Mononuclear Copper(I) 3-(2-pyridyl)pyrazole Complexes: The Crucial Role of Phosphine on Photoluminescence
Abstract
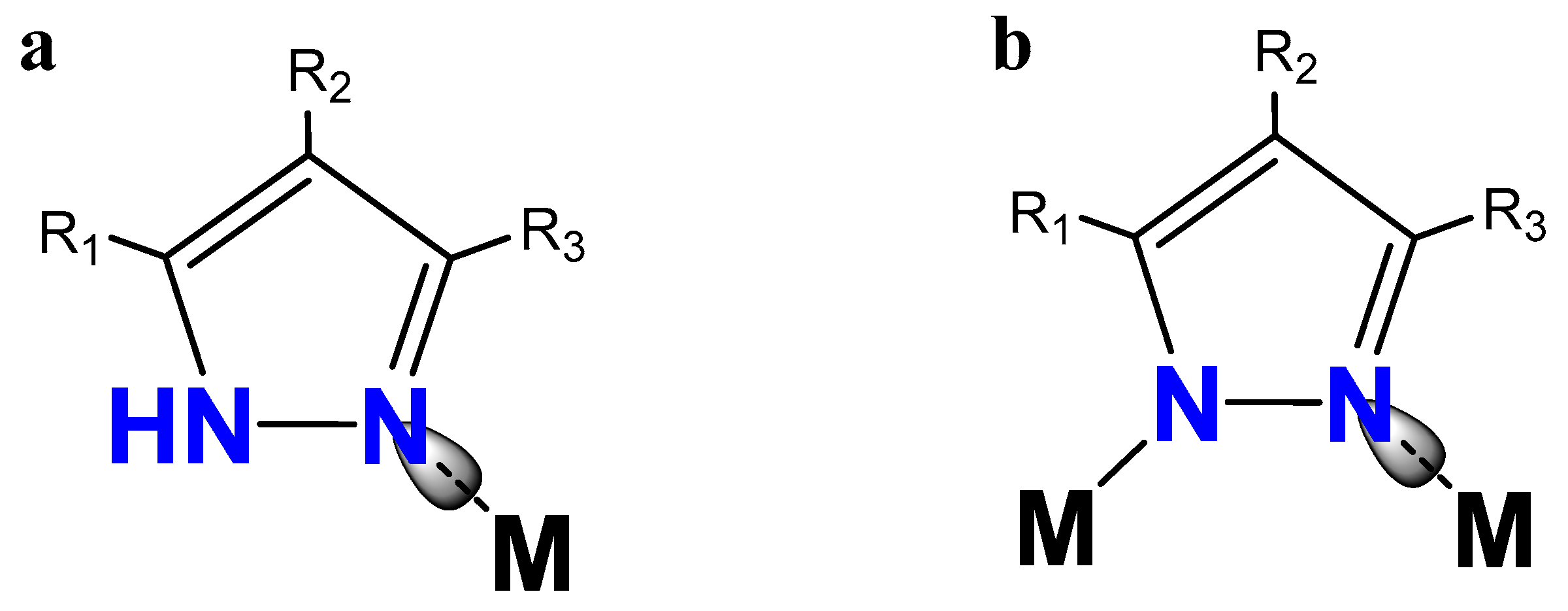
:1. Introduction
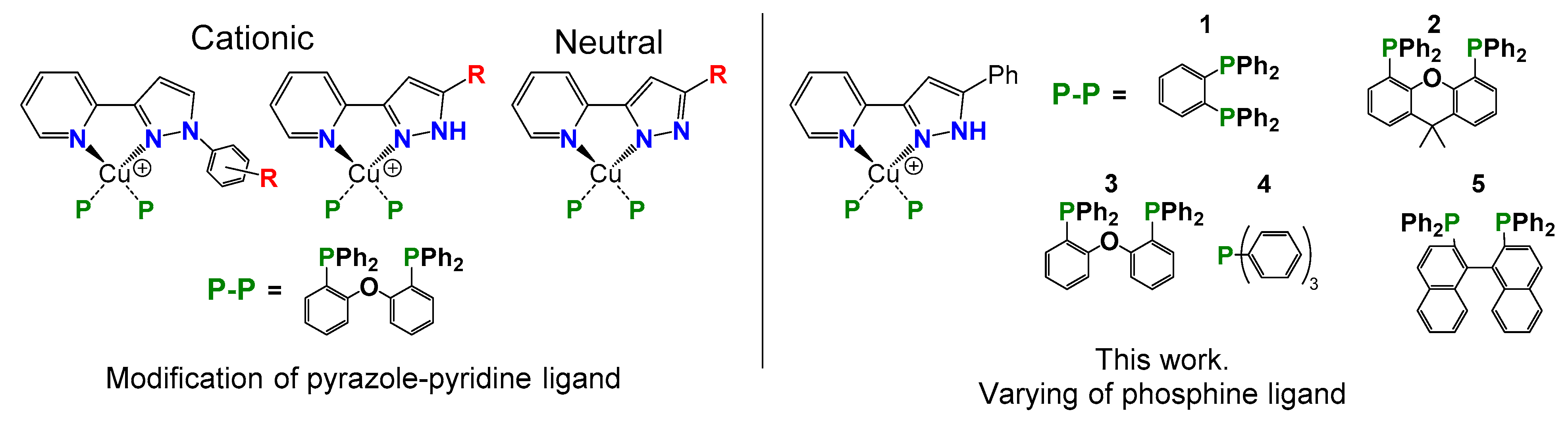
2. Results and Discussions
2.1. Synthesis
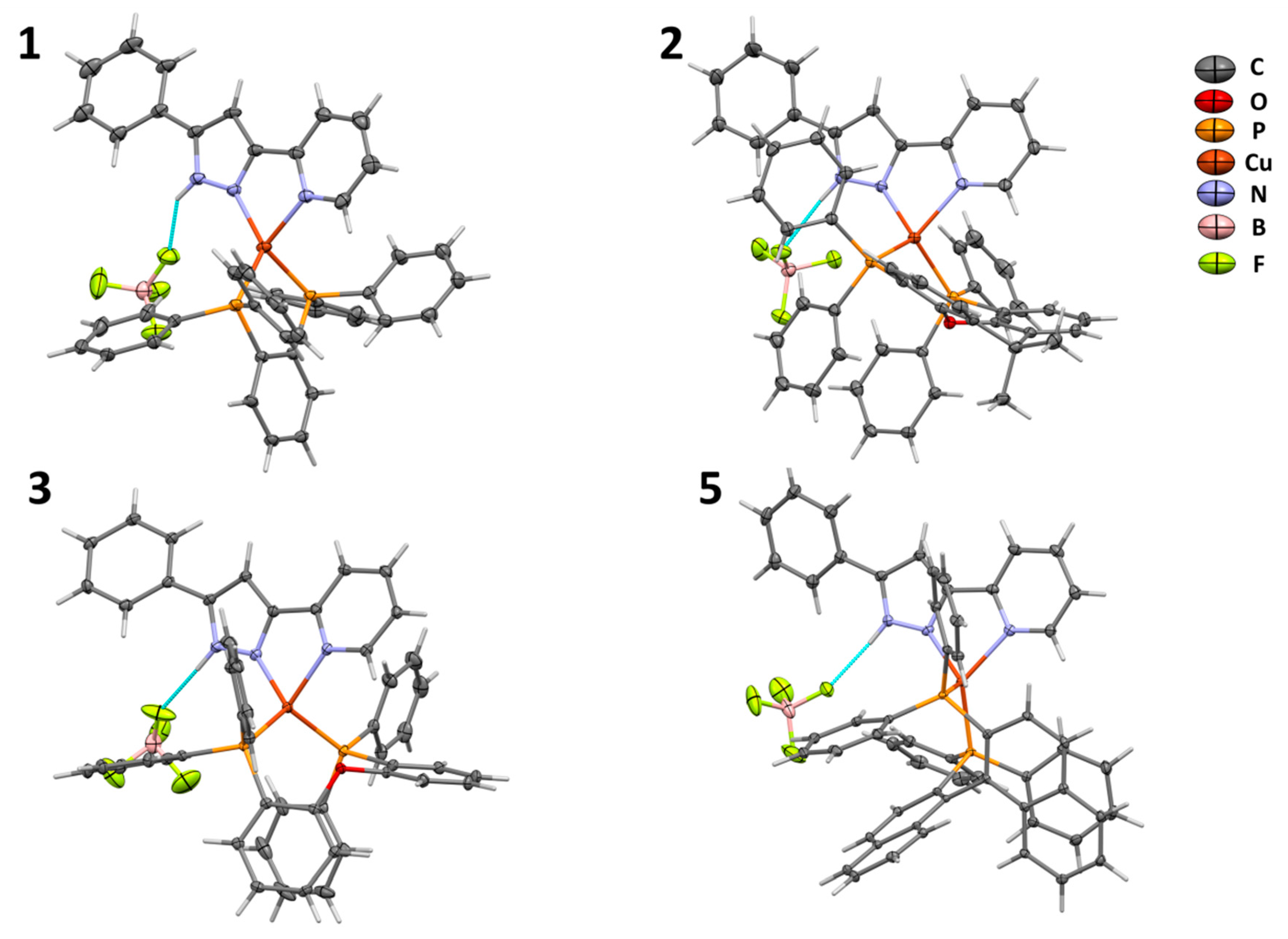
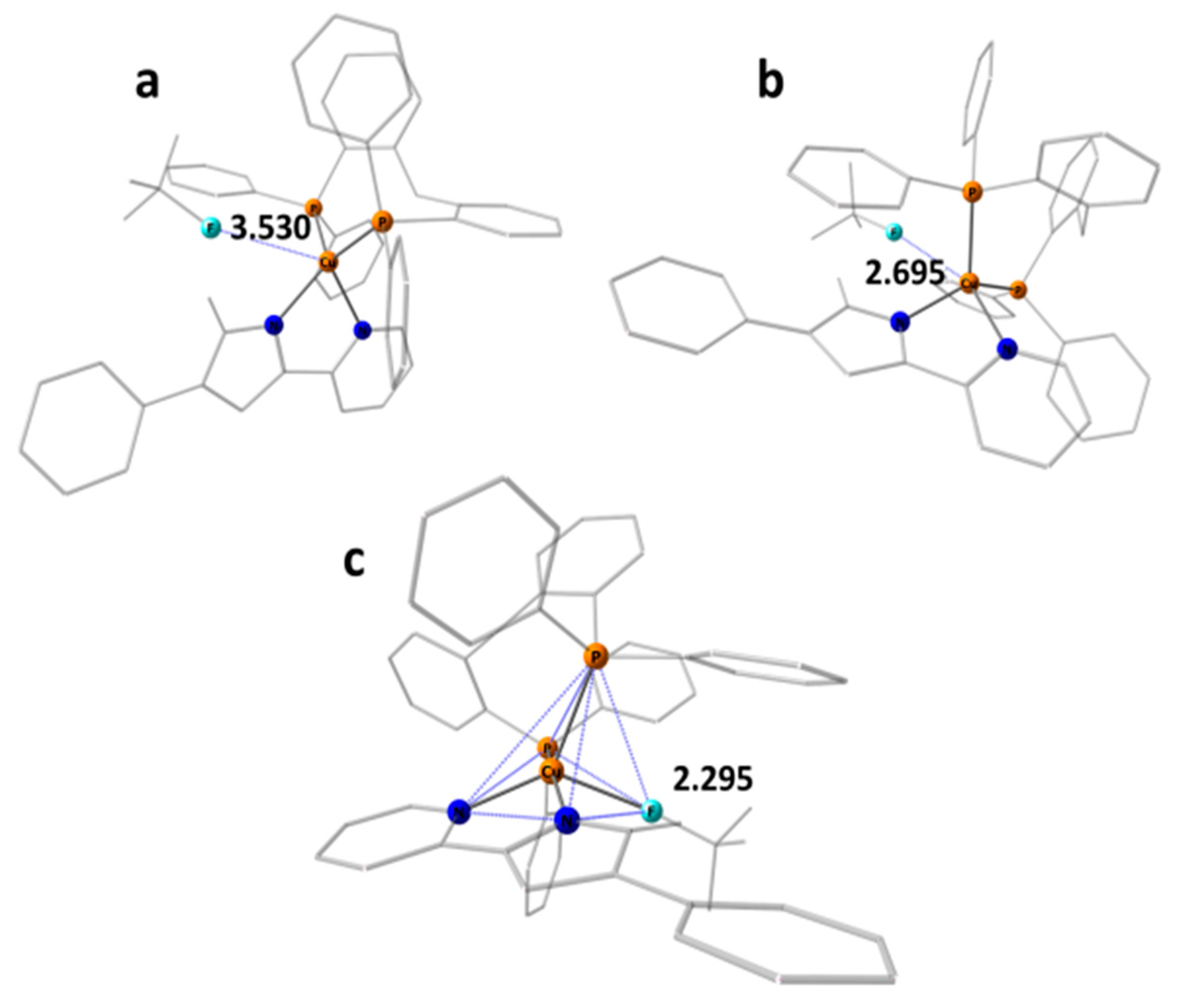
2.2. Crystal Structures
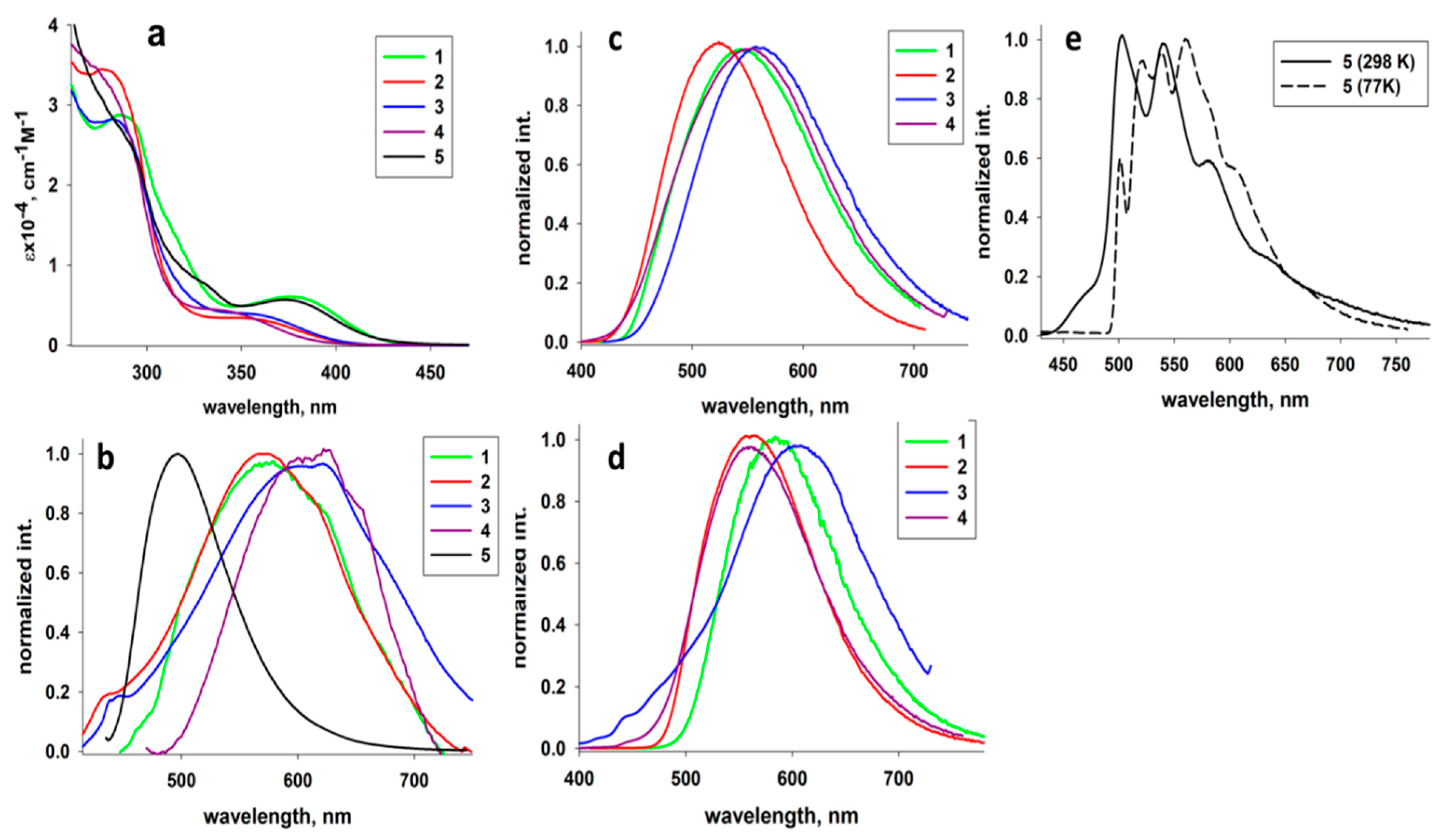
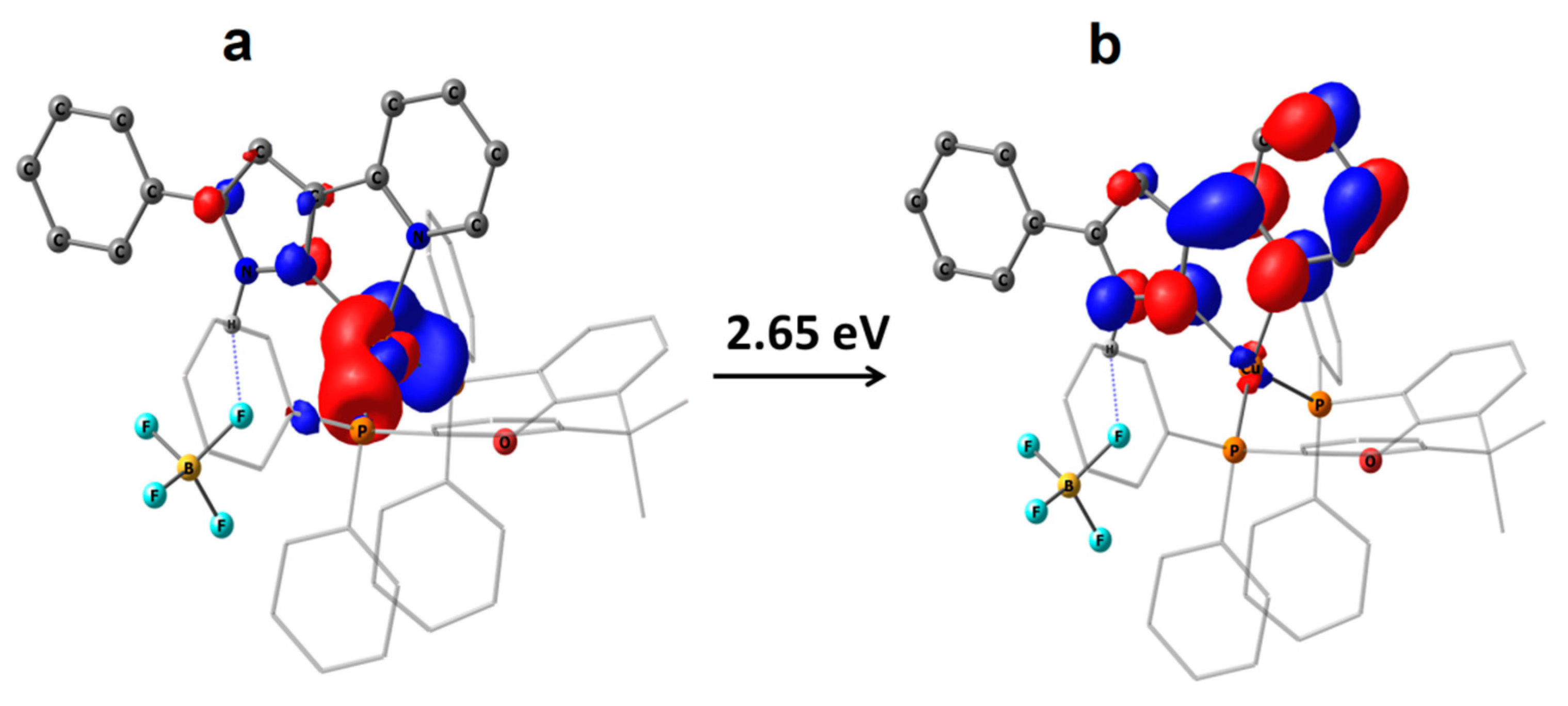
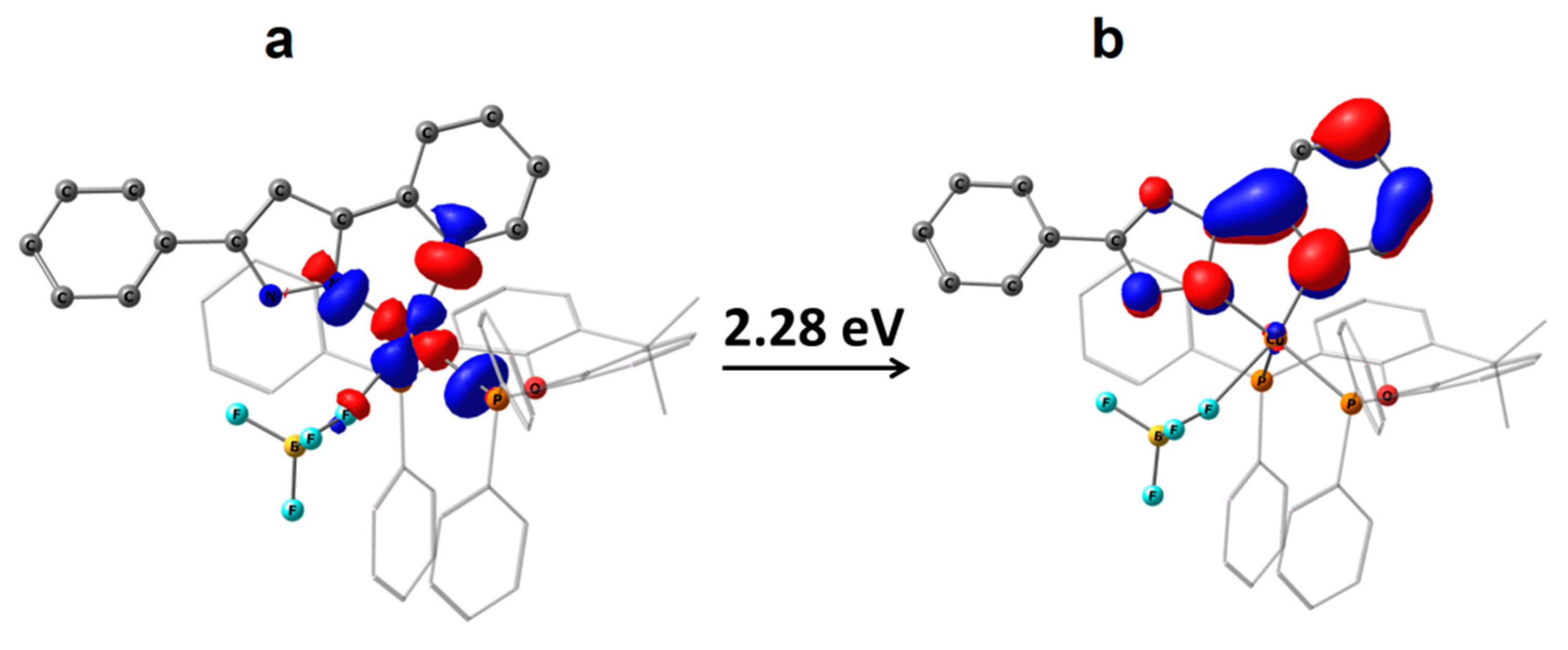
2.3. Photophysical Properties
3. Materials and Methods
3.1. Synthesis and Characterization
3.2. Computational Details
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Halcrow, M.A. Pyrazoles and pyrazolides—Flexible synthons in self-assembly. Dalton Trans. 2009, 12, 2059–2073. [Google Scholar] [CrossRef]
- Brown, A.W. Recent Developments in the Chemistry of Pyrazoles. Adv. Heterocycl. Chem. 2018, 126, 55–107. [Google Scholar]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef]
- Baranova, K.F.; Titov, A.A.; Filippov, O.A.; Smol’Yakov, A.F.; Averin, A.A.; Shubina, E.S. Dinuclear Silver(I) Nitrate Complexes with Bridging Bisphosphinomethanes: Argentophilicity and Luminescence. Crystals 2020, 10, 881. [Google Scholar] [CrossRef]
- Rasika Dias, H.V.; Polach, S.A.; Wang, Z. Coinage metal complexes of 3,5-bis(trifluoromethyl)pyrazolate ligand: Synthesis and characterization of {[3,5-(CF3)2Pz]Cu}3 and {[3,5-(CF3)2Pz]Ag}3. J. Fluor. Chem. 2000, 103, 163–169. [Google Scholar] [CrossRef]
- Mohamed, A.A. Advances in the coordination chemistry of nitrogen ligand complexes of coinage metals. Coord. Chem. Rev. 2010, 254, 1918–1947. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ishikawa, Y.; Miyashita, Y.; Okamoto, K.-I. Pyrazolate-bridged group 11 metal(I) complexes: Substituent effects on the supramolecular structures and physicochemical properties. Inorg. Chim. Acta 2010, 363, 2977–2989. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Epstein, L.M.; Belkova, N.V.; Shubina, E.S. Macrocyclic copper(I) and silver(I) pyrazolates: Principles of supramolecular assemblies with Lewis bases. Inorg. Chim. Acta 2018, 470, 22–35. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, Z.; Wu, K.; Ning, G.H.; Li, D. Coinage-Metal-Based Cyclic Trinuclear Complexes with Metal-Metal Interactions: Theories to Experiments and Structures to Functions. Chem. Rev. 2020, 120, 9675–9742. [Google Scholar] [CrossRef] [PubMed]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Averin, A.A.; Shubina, E.S. Synthesis, Structures and luminescence of multinuclear silver(i) pyrazolate adducts with 1,10-phenanthroline derivatives. Dalton Trans. 2019, 48, 8410–8417. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Godovikov, I.A.; Shakirova, J.R.; Tunik, S.P.; Podkorytov, I.S.; Shubina, E.S. Luminescent Complexes of the Trinuclear Silver(I) and Copper(I) Pyrazolates Supported with Bis(diphenylphosphino)methane. Inorg. Chem. 2019, 58, 8645–8656. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’akov, A.F.; Baranova, K.F.; Titova, E.M.; Averin, A.A.; Shubina, E.S. Dinuclear CuI and AgI Pyrazolates Supported with Tertiary Phosphines: Synthesis, Structures, and Photophysical Properties. Eur. J. Inorg. Chem. 2019, 2019, 821–827. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Diyabalanage, H.V.K.; Ghimire, M.M.; Hudson, J.M.; Parasar, D.; Palehepitiya Gamage, C.S.; Li, S.; Omary, M.A. Brightly phosphorescent tetranuclear copper(i) pyrazolates. Dalton Trans. 2019, 48, 14979–14983. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.Z.; Chen, W.; Zheng, J.; Ng, S.W.; Li, D. Luminescent polymorphic aggregates of trinuclear Cu(I)-pyrazolate tuned by intertrimeric CuNPy weak coordination bonds. Dalton Trans. 2021, 50, 1733–1739. [Google Scholar] [CrossRef]
- Chen, X.-L.; Yu, R.; Zhang, Q.-K.; Zhou, L.-J.; Wu, X.-Y.; Zhang, Q.; Lu, C.-Z. Rational Design of Strongly Blue-Emitting Cuprous Complexes with Thermally Activated Delayed Fluorescence and Application in Solution-Processed OLEDs. Chem Mater. 2013, 25, 3910–3920. [Google Scholar] [CrossRef]
- Halcrow, M.A. Recent advances in the synthesis and applications of 2,6-dipyrazolylpyridine derivatives and their complexes. New J. Chem. 2014, 38, 1868–1882. [Google Scholar] [CrossRef]
- Trofimenko, S. Recent advances in poly(pyrazolyl) borate (scorpionate) chemistry. Chem. Rev. 1993, 93, 943–980. [Google Scholar] [CrossRef]
- Dias, H.V.; Lovely, C.J. Carbonyl and olefin adducts of coinage metals supported by poly(pyrazolyl)borate and poly(pyrazolyl)alkane ligands and silver mediated atom transfer reactions. Chem. Rev. 2008, 108, 3223–3238. [Google Scholar] [CrossRef]
- Muñoz-Molina, J.M.; Belderrain, T.R.; Pérez, P.J. Trispyrazolylborate coinage metals complexes: Structural features and catalytic transformations. Coord. Chem. Rev. 2019, 390, 171–189. [Google Scholar] [CrossRef]
- Bigmore, H.R.; Lawrence, S.C.; Mountford, P.; Tredget, C.S. Coordination, Organometallic and related chemistry of tris(pyrazolyl)methane ligands. Dalton Trans. 2005, 4, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.D.R.S. C-scorpionate complexes: Ever young catalytic tools. Coord. Chem. Rev. 2019, 396, 89–102. [Google Scholar] [CrossRef]
- Wagner, H.E.; Hohnstein, S.; Schussmann, M.G.; Steppe, L.A.; Breher, F. Phosphine-functionalised tris(pyrazolyl)methane ligands and their mono- and heterobimetallic complexes. Dalton Trans. 2019, 48, 15397–15407. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Schulz, M.; Wächtler, M.; Karnahl, M.; Dietzek, B. Heteroleptic diimine–diphosphine Cu(I) complexes as an alternative towards noble-metal based photosensitizers: Design strategies, photophysical properties and perspective applications. Coord. Chem. Rev. 2018, 356, 127–146. [Google Scholar] [CrossRef]
- Sethi, S.; Jena, S.; Das, P.K.; Behera, N. Synthetic approach and structural diversities of pyridylpyrazole derived late transition metal complexes. J. Mol. Struct. 2019, 1193, 495–521. [Google Scholar] [CrossRef]
- Singh, K.; Long, J.R.; Stavropoulos, P. Ligand-Unsupported Metal−Metal (M = Cu, Ag) Interactions between Closed-Shell d10 Trinuclear Systems. J. Am. Chem. Soc. 1997, 119, 2942–2943. [Google Scholar] [CrossRef]
- Xing, L.R.; Lu, Z.; Li, M.; Zheng, J.; Li, D. Revealing High-Lying Intersystem Crossing in Brightly Luminescent Cyclic Trinuclear Cu(I)/Ag(I) Complexes. J. Phys. Chem. Lett. 2020, 11, 2067–2073. [Google Scholar] [CrossRef]
- Hsu, C.W.; Lin, C.C.; Chung, M.W.; Chi, Y.; Lee, G.H.; Chou, P.T.; Chang, C.H.; Chen, P.Y. Systematic investigation of the metal-structure-photophysics relationship of emissive d10-complexes of group 11 elements: The prospect of application in organic light emitting devices. J. Am. Chem. Soc. 2011, 133, 12085–12099. [Google Scholar] [CrossRef]
- Bergmann, L.; Braun, C.; Nieger, M.; Brase, S. The coordination- and photochemistry of copper(i) complexes: Variation of N^N ligands from imidazole to tetrazole. Dalton Trans. 2018, 47, 608–621. [Google Scholar] [CrossRef]
- Chen, J.L.; Cao, X.F.; Wang, J.Y.; He, L.H.; Liu, Z.Y.; Wen, H.R.; Chen, Z.N. Synthesis, Characterization, and photophysical properties of heteroleptic copper(I) complexes with functionalized 3-(2′-pyridyl)-1,2,4-triazole chelating ligands. Inorg. Chem. 2013, 52, 9727–9740. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.-L.; Chen, J.; Wu, X.-Y.; Yu, R.; Lu, C.-Z. Four highly efficient cuprous complexes and their applications in solution-processed organic light-emitting diodes. RSC Adv. 2015, 5, 34424–34431. [Google Scholar] [CrossRef]
- Chen, J.L.; Guo, Z.H.; Yu, H.G.; He, L.H.; Liu, S.J.; Wen, H.R.; Wang, J.Y. Luminescent dinuclear copper(I) complexes bearing 1,4-bis(diphenylphosphino)butane and functionalized 3-(2′-pyridyl)pyrazole mixed ligands. Dalton Trans. 2016, 45, 696–705. [Google Scholar] [CrossRef]
- Chen, J.-L.; Guo, Z.-H.; Luo, Y.-S.; Qiu, L.; He, L.-H.; Liu, S.-J.; Wen, H.-R.; Wang, J.-Y. Luminescent monometallic Cu(i) triphenylphosphine complexes based on methylated 5-trifluoromethyl-3-(2-pyridyl)-1,2,4-triazole ligands. New J. Chem. 2016, 40, 5325–5332. [Google Scholar] [CrossRef]
- Sun, Y.; Lemaur, V.; Beltran, J.I.; Cornil, J.; Huang, J.; Zhu, J.; Wang, Y.; Frohlich, R.; Wang, H.; Jiang, L.; et al. Neutral Mononuclear Copper(I) Complexes: Synthesis, Crystal Structures, and Photophysical Properties. Inorg. Chem. 2016, 55, 5845–5852. [Google Scholar] [CrossRef]
- He, L.H.; Luo, Y.S.; Di, B.S.; Chen, J.L.; Ho, C.L.; Wen, H.R.; Liu, S.J.; Wang, J.Y.; Wong, W.Y. Luminescent Three- and Four-Coordinate Dinuclear Copper(I) Complexes Triply Bridged by Bis(diphenylphosphino)methane and Functionalized 3-(2′-Pyridyl)-1,2,4-triazole Ligands. Inorg. Chem. 2017, 56, 10311–10324. [Google Scholar] [CrossRef]
- Donato, L.; Atoini, Y.; Prasetyanto, E.A.; Chen, P.; Rosticher, C.; Bizzarri, C.; Rissanen, K.; De Cola, L. Selective Encapsulation and Enhancement of the Emission Properties of a Luminescent Cu(I) Complex in Mesoporous Silica. Helv. Chim. Acta 2018, 101, e1700273. [Google Scholar] [CrossRef]
- Chen, X.W.; Yuan, H.L.; He, L.H.; Chen, J.L.; Liu, S.J.; Wen, H.R.; Zhou, G.; Wang, J.Y.; Wong, W.Y. A Sublimable Dinuclear Cuprous Complex Showing Selective Luminescence Vapochromism in the Crystalline State. Inorg. Chem. 2019, 58, 14478–14489. [Google Scholar] [CrossRef]
- Chen, X.-W.; He, L.-H.; Ju, P.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Mechanochromic luminescent materials of bimetallic Cu(i) complexes showing thermally activated delayed fluorescence. J. Mater. Chem. C 2020, 8, 16160–16167. [Google Scholar] [CrossRef]
- Chen, J.-L.; Zeng, X.-H.; Ganesan, P.; He, L.-H.; Liao, J.-S.; Liu, S.-J.; Wen, H.-R.; Zhao, F.; Chi, Y. Heterobimetallic copper(i) complexes bearing both 1,1-bis(diphenylphosphino)ferrocene and functionalized 3-(2-pyridyl)-1,2,4-triazole. New J. Chem. 2019, 43, 4261–4271. [Google Scholar] [CrossRef]
- Caron, A.; Morin, É.; Collins, S.K. Bifunctional Copper-Based Photocatalyst for Reductive Pinacol-Type Couplings. ACS Catal. 2019, 9, 9458–9464. [Google Scholar] [CrossRef]
- Huang, C.H.; Yang, M.; Chen, X.L.; Lu, C.Z. Bright bluish-green emitting Cu(I) complexes exhibiting efficient thermally activated delayed fluorescence. Dalton Trans. 2021, 50, 5171–5176. [Google Scholar] [CrossRef]
- Brunner, F.; Babaei, A.; Pertegas, A.; Junquera-Hernandez, J.M.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Orti, E.; Housecroft, C.E. Phosphane tuning in heteroleptic [Cu(N^N)(P^P)](+) complexes for light-emitting electrochemical cells. Dalton Trans. 2019, 48, 446–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkan-Zambada, M.; Constable, E.C.; Housecroft, C.E. The Role of Percent Volume Buried in the Characterization of Copper(I) Complexes for Lighting Purposes. Molecules 2020, 25, 2647. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mackenzie, C.F.R.; Said, S.A.; Pal, A.K.; Haghighatbin, M.A.; Babaei, A.; Sessolo, M.; Cordes, D.B.; Slawin, A.M.Z.; Kamer, P.C.J.; et al. Wide-Bite-Angle Diphosphine Ligands in Thermally Activated Delayed Fluorescent Copper(I) Complexes: Impact on the Performance of Electroluminescence Applications. Inorg. Chem. 2021, 60, 10323–10339. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wu, Z.G.; Zheng, Y.X. Highly efficient bluish green organic light-emitting diodes of iridium(iii) complexes with low efficiency roll-off. Dalton Trans. 2018, 47, 7587–7593. [Google Scholar] [CrossRef] [PubMed]
- Reineke, M.H.; Sampson, M.D.; Rheingold, A.L.; Kubiak, C.P. Synthesis and structural studies of nickel(0) Tetracarbene complexes with the introduction of a new four-coordinate geometric index, taudelta. Inorg. Chem. 2015, 54, 3211–3217. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, tau4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Yersin, H.; Czerwieniec, R.; Shafikov, M.Z.; Suleymanova, A.F. TADF Material Design: Photophysical Background and Case Studies Focusing on CuI and AgI Complexes. ChemPhysChem 2017, 18, 3508–3535. [Google Scholar] [CrossRef]
- Kunkely, H.; Pawlowski, V.; Vogler, A. Copper(I) binap complexes (binap=(2,2′-bis(diphenylphosphino)-1,1′-binaphthyl). Luminescence from IL and LLCT states. Inorg. Chem. Commun. 2008, 11, 1003–1005. [Google Scholar] [CrossRef]
- Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Towards the online computer-aided design of catalytic pockets. Nat. Chem 2019, 11, 872–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, C.T.; Moore, J.J.; Cunningham, K.L.; Fanwick, P.E.; McMillin, D.R. Structural and photophysical studies of Cu(NN)2+ systems in the solid state. Emission at last from complexes with simple 1,10-phenanthroline ligands. Inorg. Chem. 2000, 39, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, P.; van Leeuwen, P.W.N.M. The bite angle makes the difference: A practical ligand parameter for diphosphine ligands. J. Chem. Soc. Dalton Trans. 1999, 10, 1519–1530. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, B.; Lu, G.-P. A DFT study on the mechanism of rhodium-catalyzed regioselective hydrothiolation of the allyl amine. Mol. Catal. 2019, 468, 62–74. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
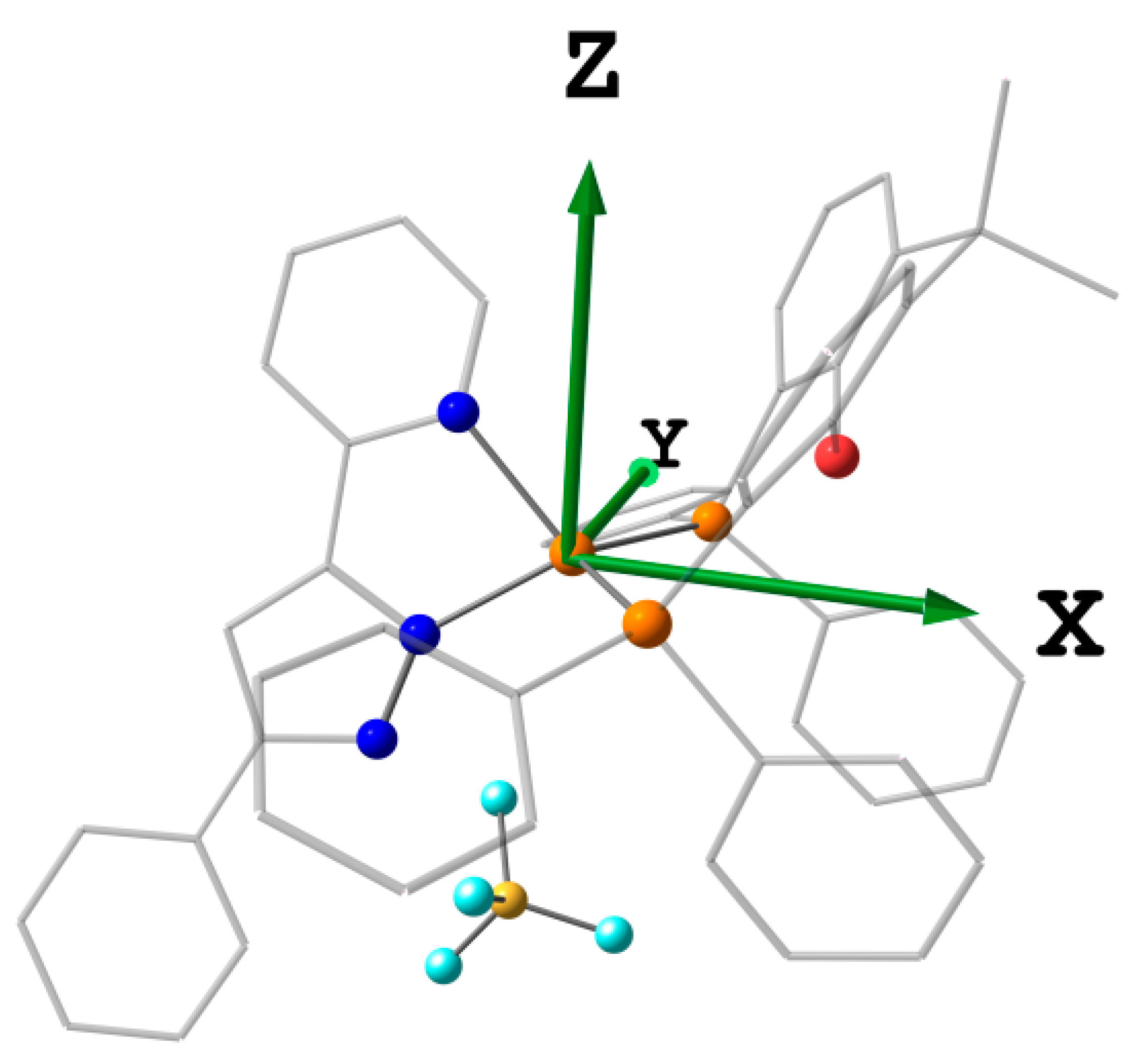
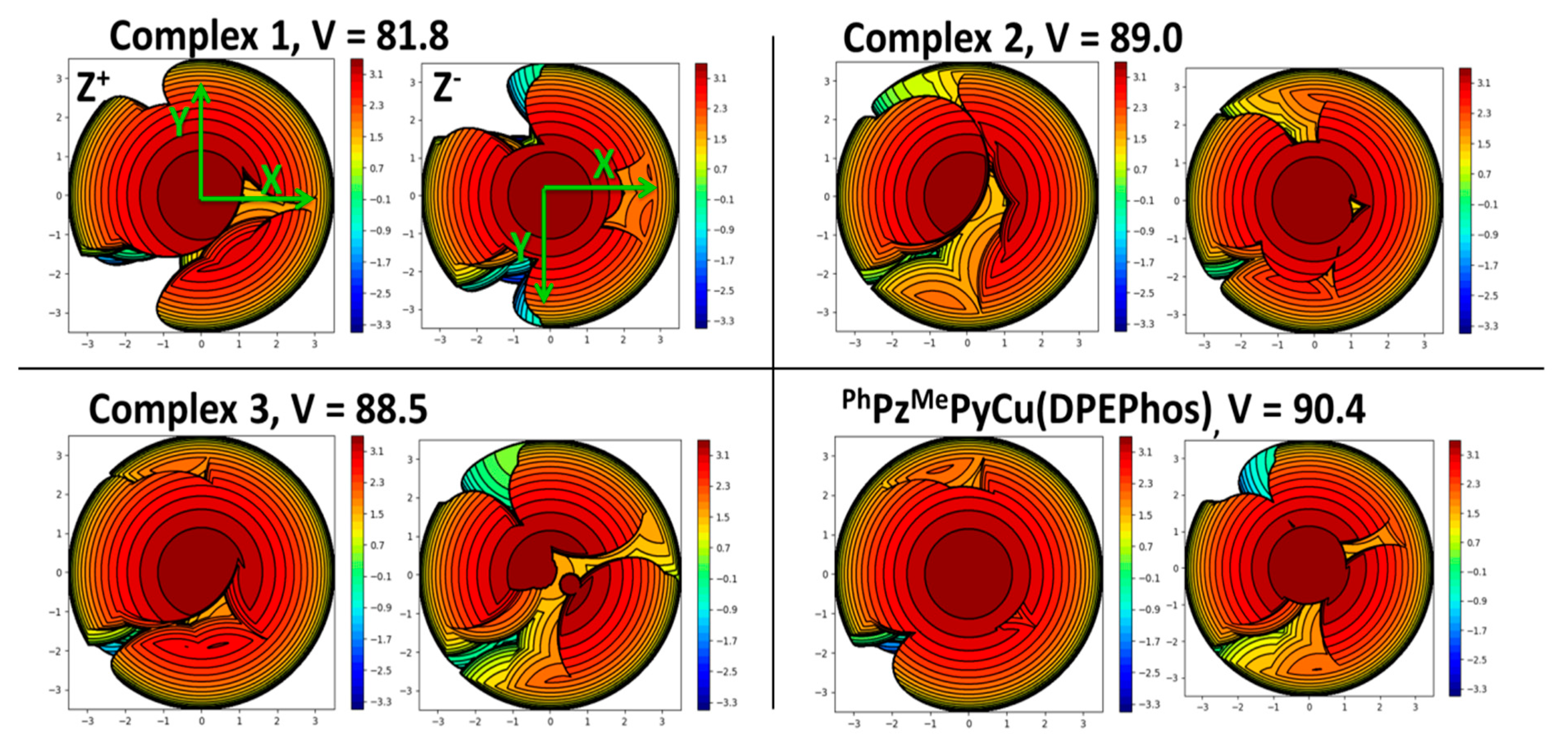
| Largest Angle, α (Deg) | Second-Largest Angle, β (Deg) | τδ | References | |
|---|---|---|---|---|
| 1 | 130.89 | 128.78 | 0.71 | this work |
| 2 | 123.21 | 121.61 | 0.81 | this work |
| 3 | 119.75 | 117.48 | 0.85 | this work |
| 5 | 128.93 | 127.18 | 0.73 | this work |
| PhPzMePyCu(DPEPhos) | 120.65 | 117.16 | 0.84 | [40] |
| FPzMePyCu(DPEPhos) 1 | 120.0 | 118.92 | 0.85 | [35] |
| FPzMePyCu(DPEPhos) 2 | 119.73 | 119.05 | 0.85 | [35] |
| PhTrzPy a Cu(DPEPhos) a | 117.1 | 116.8 | 0.89 | [33] |
| tBuTrzPyCu(dppf) b | 123.88 | 120.96 | 0.79 | [38] |
| FTrzPyCu(dppf) | 126.58 | 114.99 | 0.76 | [38] |
| Solution | Solid | ||||||
|---|---|---|---|---|---|---|---|
| λmax (ε∙10−4, M−1 cm−1), nm | λmaxem (λex), nm | λmaxem (λex), nm; 298 K | τ298, µs | λmaxem (λex), nm; 77 K | τ77, µs | Φ298, % | |
| 1 | 287 (2.86); 315 (1.33); 378 (0.59) | 574 (420) | 545 (420) | 19.4 ± 0.2 | 585 (410) | 404 ± 4 | 23 |
| 2 | 278 (3.43); 349 (0.35) | 580 (385) | 522 (370) | 66.2 ± 0.6 | 561 (370) | 252 ± 2 | 78 |
| 3 | 280 (2.8); 353 (0.41) | 605 (385) | 559 (370) | 22.7 ± 0.1 | 610 (370) | 186 ± 1 | 28 |
| 4 | 267 (3.6); 340 (0.44) | 610 (385) | 540 (370) | 28.9 ± 0.5 | 548 (370) | 132 ± 1 | 5 |
| 5 | 287 (2.73); 328 (0.78); 373 (0.60) | 495 (430) | LC bands a | 118 ± 1 | LC bands a | 4456 ± 34 | 1 |
| S1 | UDFT Tpyr a | RODFT Tpyr b | UDFT Ttetr | RODFT Ttetr | |
|---|---|---|---|---|---|
| 1 | 2.61 | 2.23 | 2.34 | - | - |
| 2 | 2.65 | 2.28 | 2.38 | 3.14 | 3.29 |
| 3 | 2.88 | 2.47 | 2.57 | 3.01 | 3.16 |
| 5 | 2.91 | 2.47 | 2.57 | - | - |
| X-Ray | DFT (S0) | TDDFT (S1) | DFT (Tpyr) | |
|---|---|---|---|---|
| 1 | 92.07(4) | 89 | 80 | 80 |
| 2 | 112.03(3) | 114 | 101 | 103 |
| 3 | 115.08(3) | 114 | 97 | 99 |
| 5 | 101.65(3) | 100 | 92 | 92 |
| S0/S1 | S0/Tpyr | S0/Ttetr | S1/Tpyr | |
|---|---|---|---|---|
| 1 | 135 | 133 | - | 5 |
| 2 | 91 | 80 | 20 | 13 |
| 3 | 107 | 100 | 44 | 11 |
| 5 | 74 | 73 | - | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, K.F.; Titov, A.A.; Smol’yakov, A.F.; Chernyadyev, A.Y.; Filippov, O.A.; Shubina, E.S. Mononuclear Copper(I) 3-(2-pyridyl)pyrazole Complexes: The Crucial Role of Phosphine on Photoluminescence. Molecules 2021, 26, 6869. https://doi.org/10.3390/molecules26226869
Baranova KF, Titov AA, Smol’yakov AF, Chernyadyev AY, Filippov OA, Shubina ES. Mononuclear Copper(I) 3-(2-pyridyl)pyrazole Complexes: The Crucial Role of Phosphine on Photoluminescence. Molecules. 2021; 26(22):6869. https://doi.org/10.3390/molecules26226869
Chicago/Turabian StyleBaranova, Kristina F., Aleksei A. Titov, Alexander F. Smol’yakov, Andrey Yu. Chernyadyev, Oleg A. Filippov, and Elena S. Shubina. 2021. "Mononuclear Copper(I) 3-(2-pyridyl)pyrazole Complexes: The Crucial Role of Phosphine on Photoluminescence" Molecules 26, no. 22: 6869. https://doi.org/10.3390/molecules26226869
APA StyleBaranova, K. F., Titov, A. A., Smol’yakov, A. F., Chernyadyev, A. Y., Filippov, O. A., & Shubina, E. S. (2021). Mononuclear Copper(I) 3-(2-pyridyl)pyrazole Complexes: The Crucial Role of Phosphine on Photoluminescence. Molecules, 26(22), 6869. https://doi.org/10.3390/molecules26226869








