T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies
Abstract
:1. Introduction
2. Structure and Physical and Chemical Properties of T-2 Toxin
3. Metabolism of T-2 Toxin
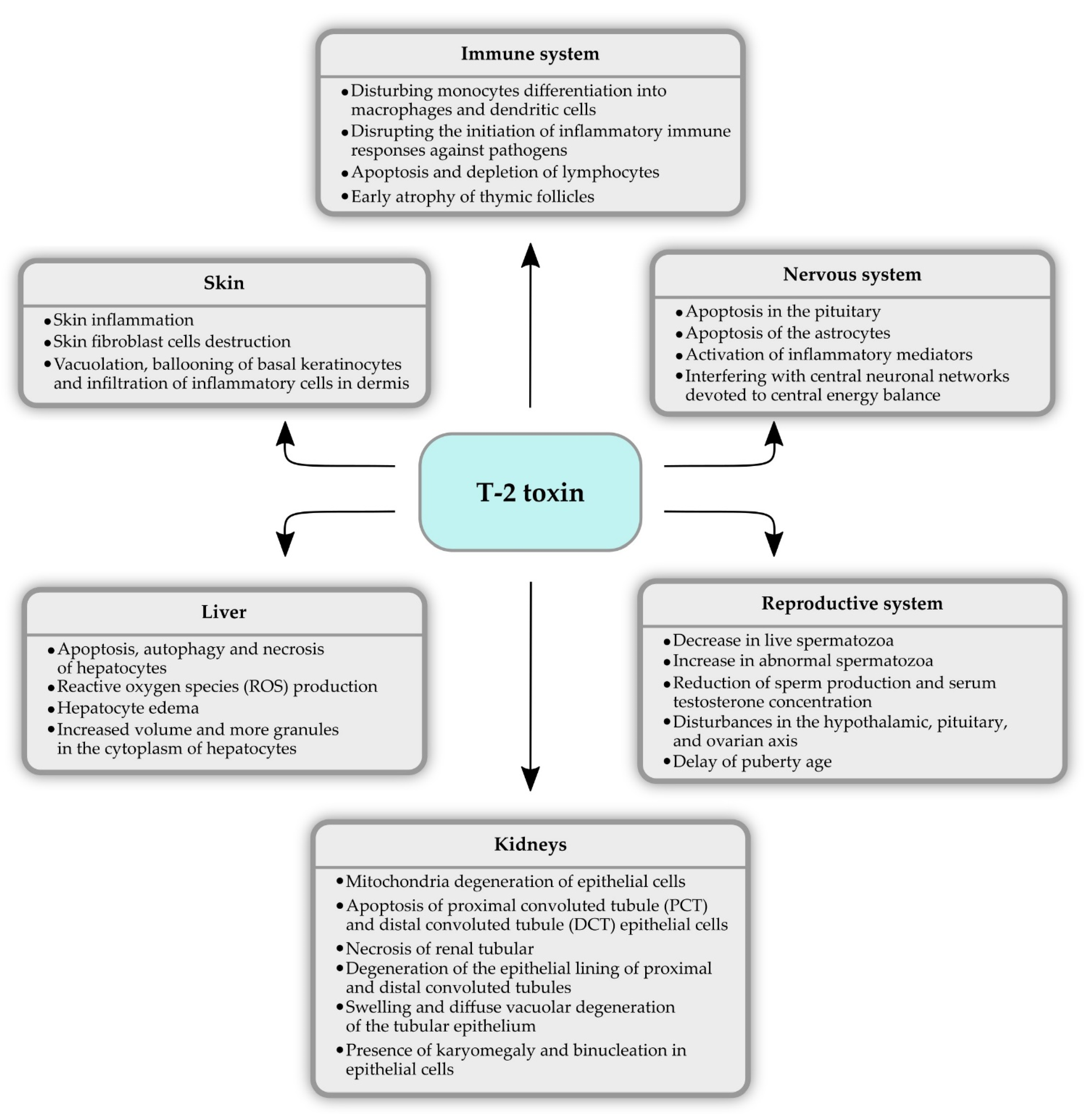
4. T-2 Toxicity
4.1. Hepatotoxicity
4.2. Nephrotoxicity
4.3. Immunotoxicity
4.4. Neurotoxicity
4.5. Reproductive System
4.6. Dermal Toxicity
5. T-2 Degradation and Mitigation Strategies
5.1. Physical Methods
5.2. Chemical Methods
5.3. Biological Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Xiang, B.; Shi, H.; Yu, P.; Song, Y.; Li, S. Recent advances on type A trichothecenes in food and feed: Analysis, prevalence, toxicity, and decontamination techniques. Food Control 2020, 118, 107371. [Google Scholar] [CrossRef]
- Milićević, D.R.; Škrinjar, M.; Baltić, T. Real and Perceived Risks for Mycotoxin Contamination in Foods and Feeds: Challenges for Food Safety Control. Toxins 2010, 2, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains—An Update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef] [Green Version]
- Arunachalam, C.; Doohan, F.M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar] [CrossRef]
- Nielsen, C.; Casteel, M.; Didier, A.; Dietrich, R.; Märtlbauer, E. Trichothecene-induced cytotoxicity on human cell lines. Mycotoxin Res. 2009, 25, 77–84. [Google Scholar] [CrossRef]
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, R.E.; Malmierca, M.G.; Hermosa, M.R.; Alexander, N.J.; McCormick, S.P.; Proctor, R.H.; Tijerino, A.M.; Rumbero, A.; Monte, E.; Gutiérrez, S. Identification of Loci and Functional Characterization of Trichothecene Biosynthesis Genes in Filamentous Fungi of the Genus Trichoderma. Appl. Environ. Microbiol. 2011, 77, 4867–4877. [Google Scholar] [CrossRef] [Green Version]
- Proctor, R.H.; McCormick, S.P.; Kim, H.-S.; Cardoza, R.E.; Stanley, A.M.; Lindo, L.; Kelly, A.; Brown, D.W.; Lee, T.; Vaughan, M.M. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog. 2018, 14, e1006946. [Google Scholar] [CrossRef] [Green Version]
- Foroud, N.A.; Shank, R.A.; Kiss, D.; Eudes, F.; Hazendonk, P. Solvent and Water Mediated Structural Variations in Deoxynivalenol and Their Potential Implications on the Disruption of Ribosomal Function. Front. Microbiol. 2016, 7, 1239. [Google Scholar] [CrossRef]
- Garvey, G.S.; McCormick, S.P.; Rayment, I. Structural and Functional Characterization of the TRI101 Trichothecene 3-O-Acetyltransferase from Fusarium sporotrichioides and Fusarium graminearum: Kinetic insights to combating fusarium head blight. J. Biol. Chem. 2008, 283, 1660–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed. Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Wan, Q.; Wu, G.; He, Q.; Tang, H.; Wang, Y. The toxicity of acute exposure to T-2 toxin evaluated by the metabonomics technique. Mol. BioSystems 2015, 11, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Nayakwadi, S.; Ramu, R.; Kumar Sharma, A.; Kumar Gupta, V.; Rajukumar, K.; Kumar, V.; Shirahatti, P.S.; Rashmi, L.; Basalingappa, K.M. Toxicopathological studies on the effects of T-2 mycotoxin and their interaction in juvenile goats. PLoS ONE 2020, 15, e0229463. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Lancova, K.; Hajslova, J.; Poustka, J.; Krplova, A.; Zachariasova, M.; Dostalek, P.; Sachambula, L. Transfer of Fusarium mycotoxins and ‘masked’ deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit. Contam. Part A 2008, 25, 732–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Male, D.; Wu, W.; Mitchell, N.J.; Bursian, S.; Pestka, J.J.; Wu, F. Modeling the emetic potencies of food-borne trichothecenes by benchmark dose methodology. Food Chem. Toxicol. 2016, 94, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Pascari, X.; Maul, R.; Kemmlein, S.; Marin, S.; Sanchis, V. The fate of several trichothecenes and zearalenone during roasting and enzymatic treatment of cereal flour applied in cereal-based infant food production. Food Control 2020, 114, 107245. [Google Scholar] [CrossRef]
- Cope, R.B. Chapter 75—Trichothecenes. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1043–1053. [Google Scholar]
- Rameshkumar, G.; Sikha, M.; Ponlakshmi, M.; Selva pandiyan, A.; Lalitha, P. A rare case of Myrothecium species causing mycotic keratitis: Diagnosis and management. Med. Mycol. Case Rep. 2019, 25, 53–55. [Google Scholar] [CrossRef]
- He, J.; Zhou, T.; Young, J.C.; Boland, G.J.; Scott, P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trends Food Sci. Technol. 2010, 21, 67–76. [Google Scholar] [CrossRef]
- Meneely, J.P.; Ricci, F.; van Egmond, H.P.; Elliott, C.T. Current methods of analysis for the determination of trichothecene mycotoxins in food. TrAC Trends Anal. Chem. 2011, 30, 192–203. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Zhang, J.; You, L.; Wu, W.; Wang, X.; Chrienova, Z.; Nepovimova, E.; Wu, Q.; Kuca, K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): Current status and future perspectives. Food Chem. Toxicol. 2020, 145, 111676. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Arcella, D.; Gergelova, P.; Innocenti, M.L.; Steinkellner, H. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, e04972. [Google Scholar] [PubMed]
- Zouagui, Z.; Asrar, M.; Lakhdissi, H.; Abdennebi, E.H. Prevention of mycotoxin effects in dairy cows by adding an anti-mycotoxin product in feed. J. Mater. Environ. Sci. 2017, 8, 3766–3770. [Google Scholar]
- Cano-Sancho, G.; Valle-Algarra, F.M.; Jiménez, M.; Burdaspal, P.; Legarda, T.M.; Ramos, A.J.; Sanchis, V.; Marín, S. Presence of trichothecenes and co-occurrence in cereal-based food from Catalonia (Spain). Food Control 2011, 22, 490–495. [Google Scholar] [CrossRef]
- Rosa Seus Arraché, E.; Fontes, M.R.V.; Garda Buffon, J.; Badiale-Furlong, E. Trichothecenes in wheat: Methodology, occurrence and human exposure risk. J. Cereal Sci. 2018, 82, 129–137. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Beier, R.C.; Shen, J.; Smet, D.D.; De Saeger, S.; Zhang, S. T-2 Toxin, a Trichothecene Mycotoxin: Review of Toxicity, Metabolism, and Analytical Methods. J. Agric. Food Chem. 2011, 59, 3441–3453. [Google Scholar] [CrossRef]
- Moss, M.O. Mycotoxin review–2. Fusarium. Mycologist 2002, 16, 158–161. [Google Scholar] [CrossRef]
- Nazari, L.; Pattori, E.; Terzi, V.; Morcia, C.; Rossi, V. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbiol. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Varga, E.; Meng-Reiterer, J.; Bueschl, C.; Michlmayr, H.; Malachova, A.; Fruhmann, P.; Jestoi, M.; Peltonen, K.; Adam, G.; et al. Metabolism of the Fusarium Mycotoxins T-2 Toxin and HT-2 Toxin in Wheat. J. Agric. Food Chem. 2015, 63, 7862–7872. [Google Scholar] [CrossRef]
- Edwards, S.G.; Imathiu, S.M.; Ray, R.V.; Back, M.; Hare, M.C. Molecular studies to identify the Fusarium species responsible for HT-2 and T-2 mycotoxins in UK oats. Int. J. Food Microbiol. 2012, 156, 168–175. [Google Scholar] [CrossRef]
- Lippolis, V.; Pascale, M.; Maragos, C.M.; Visconti, A. Improvement of detection sensitivity of T-2 and HT-2 toxins using different fluorescent labeling reagents by high-performance liquid chromatography. Talanta 2008, 74, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Kiš, M.; Vulić, A.; Kudumija, N.; Šarkanj, B.; Jaki Tkalec, V.; Aladić, K.; Škrivanko, M.; Furmeg, S.; Pleadin, J. A Two-Year Occurrence of Fusarium T-2 and HT-2 Toxin in Croatian Cereals Relative of the Regional Weather. Toxins 2021, 13, 39. [Google Scholar] [CrossRef]
- Medina, A.; Magan, N. Temperature and water activity effects on production of T-2 and HT-2 by Fusarium langsethiae strains from north European countries. Food Microbiol. 2011, 28, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Garai, E.; Risa, A.; Varga, E.; Cserháti, M.; Kriszt, B.; Urbányi, B.; Csenki, Z. Qualifying the T-2 Toxin-Degrading Properties of Seven Microbes with Zebrafish Embryo Microinjection Method. Toxins 2020, 12, 460. [Google Scholar] [CrossRef]
- Makowska, K.; Obremski, K.; Gonkowski, S. The Impact of T-2 Toxin on Vasoactive Intestinal Polypeptide-Like Immunoreactive (VIP-LI) Nerve Structures in the Wall of the Porcine Stomach and Duodenum. Toxins 2018, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Königs, M.; Mulac, D.; Schwerdt, G.; Gekle, M.; Humpf, H.-U. Metabolism and cytotoxic effects of T-2 toxin and its metabolites on human cells in primary culture. Toxicology 2009, 258, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yagen, B.; Joffe, A.Z. Screeing of toxic isolates of Fusarium poae and Fusarium sporotrichiodes involved in causing alimentary toxic aleukia. Appl. Environ. Microbiol. 1976, 32, 423–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolović, M.; Garaj-Vrhovac, V.; Šimpraga, B. T-2 toxin: Incidence and toxicity in poultry. Arhiv za Higijenu Rada i Toksikologiju 2008, 59, 43–52. [Google Scholar] [CrossRef]
- Wannemacher, R.W.; Wiener, S.L.; Sidell, F.R.; Takafuji, E.T.; Franz, D.R. Trichothecene mycotoxins. Med. Asp. Chem. Biol. Warf. 1997, 6, 655–676. [Google Scholar]
- Yang, S.; Li, Y.; Cao, X.; Hu, D.; Wang, Z.; Wang, Y.; Shen, J.; Zhang, S. Metabolic Pathways of T-2 Toxin in in Vivo and in Vitro Systems of Wistar Rats. J. Agric. Food Chem. 2013, 61, 9734–9743. [Google Scholar] [CrossRef] [PubMed]
- Kuca, K.; Dohnal, V.; Jezkova, A.; Jun, D. Metabolic pathways of T-2 toxin. Curr. Drug Metab. 2008, 9, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mackei, M.; Orbán, K.; Molnár, A.; Pál, L.; Dublecz, K.; Husvéth, F.; Neogrády, Z.; Mátis, G. Cellular Effects of T-2 Toxin on Primary Hepatic Cell Culture Models of Chickens. Toxins 2020, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- Yang, S.; De Boevre, M.; Zhang, H.; De Ruyck, K.; Sun, F.; Zhang, J.; Jin, Y.; Li, Y.; Wang, Z.; Zhang, S.; et al. Metabolism of T-2 Toxin in Farm Animals and Human In Vitro and in Chickens In Vivo Using Ultra High-Performance Liquid Chromatography- Quadrupole/Time-of-Flight Hybrid Mass Spectrometry Along with Online Hydrogen/Deuterium Exchange Technique. J. Agric. Food Chem. 2017, 65, 7217–7227. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef]
- Rai, R.B.; Rahman, S.; Dixit, H.; Rai, S.; Singh, B.; Kumar, H.; Damodaran, T.; Dhama, K. Analysis of feed ingredients for Afla and T-2 mycotoxins by ELISA in rural areas of Uttar Pradesh. Indian J. Vet. Pathol. 2011, 35, 238–240. [Google Scholar]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef] [Green Version]
- Sudakin, D.L. Trichothecenes in the environment: Relevance to human health. Toxicol. Lett. 2003, 143, 97–107. [Google Scholar] [CrossRef]
- Ueno, Y. Toxicological features of T-2 toxin and related trichothecenes. Fundam. Appl. Toxicol. 1984, 4, S124–S132. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.-Y.; Kuča, K.; Dohnal, V.; Zhang, X.-J.; Yuan, Z.-H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Ihara, T.; Sugamata, M.; Sekijima, M.; Okumura, H.; Yoshino, N.; Ueno, Y. Apoptotic cellular damage in mice after T-2 toxin-induced acute toxicosis. Nat. Toxins 1997, 5, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Han, S.; Chen, Y.; Wang, Y.; Li, D.; Zhu, Q. T-2 Toxin Induces Oxidative Stress, Apoptosis and Cytoprotective Autophagy in Chicken Hepatocytes. Toxins 2020, 12, 90. [Google Scholar] [CrossRef] [Green Version]
- Shinozuka, J.; Miwa, S.; Fujimura, H.; Toriumi, W.; Doi, K. Hepatotoxicity of T-2 toxin, trichothecene mycotoxin. Mycotoxins 2006, 2006, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Sharma, A.K.; Singh, N.D.; Prawez, S. Immunopathological effects of experimental T-2 mycotoxicosis in Wistar rats. Hum. Exp. Toxicol. 2020, 40, 772–790. [Google Scholar] [CrossRef]
- Minervini, F.; Fornelli, F.; Lucivero, G.; Romano, C.; Visconti, A. T-2 toxin immunotoxicity on human B and T lymphoid cell lines. Toxicology 2005, 210, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hymery, N.; Léon, K.; Carpentier, F.G.; Jung, J.L.; Parent-Massin, D. T-2 toxin inhibits the differentiation of human monocytes into dendritic cells and macrophages. Toxicol. In Vitro 2009, 23, 509–519. [Google Scholar] [CrossRef]
- Seeboth, J.; Solinhac, R.; Oswald, I.P.; Guzylack-Piriou, L. The fungal T-2 toxin alters the activation of primary macrophages induced by TLR-agonists resulting in a decrease of the inflammatory response in the pig. Vet. Res. 2012, 43, 35. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, M.; Bhaskar, A.S.B.; Rao, P.V.L. Involvement of Mitogen-Activated Protein Kinase Pathway in T-2 Toxin-Induced Cell Cycle Alteration and Apoptosis in Human Neuroblastoma Cells. Mol. Neurobiol. 2015, 51, 1379–1394. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Velkov, T.; Tang, S.; Dai, C. T-2 toxin-induced toxicity in neuroblastoma-2a cells involves the generation of reactive oxygen, mitochondrial dysfunction and inhibition of Nrf2/HO-1 pathway. Food Chem. Toxicol. 2018, 114, 88–97. [Google Scholar] [CrossRef]
- Weidner, M.; Lenczyk, M.; Schwerdt, G.; Gekle, M.; Humpf, H.-U. Neurotoxic Potential and Cellular Uptake of T-2 Toxin in Human Astrocytes in Primary Culture. Chem. Res. Toxicol. 2013, 26, 347–355. [Google Scholar] [CrossRef]
- Guo, P.; Liu, A.; Huang, D.; Wu, Q.; Fatima, Z.; Tao, Y.; Cheng, G.; Wang, X.; Yuan, Z. Brain damage and neurological symptoms induced by T-2 toxin in rat brain. Toxicol. Lett. 2018, 286, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Gaigé, S.; Djelloul, M.; Tardivel, C.; Airault, C.; Félix, B.; Jean, A.; Lebrun, B.; Troadec, J.-D.; Dallaporta, M. Modification of energy balance induced by the food contaminant T-2 toxin: A multimodal gut-to-brain connection. Brain Behav. Immun. 2014, 37, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Zhang, Y.F.; Liang, A.M.; Kong, X.F.; Li, Y.X.; Ma, K.W.; Jing, A.H.; Feng, S.Y.; Qiao, X.L. Toxic effects of T-2 toxin on reproductive system in male mice. Toxicol. Ind. Health 2009, 26, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Zhang, J.; Ji, Q.; Huang, W.; Zhang, X.; Li, Y. Spermatogenesis disorder caused by T-2 toxin is associated with germ cell apoptosis mediated by oxidative stress. Environ. Pollut. 2019, 251, 372–379. [Google Scholar] [CrossRef]
- Shen, J.; Perveen, A.; Kaka, N.; Li, Z.; Dai, P.; Li, C. Maternal Exposure to T-2 Toxin Induces Changes in Antioxidant System and Testosterone Synthesis in the Testes of Mice Offspring. Animals 2019, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- Perveen, A.; Shen, J.; Ali Kaka, N.; Li, C. Maternal Exposure to T-2 Toxin Affects Puberty Genes and Delays Estrus Cycle in Mice Offspring. Animals 2020, 10, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caloni, F.; Ranzenigo, G.; Cremonesi, F.; Spicer, L.J. Effects of a trichothecene, T-2 toxin, on proliferation and steroid production by porcine granulosa cells. Toxicon 2009, 54, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, A.A.; Kalantari, H.; Jalali, A.; Rezai, S.; Zadeh, H.H. Healing effect of quince seed mucilage on T-2 toxin-induced dermal toxicity in rabbit. Exp. Toxicol. Pathol. 2012, 64, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pang, V.F.; Swanson, S.P.; Beasley, V.R.; Buck, W.B.; Haschek, W.M. The toxicity of T-2 toxin in swine following topical application: I. Clinical signs, pathology, and residue concentrations. Fundam. Appl. Toxicol. 1987, 9, 41–49. [Google Scholar] [CrossRef]
- Agrawal, M.; Yadav, P.; Lomash, V.; Bhaskar, A.S.B.; Lakshmana Rao, P.V. T-2 toxin induced skin inflammation and cutaneous injury in mice. Toxicology 2012, 302, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, S.; Bai, Y.; Louzada Prates, L.; Lei, Y.; Yu, P. Mycotoxin contamination of food and feed in China: Occurrence, detection techniques, toxicological effects and advances in mitigation technologies. Food Control 2018, 91, 202–215. [Google Scholar] [CrossRef]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Lawal, I.A.; Gbashi, S.; Njobeh, P.B. Decontamination of T-2 Toxin in Maize by Modified Montmorillonite Clay. Toxins 2019, 11, 616. [Google Scholar] [CrossRef] [Green Version]
- Nathanail, A.V.; Gibson, B.; Han, L.; Peltonen, K.; Ollilainen, V.; Jestoi, M.; Laitila, A. The lager yeast Saccharomyces pastorianus removes and transforms Fusarium trichothecene mycotoxins during fermentation of brewer’s wort. Food Chem. 2016, 203, 448–455. [Google Scholar] [CrossRef]
- De Angelis, E.; Monaci, L.; Pascale, M.; Visconti, A. Fate of deoxynivalenol, T-2 and HT-2 toxins and their glucoside conjugates from flour to bread: An investigation by high-performance liquid chromatography high-resolution mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 30, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbuch, H.; Becker, S.; Schulz, M.; Cramer, B.; Humpf, H.-U. Thermal stability of T-2 and HT-2 toxins during biscuit- and crunchy muesli-making and roasting. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 2158–2167. [Google Scholar] [CrossRef]
- Wei, J.-T.; Wu, K.-T.; Sun, H.; Khalil, M.M.; Dai, J.-F.; Liu, Y.; Liu, Q.; Zhang, N.-Y.; Qi, D.-S.; Sun, L.-H. A Novel Modified Hydrated Sodium Calcium Aluminosilicate (HSCAS) Adsorbent Can Effectively Reduce T-2 Toxin-Induced Toxicity in Growth Performance, Nutrient Digestibility, Serum Biochemistry, and Small Intestinal Morphology in Chicks. Toxins 2019, 11, 199. [Google Scholar] [CrossRef] [Green Version]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Reinholds, I.; Gražina, J.; Bartkiene, E.; Zadeike, D.; Bartkevics, V.; Lele, V.; Cernauskas, D.; Cizeikiene, D. Evaluation of ozonation as a method for mycotoxins degradation in malting wheat grains. World Mycotoxin J. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Carson, M.S.; Smith, T.K. Role of bentonite in prevention of T-2 toxicosis in rats. J. Anim. Sci. 1983, 57, 1498–1506. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. New mycotoxin adsorbents based on tri-octahedral bentonites for animal feed. Anim. Feed Sci. Technol. 2019, 255, 114228. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. In Vitro Detoxification of Aflatoxin B(1), Deoxynivalenol, Fumonisins, T-2 Toxin and Zearalenone by Probiotic Bacteria from Genus Lactobacillus and Saccharomyces cerevisiae Yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, Z.U.; Al Thani, R.; Alsafran, M.; Migheli, Q.; Jaoua, S. Selection of Bacillus spp. with decontamination potential on multiple Fusarium mycotoxins. Food Control 2021, 127, 108119. [Google Scholar] [CrossRef]
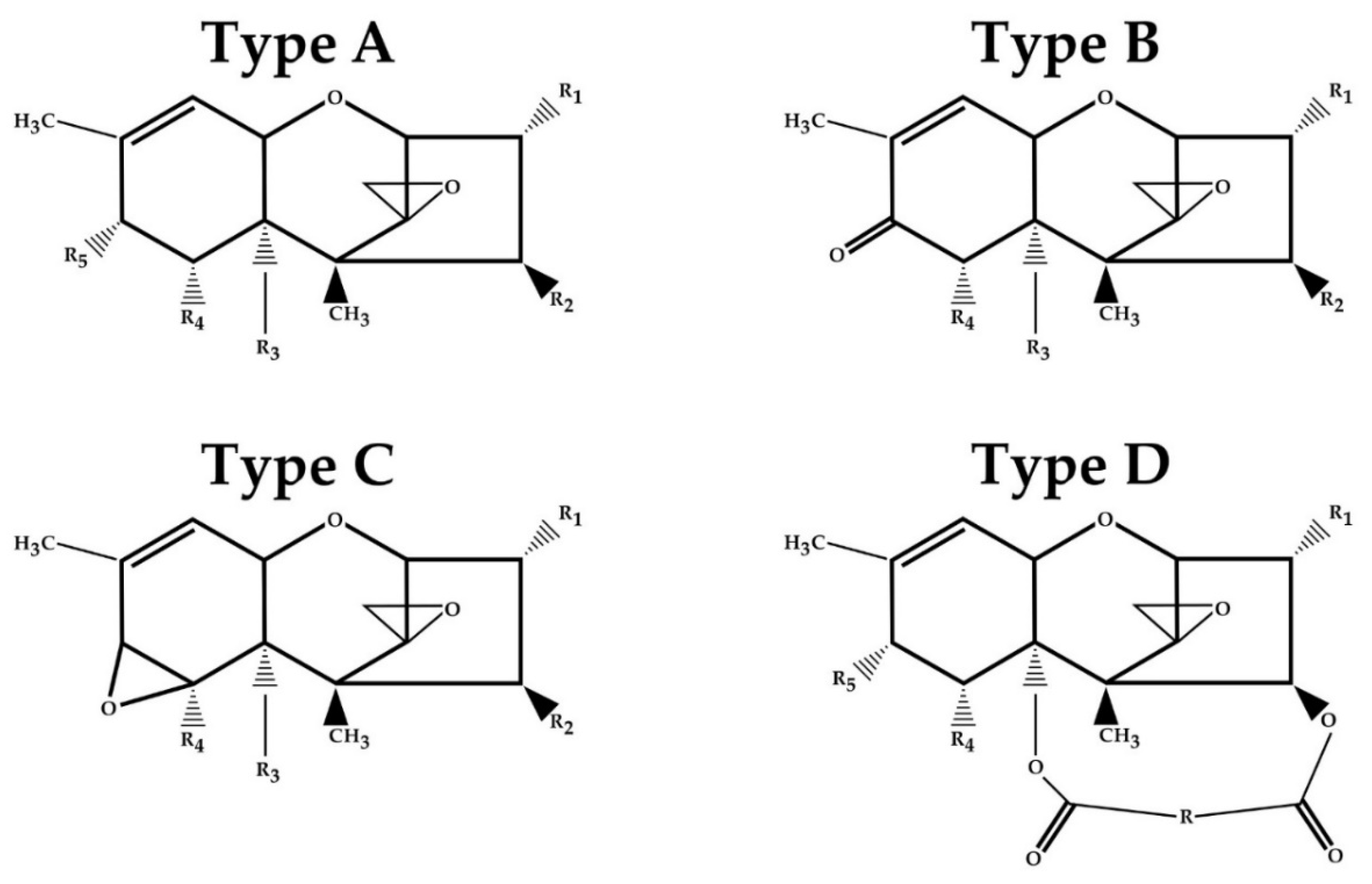
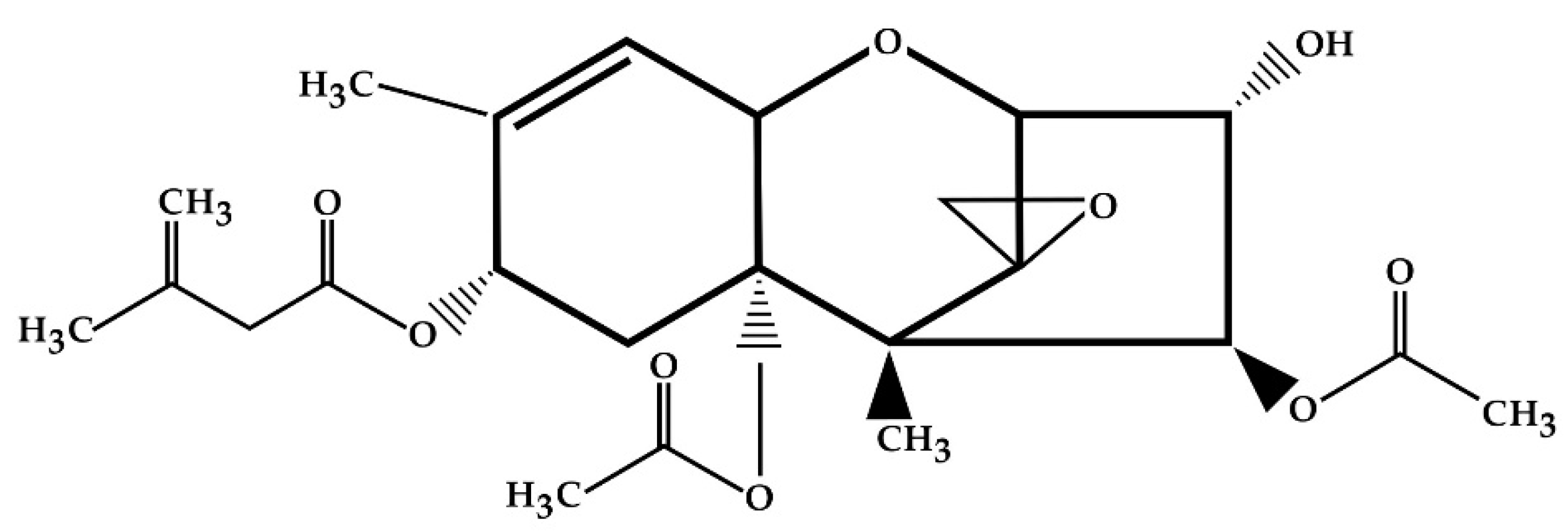
| Toxin | Type | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|
| T-2 | A | OH | OCOCH3 | OCOCH3 | H | OCOCH2CH(CH3)2 |
| HT-2 | A | OH | OH | OCOCH3 | H | OCOCH2CH(CH3)2 |
| Neosolaniol (NEO) | A | OH | OCOCH3 | OCOCH3 | H | OH |
| Diacetoxyscirpenol (DAS) | A | OH | OCOCH3 | OCOCH3 | H | H |
| Nivalenol (NIV) | B | OH | OH | OH | OH | =O |
| Deoxynivalenol (DON) | B | OH | H | OH | OH | =O |
| 3-Acetyldeoxynivalenol (3-ADON) | B | OCOCH3 | H | OH | OH | =O |
| 15-Acetyldeoxynivalenol (15-AcDON) | B | OH | H | OCOCH3 | OH | =O |
| Crotocin | C | H | OCOCH-CHCH3 | H | Epoxide | |
| Roridin E | D | H | Macrocyclic ring | H | H | |
| Verrucarin A | D | H | Macrocyclic ring | H | H | |
| Number of Metabolite | Metabolite | Metabolic Pathway |
|---|---|---|
| 1 | HT-2 toxin (HT-2) | Hydrolysis |
| 2 | Neosolaniol (NEO) | |
| 3 | 4-deacetylneosolaniol (4-deAc-NEO) | |
| 4 | 3′-hydroxy-T-2 (3′-OH-T-2) | Hydroxylation |
| 5 | 3′-hydroxy-HT-2 (3′-OH-HT-2) | |
| 6 | 3′-Hydroxy-T-2-3-sulfate (3′-OH-T-2 3-SO3H) | Sulfonation |
| 7 | 3′-Hydroxy-HT-2-3-sulfate (3′-OH-HT-2 3-SO3H) | |
| 8 | 4′-Hydroxy-HT-2 (4′-OH-HT-2) | Hydroxylation |
| 9 | 4′-OH-HT-2 isomer | |
| 10 | 4′-Carboxyl-T-2 (4′-COOH-T-2) | Carboxylation |
| 11 | 4′-COOH-T-2 isomer | |
| 12 | 4′-Carboxyl-HT-2 (4′-COOH-HT-2) | |
| 13 | 4′-COOH-HT-2 isomer | |
| 14 | 4′-Carboxyl-3′-hydroxy-T-2 (4′-COOH-3′-OH-T-2) | |
| 15 | 4′-COOH-3′-OH-T-2 isomer | |
| 16 | 3′,4′-Dihydroxy-T-2 (3′,4′-di-OH-T-2) | Hydroxylation |
| 17 | 3′,4′-di-OH-T-2 isomer | |
| 18 | 4′,4′-Dihydroxy-T-2 (4′,4′-di-OH-T-2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules 2021, 26, 6868. https://doi.org/10.3390/molecules26226868
Janik E, Niemcewicz M, Podogrocki M, Ceremuga M, Stela M, Bijak M. T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules. 2021; 26(22):6868. https://doi.org/10.3390/molecules26226868
Chicago/Turabian StyleJanik, Edyta, Marcin Niemcewicz, Marcin Podogrocki, Michal Ceremuga, Maksymilian Stela, and Michal Bijak. 2021. "T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies" Molecules 26, no. 22: 6868. https://doi.org/10.3390/molecules26226868
APA StyleJanik, E., Niemcewicz, M., Podogrocki, M., Ceremuga, M., Stela, M., & Bijak, M. (2021). T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules, 26(22), 6868. https://doi.org/10.3390/molecules26226868






