Hazelnut Shells as Source of Active Ingredients: Extracts Preparation and Characterization
Abstract
:1. Introduction
2. Results and Discussions
2.1. Hazelnut Shell Grinding and Sieving
2.2. Extracts Preparation
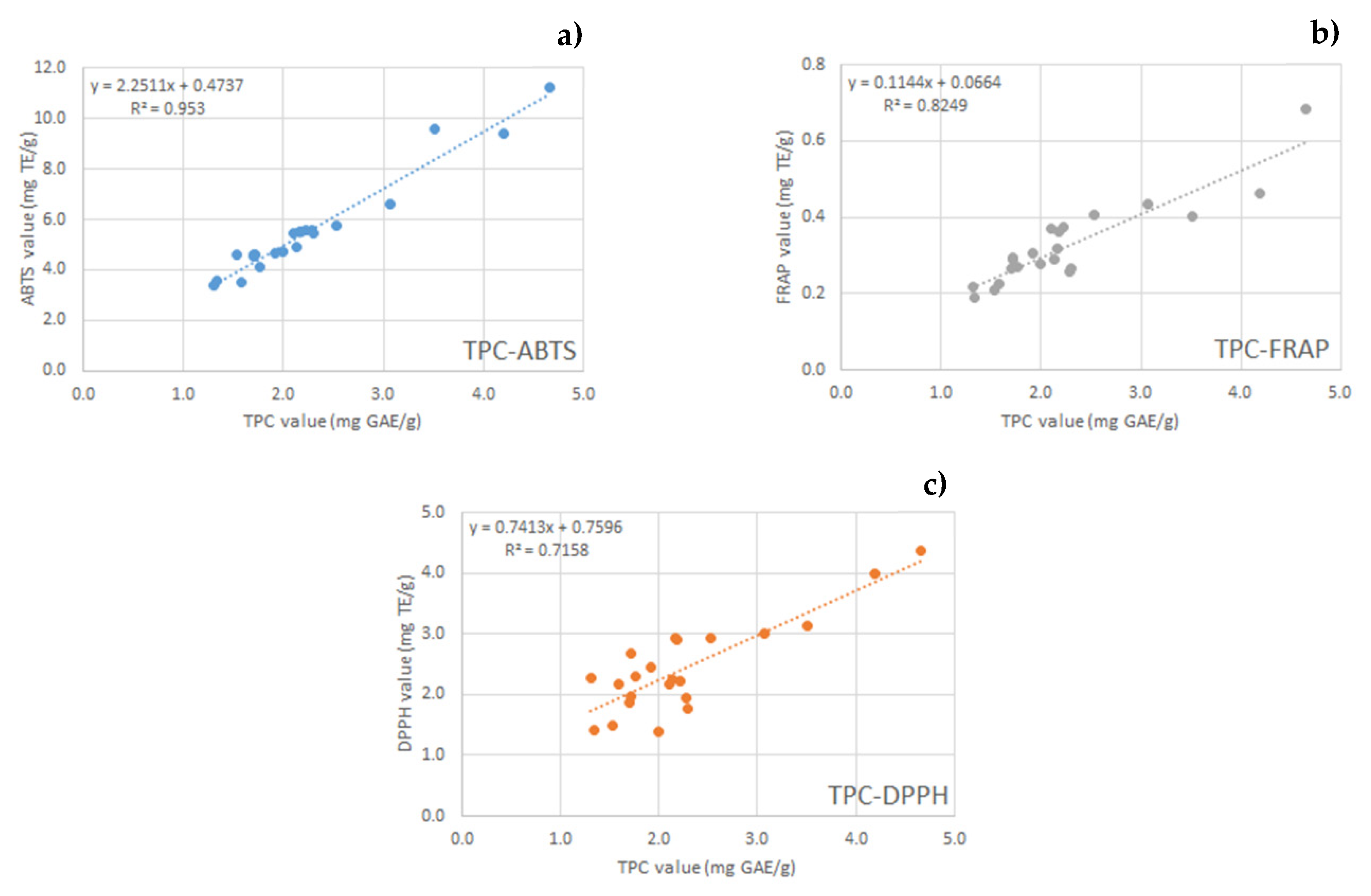
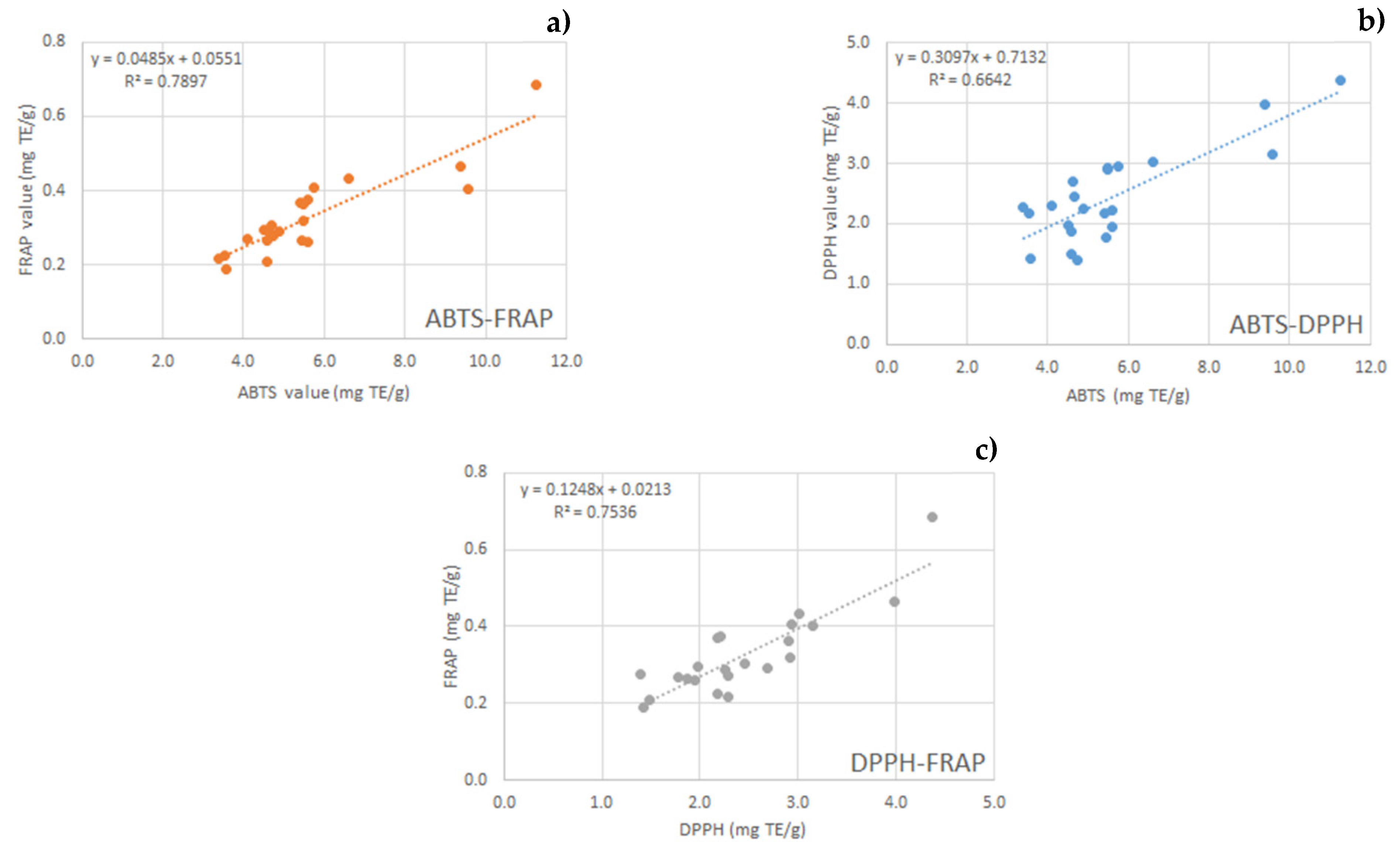
2.3. Total Phenol Content and Antioxidant Activity
2.4. Chemical Analysis
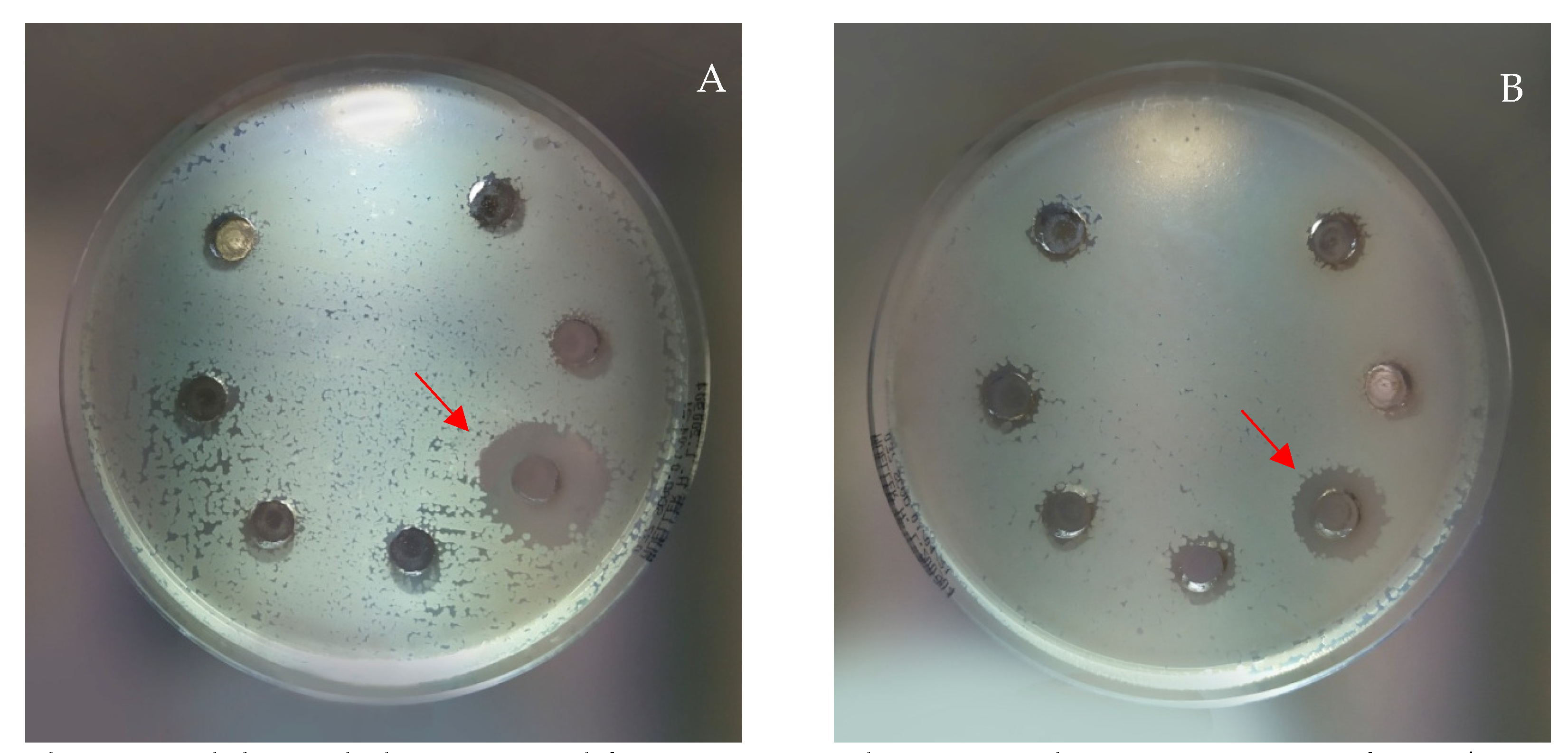
2.5. Antimicrobial Activity
3. Materials and Methods
3.1. Reagents
3.2. Hazelnut Shells Grinding and Sieving
3.3. Extracts Preparation
3.4. Total Phenol Content and Antioxidant Activity
3.5. Chemical Analysis
- Linear increase of B phase from 5% to 30% in 3.0 min;
- Linear increase of B phase from 30% to 40% in 1.4 min;
- Linear increase of B phase from 40% to 60% in 2.5 min;
- Linear increase of B phase from 60% to 99% in 1.5 min;
- Isocratic of B phase at 99% for 0.5 min.
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bottone, A.; Cerulli, A.; Durso, G.; Masullo, M.; Montoro, P.; Napolitano, A.; Piacente, S. Plant Specialized Metabolites in Hazelnut (Corylus avellana) Kernel and Byproducts: An Update on Chemistry, Biological Activity, and Analytical Aspects. Planta Med. 2019, 85, 840–855. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef]
- Ramalhosa, E.; Delgado, T.; Estevinho, L.; Pereira, J.A. Hazelnut (Corylus avellana L.) Cultivars and Antimicrobial Activity. In Nuts and Seeds in Health and Disease Prevention, 1th ed.; Academic Press: Cambrodge, MA, USA, 2011; pp. 627–636. [Google Scholar]
- Ciemniewska-Zytkiewicz, H.; Verardo, V.; Pasini, F.; Bryś, J.; Koczoń, P.; Caboni, M.F. Determination of lipid and phenolic fraction in two hazelnut (Corylus avellana L.) cultivars grown in Poland. Food Chem. 2015, 168, 615–622. [Google Scholar] [CrossRef]
- Şenol, H. Biogas potential of hazelnut shells and hazelnut wastes in Giresun City. Biotechnol. Reports 2019, 24, e00361. [Google Scholar] [CrossRef]
- Demirbas, A. Furfural production from fruit shells by acid-catalyzed hydrolysis. Energy Sources Part A Recover. Util. Environ. Eff. 2006, 28, 157–165. [Google Scholar] [CrossRef]
- Fanali, C.; Gallo, V.; Posta, S.D.; Dugo, L.; Mazzeo, L.; Cocchi, M.; Piemonte, V.; De Gara, L. Choline chloride–lactic acid-based NADES as an extraction medium in a response surface methodology-optimized method for the extraction of phenolic compounds from hazelnut skin. Molecules 2021, 26, 2652. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Koundal, R.; Agnihotri, V.K. Antioxidant properties and UPLC–MS/MS profiling of phenolics in jacquemont’s hazelnut kernels (Corylus jacquemontii) and its byproducts from western Himalaya. J. Food Sci. Technol. 2016, 53, 3522–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.M.; Mocan, A.; Crișan, G.; Vlase, L.; Loghin, F.; Popa, D.S.; Tomuta, I. Enhanced recovery of antioxidant compounds from hazelnut (Corylus avellana L.) involucre based on extraction optimization: Phytochemical profile and biological activities. Antioxidants 2019, 8, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects-A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Herrera, R.; Hemming, J.; Smeds, A.; Gordobil, O.; Willför, S.; Labidi, J. Recovery of bioactive compounds from hazelnuts and walnuts shells: Quantitative–qualitative analysis and chromatographic purification. Biomolecules 2020, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Colucci, G.; Apone, F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R.; et al. Bioadhesive Polymeric Films Based on Red Onion Skins Extract for Wound Treatment: An Innovative and Eco-Friendly Formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Calarco, P.; Ceccarini, M.R.; Cossignani, L.; di Michele, A.; et al. Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharm. 2020, 587, e119700. [Google Scholar] [CrossRef]
- Pagano, C.; Baiocchi, C.; Beccari, T.; Blasi, F.; Cossignani, L.; Ceccarini, M.R.; Orabona, C.; Orecchini, E.; Di Raimo, E.; Primavilla, S.; et al. Emulgel loaded with flaxseed extracts as new therapeutic approach in wound treatment. Pharmaceutics 2021, 13, 1107. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Hanna, M.A. Valorization of hazelnut shells into natural antioxidants by ultrasound-assisted extraction: Process optimization and phenolic composition identification. J. Food Process. Eng. 2018, 41, e12692. [Google Scholar] [CrossRef]
- Encyclopedia Britannica online. Choice Rev. Online 2004, 41.
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. Phenolic Compd. Biol. Act. 2017, 1–24. [Google Scholar]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef]
- Masullo, M.; Cerulli, A.; Mari, A.; de Souza Santos, C.C.; Pizza, C.; Piacente, S. LC-MS profiling highlights hazelnut (Nocciola di Giffoni PGI) shells as a byproduct rich in antioxidant phenolics. Food Res. Int. 2017, 101, 180–187. [Google Scholar] [CrossRef]
- Pérez-Armada, L.; Rivas, S.; González, B.; Moure, A. Extraction of phenolic compounds from hazelnut shells by green processes. J. Food Eng. 2019, 255, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Sismour, E.N.; Parry, J.; Hanna, M.A.; Li, H. Nutritional composition and antioxidant activity in hazelnut shells from US-grown cultivars. Int. J. Food Sci. Technol. 2012, 47, 940–946. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Nazzaro, M.; Valentina Mottola, M.; La Cara, F.; Del Monaco, G.; Patrizia Aquino, R.; Volpe, M.G. Extraction and characterization of biomolecules from agricultural wastes. Chem. Eng. Trans. 2012, 27. [Google Scholar]
- Perva-Uzunalić, A.; Škerget, M.; Knez, Ž.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Morais, J.S.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Chemical composition, and antioxidant and antimicrobial activities of three hazelnut (Corylus avellana L.) cultivars. Food Chem. Toxicol. 2008, 46, 1801–1817. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.I.; Nihei, K.I.; Nihei, A. Antibacterial Activity of Akyl Gallates against Bacillus subtilis. J. Agric. Food Chem. 2004, 52, 1072–1076. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Roila, R.; Ranucci, D.; Valiani, A.; Galarini, R.; Servili, M.; Branciari, R. Antimicrobial and anti-biofilm activity of olive oil by-products against Campylobacter spp. isolated from chicken meat. Acta Sci. Pol. Technol. Aliment. 2019, 18, 43–52. [Google Scholar]
- Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Servili, M.; Veneziani, G.; Branciari, R. Antimicrobial efficacy of a polyphenolic extract from olive oil by-product against “Fior di latte” cheese spoilage bacteria. Int. J. Food Microbiol. 2019, 295, 49–53. [Google Scholar] [CrossRef]
- Kayumov, A.R.; Khakimullina, E.N.; Sharafutdinov, I.S.; Trizna, E.Y.; Latypova, L.Z.; Thi Lien, H.; Margulis, A.B.; Bogachev, M.I.; Kurbangalieva, A.R. Inhibition of biofilm formation in Bacillus subtilis by new halogenated furanones. J. Antibiot. 2015, 68, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, A.; Cirioni, O.; Schimizzi, A.M.; Del Prete, M.S.; Barchiesi, F.; D’Errico, M.M.; Petrelli, E.; Scalise, G. Epidemiology and microbiology of surgical wound infections. J. Clin. Microbiol. 2000, 38, 918–922. [Google Scholar] [CrossRef] [Green Version]
- Ronco, T.; Aragao, M.F.; Svenningsen, S.; Christensen, J.B.; Permin, A.; Saaby, L.; Bionda, N.; Lantz, E.E.; Olsen, R.H. Efficacy of a novel antimicrobial hydrogel for eradication of Staphylococcus epidermidis, Staphylococcus aureus and Cutibacterium acnes from preformed biofilm and treatment performance in an in vivo MRSA wound model. JAC-Antimicrobial Resist. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Pîrvǎnescu, H.; BǎlǎŞoiu, M.; Ciurea, M.E.; Bǎlǎşoiu, A.T.; Mǎnescu, R. Wound infections with multi-drug resistant bacteria. Chir. 2014, 109, 73–79. [Google Scholar]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [Green Version]
- Pagano, C.; Perioli, L.; Blasi, F.; Bastianini, M.; Chiesi, C.; Cossignani, L. Optimisation of phenol extraction from wine using layered double hydroxides and technological evaluation of the bioactive-rich powder. Int. J. Food Sci. Technol. 2017, 52, 2582–2588. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pagnossa, J.P.; Blasi, F.; Cossignani, L.; Piccoli, R.H.; Zengin, G.; Montesano, D.; Cocconcelli, P.S.; Lucini, L. Phenolic profiling and in vitro bioactivity of Moringa oleifera leaves as affected by different extraction solvents. Food Res. Int. 2020, 127, 108712. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Blasi, F.; Angelini, P.; Di Simone, S.C.; Flores, G.A.; Cossignani, L.; Venanzoni, R. Extraction optimization by experimental design of bioactives from pleurotus ostreatus and evaluation of antioxidant and antimicrobial activities. Processes 2021, 9, 743. [Google Scholar] [CrossRef]
- Oliva, E.; Viteritti, E.; Fanti, F.; Eugelio, F.; Pepe, A.; Palmieri, S.; Sergi, M.; Compagnone, D. Targeted and semi-untargeted determination of phenolic compounds in plant matrices by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1651, 462315. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Phenolic Compounds | More Abundant Components |
|---|---|
| Phenolic Acids | gallic acid, vanillic acid, methyl gallate, veratric acid, galloylquinic acid, coumaroyl acid, quinic acid, feruloylquinic acid, protocatechuic acid |
| Flavonoids | quercetin, myricetin, quercetin 3-rhamnoside, myricetin 3-rhamnoside, rutin, taxifolin, naringin, catechin, epicatechin, epigallocatechin |
| Tannins | four isomers of B-type procyanidin |
| Diaryleptanoids | giffonin V, giffonin P, carpinontriol B |
| Lignans | ficusal, erythro-(7S,8R)-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol, erythro-(7S,8R)-guaiacylglycerol-β-coniferyl aldehyde ether, erythro-(7R,8S)-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol, dihydrodehydrodiconiferyl alcohol, balanophonin |
| Extract | Extraction Method | Extraction Temperature (°C) | Extraction Time (min) | Preventive Maceration | Yield of Freeze-Dried (%w/w) ± SD |
|---|---|---|---|---|---|
| A | MAC | 25 | 60 | no | 0.32 ± 0.02 |
| B | MAC | 25 | 60 | yes | 0.60 ± 0.03 |
| C | MAC | 25 | 180 | no | 0.51 ± 0.06 |
| D | MAC | 25 | 180 | yes | 0.64 ± 0.01 |
| E | MAC | 45 | 60 | no | 0.68 ± 0.03 |
| F | MAC | 45 | 60 | yes | 0.70 ± 0.06 |
| G | MAC | 45 | 180 | no | 0.81 ± 0.03 |
| H | MAC | 45 | 180 | yes | 1.10 ± 0.02 |
| I | MAC | 45 | 300 | no | 0.88 ± 0.05 |
| J | MAC | 45 | 300 | yes | 1.42 ± 0.07 |
| K | UB | 25 | 60 | no | 0.37 ± 0.19 |
| L | UB | 25 | 60 | yes | 0.50 ± 0.15 |
| M | UB | 25 | 120 | no | 0.57 ± 0.13 |
| N | UB | 25 | 120 | yes | 0.72 ± 0.15 |
| O | UB | 25 | 180 | no | 0.64 ± 0.07 |
| P | UB | 25 | 180 | yes | 0.77 ± 0.03 |
| Q | HPU | 25 | 5 | no | 0.47 ± 0.04 |
| R | HPU | 25 | 5 | yes | 0.96 ± 0.06 |
| S | HPU | 25 | 30 | no | 0.80 ± 0.22 |
| T | HPU | 25 | 30 | yes | 0.82 ± 0.27 |
| U | HPU | 25 | 60 | no | 0.96 ± 0.03 |
| V | HPU | 25 | 60 | yes | 0.94 ± 0.21 |
| Sample | TPC mg GAE/g | ABTS mg TE/g | DPPH mg TE/g | FRAP mg TE/g |
|---|---|---|---|---|
| A | 1.34 ± 0.03 | 3.57 ± 0.07 | 1.42 ± 0.09 | 0.19 ± 0.00 |
| B | 2.30 ± 0.07 | 5.46 ± 0.14 | 1.78 ± 0.14 | 0.27 ± 0.0 |
| C | 1.54 ± 0.05 | 4.59 ± 0.14 | 1.48 ± 0.01 | 0.21 ± 0.00 |
| D | 1.99 ± 0.01 | 4.73 ± 0.13 | 1.22 ± 0.26 | 0.28 ± 0.00 |
| E | 3.51 ± 0.24 | 9.57 ± 0.18 | 3.14 ± 0.01 | 0.40 ± 0.01 |
| F | 2.14 ± 0.05 | 4.89 ± 0.08 | 2.25 ± 0.03 | 0.29 ± 0.00 |
| G | 4.20 ± 0.05 | 9.37 ± 0.01 | 3.99 ± 0.29 | 0.46 ± 0.02 |
| H | 2.28 ± 0.04 | 5.59 ± 0.11 | 1.95 ± 0.16 | 0.26 ± 0.00 |
| I | 3.07 ± 0.04 | 6.61 ± 0.20 | 3.02 ± 0.15 | 0.43 ± 0.00 |
| J | 4.66 ± 0.08 | 11.25 ± 0.34 | 4.37 ± 0.35 | 0.68 ± 0.01 |
| K | 1.70 ± 0.06 | 4.59 ± 0.43 | 1.86 ± 0.13 | 0.26 ± 0.00 |
| L | 1.77 ± 0.01 | 4.10 ± 0.09 | 2.29 ± 0.10 | 0.27 ± 0.01 |
| M | 1.58 ± 0.04 | 3.54 ± 0.00 | 2.18 ± 0.16 | 0.22 ± 0.00 |
| N | 1.92 ± 0.06 | 4.68 ± 0.25 | 2.45 ± 0.19 | 0.30 ± 0.01 |
| O | 2.18 ± 0.06 | 5.50 ± 0.03 | 2.91 ± 0.09 | 0.36 ± 0.01 |
| P | 2.16 ± 0.05 | 5.49 ± 0.22 | 2.92 ± 0.07 | 0.32 ± 0.01 |
| Q | 1.31 ± 0.04 | 3.39 ± 0.10 | 2.28 ± 0.14 | 0.22 ± 0.01 |
| R | 1.71 ±0.00 | 4.51 ± 0.08 | 1.98 ± 0.02 | 0.30 ± 0.00 |
| S | 1.72 ± 0.03 | 4.63 ± 0.33 | 2.69 ± 0.04 | 0.29 ± 0.00 |
| T | 2.10 ± 0.05 | 5.43 ± 0.05 | 2.18 ± 0.09 | 0.37 ± 0.00 |
| U | 2.53 ± 0.03 | 5.74 ± 0.13 | 2.94 ± 0.09 | 0.41 ± 0.00 |
| V | 2.22 ± 0.04 | 5.59 ± 0.06 | 2.22 ± 0.07 | 0.37 ± 0.00 |
| Sample | Gallic Acid | Chlorogenic Acid | Catechin | Caffeic Acid | EGCG | Syringic Acid | Rutin | Isoquercetin | 3-OH-Benzoic Acid |
|---|---|---|---|---|---|---|---|---|---|
| A | 2022.0 ± 319.2 | 317.7 ± 47.4 | 688.1 ± 135.7 | 3.4 ± 0.5 | 44.8 ± 5.2 | 108.5 ± 11.0 | 1897.0 ± 211.8 | 76.14 ± 11.9 | 173.6 ± 22.0 |
| B | 2683.0 ± 495.6 | 348.2 ± 44.5 | 1267.5 ± 234.2 | 4.7 ± 0.5 | 81.9 ± 8.7 | 118.9 ± 16.3 | 1343.5 ± 165.4 | 72.87 ± 16.4 | 451.6 ± 70.0 |
| C | 1797.0 ± 346.6 | 354.2 ± 79.9 | 1067.0 ± 123.1 | 9.2 ± 1.0 | 142.1 ± 15.8 | 127.5 ± 12.8 | 2611.0 ± 261.8 | 111.4 ± 11.8 | 150.3 ± 17.8 |
| D | 1708.0 ± 245.4 | 366.5 ± 45.5 | 547.1 ± 90.0 | 2.7 ± 0.3 | 71.9 ± 10.6 | 121.7 ± 18.3 | 1223.5 ± 153.9 | 229.2 ± 26.0 | 143.4 ± 21.8 |
| E | 2334.0 ± 321.6 | 327.6 ± 53.8 | 1644.0 ± 226.9 | 9.4 ± 1.1 | 328.3 ± 11.3 | 209.0 ± 21.7 | 1442.5 ± 281.2 | 29.35 ± 4.4 | 35.56 ± 5.8 |
| F | 2201.5 ± 231.2 | 377.2 ± 87.2 | 1133.0 ± 195.1 | 1.1 ± 0.1 | 33.0 ± 5.7 | 135.1 ± 17.8 | 226.7 ± 58.2 | 107.6 ± 11.5 | 170.8 ± 20.4 |
| G | 1771.0 ± 294.2 | 351.9 ± 35.5 | 894.4 ± 101.8 | 4.9 ± 0.6 | 108.4 ± 8.1 | 125.2 ± 12.8 | 715.8 ± 133.8 | 19.04 ± 2.4 | 109.1 ± 11.4 |
| H | 1958.5 ± 356.4 | 394.2 ± 67.2 | 1115.0 ± 145.1 | 2.8 ± 0.3 | 88.8 ± 9.2 | 143.3 ± 14.9 | 193.0 ± 20.7 | 506.4 ± 115.2. | <LOQ |
| I | 10077.5 ± 1266.7 | 1345.5 ± 218.2 | 2760.0 ± 440.8 | 3.7 ± 0.5 | 51.0 ± 7.9 | 5.5 ± 1.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 108.4 ± 11.4 |
| J | 6759.0 ± 853.1 | 264.8 ± 53.8 | 1385.5 ± 139.9 | 1.6 ± 0.2 | 54.6 ± 3.5 | 4.6 ± 0.5 | 0.1 ± 0.1 | 0.9 ± 0.1 | 109.5 ± 16.2 |
| K | 8469.5 ± 1007.0 | 116.6 ± 15.6 | 1367.5 ± 233.4 | 1.2 ± 0.1 | 45.1 ± 2.3 | 7.1 ± 1.4 | 0.3 ± 0.1 | 0.2 ± 0.1 | 122.1 ± 13.1 |
| L | 11785.0 ± 1715.9 | 335.1 ± 40.6 | 1762.0 ± 238.6 | 9.7 ± 1.1 | 38.2 ± 2.2 | 8.2 ± 0.8 | 0.2 ± 0.1 | 0.8 ± 0.1 | 351.6 ± 46.3 |
| M | 12940.0 ± 2202.9 | 224.0 ± 24.9 | 1641.5 ± 293.6 | 2.2 ± 0.3 | 40.2 ± 2.7 | 7.2 ± 0.7 | 0.3 ± 0.1 | 0.4 ± 0.1 | 124.2 ± 15.5 |
| N | 7387.0 ± 820.7 | 565.4 ± 70.4 | 2005.5 ± 384.9 | 7.5 ± 0.7 | 53.5 ± 3.3 | 6.9 ± 0.7 | 0.1 ± 0.1 | 0.2 ± 0.1 | 142.5 ± 21.9 |
| O | 11710.0 ± 2036.4 | 198.1 ± 22.6 | 1588.5 ± 155.0 | 2.3 ± 0.3 | 35.2 ± 2.1 | 6.5 ± 0.7 | 0.2 ± 0.1 | 0.2 ± 0.1 | 159.6 ± 26.0 |
| P | 7220.0 ± 745.1 | 521.7 ± 58.4 | 2227.5 ± 304.9 | 2.7 ± 0.4 | 44.9 ± 3.7 | 7.6 ± 0.8 | <LOQ | 0.4 ± 0.1 | 256.3 ± 31.4 |
| Q | 5692.5 ± 884.6 | 112.7 ± 16.2 | 639.6 ± 88.3 | 3.5 ± 0.6 | 64.0 ± 3.3 | 4.4 ± 0.6 | 0.1 ± 0.1 | 0.3 ± 0.1 | 167.5 ± 27.9 |
| R | 7086.5 ± 1073.6 | 207.1 ± 34.7 | 1307.1 ± 241.0 | 2.4 ± 0.3 | 23.4 ± 1.1 | 7.2 ± 1.2 | 0.5 ± 0.1 | 0.2 ± 0.1 | 111.3 ± 12.2 |
| S | 5573.0 ± 669.9 | 289.3 ± 52.5 | 749.6 ± 125.2 | 13.8 ± 2.8 | 61.5 ± 1.8 | 6.9 ± 0.8 | 0.2 ± 0.1 | 0.2 ± 0.1 | 105.4 ± 23.4 |
| T | 8099.5 ± 852.9 | 1990.5 ± 224.0 | 1265.0 ± 275.0 | 2.7 ± 0.4 | 30.5 ± 2.6 | 7.9 ± 1.0 | <LOQ | 0.2 ± 0.1 | 104.1 ± 24.6 |
| U | 8318.5 ± 1461.7 | 139.9 ± 15.3 | 318.5 ± 46.6 | 7.5 ± 1.0 | 45.2 ± 4.1 | 2.2 ± 0.2 | 0.2 ± 0.1 | <LOQ | 145.7 ± 23.8 |
| V | 5736.5 ± 785.3 | 115.5 ± 21.1 | 1263.0 ± 180.4 | 8.4 ± 1.7 | 54.6 ± 3.6 | 5.4 ± 0.7 | 0.3 ± 0.1 | 0.4 ± 0.1 | 84.2 ± 10.2 |
| Sample | p-Coumaric Acid | Ferulic Acid | Luteolin | Quercetin | Apigenin | Diosmetin | Kampferol | Protocatechuic Acid | Naringenin |
| A | 124.8 ± 16.8 | 5.1 ± 0.5 | 57.0 ± 6.1 | 35.4 ± 4.2 | 18.1 ± 2.5 | 25.0 ± 2.6 | 4.2 ± 0.5 | 1406.0 ± 159.7 | 41.5 ± 4.7 |
| B | 97.4 ± 10.7 | 8.7 ± 1.1 | 69.1 ± 7.6 | 330.2 ± 41.5 | 66.4 ± 7.0 | 130.4 ± 13,4 | 112.5 ± 16.6 | 1794.5 ± 226.3 | 40.2 ± 5.1 |
| C | 113.8 ± 16.7 | 18.0 ± 1.8 | 64.7 ± 10.0 | 331.6 ± 43.6 | 14.8 ± 1.8 | 46.7 ± 8.2 | 2.3 ± 0.3 | 1419.5 ± 184.5 | 34.9 ± 5.8 |
| D | 98.9 ± 11.1 | 24.3 ± 3.7 | 108.4 ± 13.5 | 75.2 ± 7.7 | 24.2 ± 3.7 | 16.9 ± 2.0 | 28.2 ± 3.8 | 853.9 ± 125.4 | 42.9 ± 5.6 |
| E | 102.2 ± 12.8 | 58.1 ± 6.0 | 332.9 ± 36.0 | 493.4 ± 58.3 | 21.5 ± 3.5 | 27.5 ± 2.8 | 165.1 ± 20.4 | 1311.5 ± 172.1 | 33.0 ± 6.1 |
| F | 142.5 ± 18.4 | 4.8 ± 0.5 | 21.7 ± 2.3 | 608.5 ± 112.0 | 25.0 ± 2.6 | 18.5 ± 2.8 | 18.2 ± 2.3 | 1237.5 ± 198.4 | 36.1 ± 4.2 |
| G | 72.7 ± 7.3 | 21.3 ± 2.8 | 93.1 ± 12.3 | 227.7 ± 25.1 | 16.3 ± 2.4 | 27.8 ± 3.1 | 26.9 ± 4.8 | 1023.5 ± 107.8 | 28.8 ± 3.2 |
| H | 112.5 ± 17.3 | 41.0 ± 7.0 | 106.9 ± 13.2 | 2015.5 ± 208.2 | 24.8 ± 3.2 | 35.3 ± 4.6 | 211.5 ± 24.2 | 1210.0 ± 161.3 | 48.2 ± 6.9 |
| I | 10.4 ± 1.2 | 7.0 ± 0.8 | 5.8 ± 0.7 | 44.9 ± 6.9 | 0.1 ± 0.1 | 10.4 ± 1.3 | <LOQ | 297.5 ± 36.0 | 48.0 ± 8.0 |
| J | 16.4 ± 1.8 | 3.8 ± 0.4 | 9.4 ± 1.0 | 22.5 ± 5.8 | 0.1 ± 0.1 | 16.9 ± 2.0 | <LOQ | 242.3 ± 29.6 | 37.2 ± 5.0 |
| K | 14.5 ± 2.0 | 7.7 ± 1.5 | 7.1 ± 0.9 | 10.6 ± 1.2 | 0.1 ± 0.1 | 5.3 ± 1.0 | <LOQ | 510.1 ± 74.3 | 37.8 ± 3.9 |
| L | 16.3 ± 1.7 | 6.8 ± 0.8 | 16.1 ± 1.8 | 280.5 ± 28.4 | 0.2 ± 0.1 | 22.0 ± 2.5 | 1.1 ± 0.1 | 534.3 ± 57.1 | 46.8 ± 8.5 |
| M | 16.6 ± 1.7 | 7.0 ± 0.7 | 10.0 ± 1.0 | 23.3 ± 1.8 | 0.1 ± 0.1 | 12.2 ± 1.5 | 0.3 ± 0.1 | 667.4 ± 82.4 | 52.5 ± 6.3 |
| N | 9.3 ± 1.5 | 5.0 ± 0.8 | 8.9 ± 1.1 | 30.3 ± 3.3 | 0.1 ± 0.1 | 11.1 ± 2.0 | <LOQ | 280.7 ± 38.3 | 50.2 ± 6.0 |
| O | 12.7 ± 1.6 | 6.1 ± 0.6 | 6.2 ± 1.1 | 21.8 ± 3.1 | 0.1 ± 0.1 | 9.5 ± 1.1 | <LOQ | 590.3 ± 129.4 | 35.4 ± 5.9 |
| P | 11.8 ± 1.5 | 7.8 ± 0.9 | 6.9 ± 1.0 | 193.7 ± 23.8 | 0.1 ± 0.1 | 11.8 ± 1.3 | <LOQ | 291.1 ± 36.6 | 61.5 ± 15.5 |
| Q | 8.5 ± 1.3 | 5.2 ± 0.6 | 8.0 ± 1.0 | 23.4 ± 3.2 | 0.1 ± 0.1 | 11.3 ± 2.1 | <LOQ | 303.2 ± 54.9 | 42.6 ± 6.8 |
| R | 9.2 ± 1.3 | 6.8 ± 0.9 | 7.8 ± 1.2 | 27.0 ± 4.1 | 0.1 ± 0.1 | 10.5 ± 1.5 | <LOQ | 322.5 ± 44.9 | 54.2 ± 6.3 |
| S | 9.2 ± 1.2 | 3.5 ± 0.4 | 7.9 ± 1.7 | 13.5 ± 2.0 | 0.1 ± 0.1 | 8.5 ± 1.0 | <LOQ | 285.7 ± 39.1 | 31.3 ± 3.8 |
| T | 7.6 ± 1.4 | 6.3 ± 0.9 | 3.5 ± 0.4 | 12.9 ± 1.8 | 0.1 ± 0.1 | 7.0 ± 0.9 | <LOQ | 388.7 ± 63.8 | 50.9 ± 5.5 |
| U | 10.5 ± 1.3 | 3.8 ± 0.5 | 6.8 ± 0.7 | 20.0 ± 2.4 | 0.1 ± 0.1 | 12.2 ± 1.4 | 0.2 ± 0.1 | 255.4 ± 38.9 | 32.9 ± 5.2 |
| V | 7.0 ± 0.8 | 6.4 ± 0.6 | 9.8 ± 1.5 | 284.2 ± 57.3 | 0.1 ± 0.1 | 17.3 ± 2.6 | 1.6 ± 0.1 | 305.4 ± 47.0 | 51.0 ± 5.9 |
| Extract | S. aureus | S. epidermidis | S. pyogenes | E. faecalis | B. subtilis | B. cereus | P. aeruginosa | E. coli | C. albicans |
|---|---|---|---|---|---|---|---|---|---|
| A | - | - | - | - | - | 8 | - | - | - |
| B | - | - | - | - | - | - | - | - | - |
| C | - | - | - | - | - | 9 | - | - | - |
| D | - | - | - | - | - | 9 | - | - | - |
| E | - | - | - | - | - | - | - | - | - |
| F | - | - | - | - | - | 9 | - | - | - |
| G | - | - | - | - | - | 9 | - | - | - |
| H | - | - | - | - | - | 9 | - | - | - |
| I | 9 | - | - | - | 12 | 8 | - | - | - |
| J | 9 | - | - | - | 12 | 8 | - | - | - |
| K | 9 | - | - | - | 17 | - | - | - | - |
| L | - | - | - | - | 11 | - | - | - | - |
| M | 9 | 14 | - | - | 12 | 9 | - | - | - |
| N | - | - | - | - | 12 | - | - | - | - |
| O | - | - | - | - | 11 | - | - | - | - |
| P | - | - | - | - | 14 | - | - | - | - |
| Q | - | 11 | - | - | 13 | - | - | - | - |
| R | - | - | - | - | 13 | - | - | - | - |
| S | - | - | - | - | 8 | - | - | - | - |
| T | - | - | - | - | 10 | - | - | - | - |
| U | - | - | - | - | 10 | - | - | - | - |
| V | - | - | - | - | 8 | - | - | - | - |
| Strains | Growth Conditions |
|---|---|
| Gram-positive bacteria Staphylococcus aureus WDCM 00034 | 37 °C for 24 h |
| Staphylococcus epidermidis WDCM 00036 | 37 °C for 24 h |
| Streptococcus pyogenes ATCC 19615 | 37 °C for 24–48 h |
| Enterococcus faecalis WDCM 00087 | 37 °C for 24 h |
| Bacillus subtilis WDCM 00003 | 30 °C for 24 h |
| Bacillus cereus WDCM 00001 | 30 °C for 24 h |
| Gram-negative bacteria | |
| Pseudomonas aeruginosa WDCM 00025 | 25 °C for 24–48 h |
| Escherichia coli WDCM 00013 | 37 °C for 24 h |
| Yeast | |
| Candida albicans WDCM 00054 | 25 °C for 48–72 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Michele, A.; Pagano, C.; Allegrini, A.; Blasi, F.; Cossignani, L.; Raimo, E.D.; Faieta, M.; Oliva, E.; Pittia, P.; Primavilla, S.; et al. Hazelnut Shells as Source of Active Ingredients: Extracts Preparation and Characterization. Molecules 2021, 26, 6607. https://doi.org/10.3390/molecules26216607
Di Michele A, Pagano C, Allegrini A, Blasi F, Cossignani L, Raimo ED, Faieta M, Oliva E, Pittia P, Primavilla S, et al. Hazelnut Shells as Source of Active Ingredients: Extracts Preparation and Characterization. Molecules. 2021; 26(21):6607. https://doi.org/10.3390/molecules26216607
Chicago/Turabian StyleDi Michele, Alessandro, Cinzia Pagano, Agnese Allegrini, Francesca Blasi, Lina Cossignani, Enrico Di Raimo, Marco Faieta, Eleonora Oliva, Paola Pittia, Sara Primavilla, and et al. 2021. "Hazelnut Shells as Source of Active Ingredients: Extracts Preparation and Characterization" Molecules 26, no. 21: 6607. https://doi.org/10.3390/molecules26216607
APA StyleDi Michele, A., Pagano, C., Allegrini, A., Blasi, F., Cossignani, L., Raimo, E. D., Faieta, M., Oliva, E., Pittia, P., Primavilla, S., Sergi, M., Vicino, C., Ricci, M., Schirone, B., & Perioli, L. (2021). Hazelnut Shells as Source of Active Ingredients: Extracts Preparation and Characterization. Molecules, 26(21), 6607. https://doi.org/10.3390/molecules26216607











