]
- Koh, J.J.; Zhang, X.; He, C. Fully biodegradable Poly(lactic acid)/Starch blends: A review of toughening strategies. Int. J. Biol. Macromol. 2018, 109, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Q.; Li, T.; Ma, P.; Zhang, S.; Du, M.; Chen, M.; Zhang, H.; Dong, W. Core–Shell Starch Nanoparticles and Their Toughening of Polylactide. Ind. Eng. Chem. Res. 2018, 57, 13048–13054. [Google Scholar] [CrossRef]
- Thielemans, W.; Belgacem, M.N.; Dufresne, A. Starch nanocrystals with large chain surface modifications. Langmuir 2006, 22, 4804–4810. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Dai, X.; Zhang, R.; Tang, Z.; Na, H. Preparation of Biobased Monofunctional Compatibilizer from Cardanol to Fabricate Polylactide/Starch Blends with Superior Tensile Properties. Ind. Eng. Chem. Res. 2014, 53, 10653–10659. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Chirachanchai, S. Silane modified starch for compatible reactive blend with poly(lactic acid). Carbohydr. Polym. 2014, 106, 255–263. [Google Scholar] [CrossRef]
- Meyvazeybek, Y.; Kaynak, C. Loss of thermoplastic elastomer toughening in polylactide after weathering. J. Appl. Polym. Sci. 2019, 136, 47177. [Google Scholar] [CrossRef]
- 17. Halimatul, M.J.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Ilyas, R.A. Water absorption and water solubility properties of sago starch biopolymer composite films filled with sugar palm particles. Polimery 2019, 64, 596–604. [Google Scholar] [CrossRef]
- Ferrarezi, M.M.; Taipina, M.D.; Silva, L.C.; Gonçalves, M.D. Poly(Ethylene Glycol) as a Compatibilizer for Poly(Lactic Acid)/Thermoplastic Starch Blends. J. Polym. Environ. 2013, 21, 151–159. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.X.; Han, N.; Fang, J.M. Effects of Water on the Properties of Thermoplastic Starch Poly(lactic acid) Blend Containing Citric Acid. J. Thermoplast. Compos. Mater. 2010, 23, 19–34. [Google Scholar] [CrossRef]
- Avolio, R.; Castaldo, R.; Gentile, G.; Ambrogi, V.; Fiori, S.; Avella, M.; Cocca, M.; Errico, E. Plasticization of poly(lactic acid) through blending with oligomers of lactic acid: Effect of the physical aging on properties. Eur. Polym. J. 2015, 66, 533–542. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Zhang, Z.; Jiangming, F. High-performance fully bio-based poly (lactic acid)/polyamide11 (PLA/PA11) blends by reactive blending with multi-functionalized epoxy. Polym. Test. 2019, 78, 105980. [Google Scholar] [CrossRef]
- Noivoil, N.; Yoksan, R. Oligo (lactic acid)-grafted starch: A compatibilizer for poly (lactic acid)/thermoplastic starch blend. Int. J. Biol. Macromol. 2020, 160, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Aouat, T.; Kaci, M.; Devaux, E.; Campagne, C.; Lopez-Cuesta, J. Morphological, Mechanical, and Thermal Characterization of Poly (Lactic Acid)/Cellulose Multifilament Fibers Prepared by Melt Spinning. Adv. Polym. Technol. 2016, 37, 1193–1205. [Google Scholar] [CrossRef]
- Chotiprayon, P.; Chaisawad, B.; Yoksan, R. Thermoplastic cassava starch/poly(lactic acid) blend reinforced with coir fibres. Int. J. Biol. Macromol. 2020, 156, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liang, J.; Zhang, S.; Zhu, B. Tensile Properties of Polylactide/Poly(ethylene glycol) Blends. J. Polym. Environ. 2015, 23, 407–415. [Google Scholar] [CrossRef]
- Wu, X.S.; Wu, X.S. Effect of Glycerin and Starch Crosslinking on Molecular Compatibility of Biodegradable Poly (lactic acid)-Starch Composites. J. Polym. Environ. 2011, 19, 912–917. [Google Scholar] [CrossRef]
- Li, F.J.; Tan, L.C.; Zhang, S.D.; Zhu, B. Compatibility, steady and dynamic rheological behaviors of polylactide/poly (ethylene glycol) blends. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Fang, H.; Jiang, F.; Wu, Q.; Ding, Y.; Wang, Z. Supertough Polylactide Materials Prepared through In Situ Reactive Blending with PEG-Based Diacrylate Monomer. ACS Appl. Mater. Interfaces 2014, 6, 13552–13563. [Google Scholar] [CrossRef]
- Nouri, S.; Dubois, C.; Lafleur, P.G. Effect of chemical and physical branching on rheological behavior of polylactide. J. Rheol. 2015, 59, 1045–1063. [Google Scholar] [CrossRef]
- Liu, W.; He, S.; Yang, Y. Effect of stereocomplex crystal on foaming behavior and sintering of poly(lactic acid) bead foams. Polym. Int. 2019, 68, 516–526. [Google Scholar] [CrossRef]
- Cai, J.; Liu, M.; Wang, L.; Yao, K.; Xiong, H. Isothermal crystallization kinetics of thermoplastic starch/poly(lactic acid) composites. Carbohydr. Polym. 2011, 86, 941–947. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Yuan, Y.; Peng, S.; Zhao, X. Synergistic effects of polyethylene glycol and organic montmorillonite on the plasticization and enhancement of poly(lactic acid). J. Appl. Polym. Sci. 2019, 136, 47576. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Yang, L.; Qiao, Z.; Tan, H.; Zhang, Y. Preparation and characterization of dry method esterified starch/polylactic acid composite materials. Int. J. Biol. Macromol. 2014, 64, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wootthikanokkhan, J.; Kasemwananimit, P.; Sombatsompop, N.; Kositchaiyong, A.; Isarankura na Ayutthaya, S.; Kaabbuathong, N. Preparation of modified starch-grafted poly(lactic acid) and a study on compatibilizing efficacy of the copolymers in poly(lactic acid)/thermoplastic starch blends. J. Appl. Polym. Sci. 2012, 126, E389–E396. [Google Scholar] [CrossRef]
- Shirai, M.A.; Grossmann, M.V.E.; Mali, S.; Yamashita, F.; Garcia, P.S.; Müller, C.M.O. Development of biodegradable flexible films of starch and poly(lactic acid) plasticized with adipate or citrate esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Gou, M.; Zhang, G.; Li, W.; Jiang, H. Physical and structural properties of potato starch modified by dielectric treatment with different moisture content. Int. J. Biol. Macromol. 2018, 118, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cheng, Y.; Ren, J.; Cao, E.; Fu, X.; Guo, W. Plasticizing effect of poly(ethylene glycol)s with different molecular weights in poly(lactic acid)/starch blends. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (arenga pinnata) starch for food packaging. J. Food Sci. Technol. Mysore 2016, 53, 326–336. [Google Scholar] [CrossRef]




| Material | PLA (wt%) | PSt (wt%) | PEG (wt%) | CA (wt%) | St (wt%) | VOP (wt%) | |
|---|---|---|---|---|---|---|---|
| Sample | |||||||
| 1 | 100 | - | - | - | - | - | |
| 2 | 100 | - | 1.19 | 0.17 | - | 1.19 | |
| 3 | 90 | 10 | 1.19 | 0.17 | 10 | 1.19 | |
| 4 | 80 | 20 | 1.19 | 0.17 | 20 | 1.19 | |
| 5 | 70 | 30 | 1.19 | 0.17 | 30 | 1.19 | |
| 6 | 60 | 40 | 1.19 | 0.17 | 40 | 1.19 | |
| 7 | 50 | 50 | 1.19 | 0.17 | 50 | 1.19 | |
| 8 | 70 | 30 | - | - | 30 | - | |
| 9 | 70 | 30 | 1.19 | - | 30 | 1.19 | |
| 10 | 70 | 30 | - | 0.17 | - | - | |
| 11 | 100 | - | 1.19 | - | - | - | |
| 12 | 100 | - | - | 0.17 | - | - | |
| 13 | 70 | - | - | - | 30 | - | |
| 14 | 100 | - | - | - | - | 5 | |
| 15 | 70 | - | 1.19 | - | 30 | - | |
| 16 | 70 | - | - | 0.17 | 30 | - | |
| 17 | 100 | - | - | 0.17 | - | 5 | |
| 18 | 70 | - | 1.19 | 0.17 | 30 | - | |
| Materials | Massratio | Tensile Strength/MPa | Elongation at Break/% | Notched Impact Strength/kJ·m−2 |
|---|---|---|---|---|
| Pure PLA | 100 | 70.64 ± 2.5 | 10.45 ± 2.44 | 2.02 ± 0.15 |
| PLA/PSt | 70/30 | 45.65 ± 3.27 | 5.04 ± 0.12 | 2.08 ± 0.12 |
| PLA/PSt/PEG | 70/30/1.19 | 43.5 ± 1.22 | 7.15 ± 1.28 | 2.20 ± 0.05 |
| PLA/PSt/CA | 70/30/0.17 | 28.86 ± 2 | 54.47 ± 9.87 | 3.18 ± 0.01 |
| PLA/PSt/PEG/CA | 70/30/1.19/0.17 | 28.58 ± 1.38 | 140.51 ± 10.41 | 3.56 ± 0.06 |
| PLA/PEG | 100/1.19 | 65.6 ± 3.7 | 7.6 ± 0.67 | - |
| PLA/CA | 100/0.17 | 65.41 ± 2.76 | 8.88 ± 0.76 | - |
| PLA/St | 70/30 | 55.08 ± 4.9 | 5.55 ± 0.17 | - |
| PLA/VOP | 100/5 | 66.52 ± 2.39 | 10.41 ± 0.95 | - |
| PLA/St/PEG | 70/30/1.19 | 49.84 ± 2.33 | 4.41 ± 0.31 | - |
| PLA/St/CA | 70/30/0.17 | 47.66 ± 1.69 | 4.20 ± 0.19 | - |
| PLA/CA/VOP | 100/0.17/5 | 66.78 ± 0.64 | 7.5 ± 0.45 | - |
| PLA/St/PEG/CA | 70/30/1.19/0.17 | 33.48 ± 5.14 | 3.43 ± 0.67 | - |
| Sample Code | Tg | Tc | ΔHc | Tm | ΔHm | Xc |
|---|---|---|---|---|---|---|
| (°C) | (°C) | (J/g) | (°C) | (J/g) | (%) | |
| PLA | 62.68 | 123.63 | −9.07 | 150.05 | 13.09 | 6.14 |
| PLA-PSt-30% | 55.92 | 121.95 | −5.69 | 147.66 | 7.85 | 3.30 |
| PLA-PSt-PEG-30% | 52.97 | 119.05 | −9.94 | 146.54 | 15.04 | 7.78 |
| PLA-PSt-CA-30% | 55.98 | 126.14 | −2.11 | 148.23 | 3.14 | 1.57 |
| PLA-PSt-PEG-CA-30% | 52.80 | 123.24 | −3.64 | 147.01 | 6.01 | 3.62 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Xu, A.; Zhang, D.; Zhou, W.; Peng, S.; Zhao, X. High-Toughness Poly(lactic Acid)/Starch Blends Prepared through Reactive Blending Plasticization and Compatibilization. Molecules 2020, 25, 5951. https://doi.org/10.3390/molecules25245951
Hu H, Xu A, Zhang D, Zhou W, Peng S, Zhao X. High-Toughness Poly(lactic Acid)/Starch Blends Prepared through Reactive Blending Plasticization and Compatibilization. Molecules. 2020; 25(24):5951. https://doi.org/10.3390/molecules25245951
Chicago/Turabian StyleHu, Huan, Ang Xu, Dianfeng Zhang, Weiyi Zhou, Shaoxian Peng, and Xipo Zhao. 2020. "High-Toughness Poly(lactic Acid)/Starch Blends Prepared through Reactive Blending Plasticization and Compatibilization" Molecules 25, no. 24: 5951. https://doi.org/10.3390/molecules25245951
APA StyleHu, H., Xu, A., Zhang, D., Zhou, W., Peng, S., & Zhao, X. (2020). High-Toughness Poly(lactic Acid)/Starch Blends Prepared through Reactive Blending Plasticization and Compatibilization. Molecules, 25(24), 5951. https://doi.org/10.3390/molecules25245951





