Abstract
Arsenic (As) poisoning is widespread due to exposure to pollution. The toxic level of (As) causes oxidative stress-induced aging and tissue damage. Since melatonin (MLT) has anti-oxidant and anti-aging properties, we aimed to evaluate the protective effect of MLT against the toxicity of sodium arsenite (NaAsO2). Healthy male NMRI mice were divided into eight different groups. The control group received a standard regular diet. Other groups were treated with varying diets, including MLT alone, NaAsO2, and NaAsO2 plus MLT. After one month of treatment, biochemical and pathological tests were performed on blood, heart, and lung tissue samples. NaAsO2 increased the levels of TNF-α, 8-hydroxy-2-deoxy guanosine (8OHdG), malondialdehyde (MDA), reactive oxygen species (ROS), and high mobility group box 1 (HMGB1), increased the expression of TNF receptor type 1-associated death domain (TRADD) mRNA and telomerase reverse transcriptase, and decreased the expression of Klotho (KL) mRNA in both plasma and tissues. In contrast, MLT reduced MDA, ROS, HMGB1, lactate, and TNF-α enhanced the mRNA expression of KL, and suppressed the mRNA expression of the TERT and TRADD genes. Thus, MLT confers potent protection against NaAsO2- induced tissue injury and oxidative stress.
1. Introduction
Arsenic is a metalloid and the number one toxin on the United States Environmental Protection Agency (USEPA) list of prioritized contaminants [1]. It remains one of the most important pollutants in groundwater [2]. Its concentration in drinking water of more than 50 µg/L is not considered safe for public health, but such levels are still common in many countries [1]. It has also been detected in several cosmetics, skin, face, hair, and herbal products. It is absorbed directly into the bloodstream through the skin and then accumulates in the body, causing toxic effects in different organs [3]. The inactivation of approximately 200 enzymes causes arsenic’s acute and chronic toxicity; many of these are involved in cellular energy pathways and in DNA synthesis and repair. Nausea, vomiting, stomach pain, and diarrhea are examples of acute toxicity, while chronic toxicity can lead to multisystem diseases and cancer affecting multiple organs [4]. Arsenic is cytotoxic, mutagenic, and genotoxic because it causes oxidative DNA damage, reduces anti-oxidant enzymes, and generates reactive oxygen species (ROS) [5]. The activation of telomerase is a crucial step in the progression of human cancers. The telomerase reverse transcriptase (TERT) gene’s transcriptional repression of its catalytic portion keeps it silent in healthy cells [6,7]. As a clastogenic agent, arsenic reduces telomerase expression and telomere length, causing apoptosis, necrosis, and the generation of ROS, and induces cell death [8].
Melatonin (MLT), a hormone secreted by the pineal gland, plays an essential role in circadian regulation. It also has anti-oxidant, anti-aging, immunomodulation, and cancer-fighting effects [9,10]. MLT helps avoid cellular aging by scavenging radicals and reducing oxidative damage to mitochondria [10]. MLT also helps in the reduction of mitomycin-C-induced genotoxic damage in peripheral blood in rats [11]. MLT can inhibit breast cancer cell proliferation and angiogenesis, cancer cell invasion, and telomerase function [12]. MLT decreases telomerase activity in tumor cells and mRNA expression of the TERT and TR subunits, restoring telomerase function [13,14]. The evidence indicates that MLT exposure can play a role in preventing oxidative stress-induced aging. This study is designed to evaluate whether MLT treatment can protect against oxidative stress-induced aging triggered by sodium arsenite (NaAsO2).
Arsenic causes genotoxicity in human peripheral blood, while co-treatment with MLT significantly reduces genotoxic measures [15] and brain tissue damage [16,17]. In vitro studies have shown that MLT decreases arsenite-induced autophagy and promotes mitochondrial biogenesis in primary cultured neurons [18]. MLT treatment reduces pro-inflammatory cytokines, suggesting that it can protect the CNS from arsenic-induced oxidative stress, DNA damage, and apoptosis [19]. MLT has also shown protective effects against arsenic-induced metabolic toxicity [20], arsenic-induced liver/kidney toxicity, and arsenic-induced testicular injury [21]. These studies reveal that MLT protects against arsenic-induced toxicity in blood, liver, kidney, and testicular tissues. This study aims to evaluate whether MLT treatment protects the heart and lung tissues against oxidative stress-induced aging triggered by sodium arsenite (NaAsO2) as a potential protective effect of MLT against arsenic-induced aging in these tissues has not yet been explored.
2. Results
2.1. Total Tissue Arsenic Using an Inductively Coupled Plasma Mass Spectrometer
The concentrations of As in the heart and lung tissue samples exposed to 1/2, 1/3, and 1/10 LD50 NaAsO2, with and without MLT treatment, are shown in Table 1. The total As tissue concentration was found to be increased in both heart and lung tissue in a dose-dependent manner compared to the control group. MLT treatment, on the other hand, was shown to lower As tissue concentration.

Table 1.
Total As concentration in heart and lung tissues.
2.2. Oxidative Stress Biomarkers
Malondialdehyde (MDA) is known as one of the crucial markers for oxidative stress assessment. As a high-grade toxicant, this chemical compound is a byproduct of cellular lipid peroxidation [22]. Heart and lung samples were examined after treatment with different doses of NaAsO2 alone or combined with MLT to assess oxidative stress biomarkers. In both heart and lung samples, we found similar levels of oxidative stress with varying quantities of NaAsO2 (1/2, 1/3, and 1/10 LD50). There was no statistical difference between the MDA levels of the control and MLT groups. A significant dose-dependent increase in the MDA levels of the 1/10 (both p < 0.05), 1/3 (both p < 0.0001), and ½ LD50 (both p < 0.0001) NaAsO2 groups was observed, in contrast to the control and MLT groups. However, MLT treatment prevented the increase in NaAsO2-induced MDA levels in both heart and lung samples compared to the ½ LD50 NaAsO2 group. A significant decrease in MDA levels in the MLT + NaAsO2 group was observed. These findings indicate that exposure to MLT can help reverse higher oxidative stress levels caused by NaAsO2 (Table 2).

Table 2.
Assessment of oxidative stress biomarkers.
2.3. Reactive Oxygen Species
Reactive oxygen species (ROS) belong to the oxygen derivates; they damage cellular functions and have toxic effects. ROS molecules are composed of one oxygen and several unpaired electrons during inflammation [23]. Heart and lung samples were examined after treatment with different doses of NaAsO2 alone or in combination with MLT to assess reactive oxygen species. In both heart and lung samples, we found similar levels of ROS with varying quantities of NaAsO2 (1/2, 1/3, and 1/10 LD50). There was no statistical difference between the ROS levels of the control and the MLT groups. A significant dose-dependent increase in the ROS levels of the 1/10, 1/3, and ½ LD50 NaAsO2 groups (all p < 0.0001) was observed, in contrast to the control and MLT groups. However, MLT treatment prevented the increase in NaAsO2-induced ROS levels in both heart and lung samples compared to the ½ LD50 NaAsO2 group. A significant decrease in ROS levels in the MLT + NaAsO2 group (all p < 0.0001) was observed. These findings indicate that exposure to MLT can help reverse the increase in reactive oxygen species levels caused by NaAsO2 (Table 2).
2.4. Pro-Inflammatory TNF-α Cytokine
One of the essential pro-inflammatory cytokines in the early stage of inflammation is tumor necrosis factor-alpha (TNF-α). This cytokine is released from the macrophage when an inflammatory pathway is initiated, which causes cellular dysfunction. TNF-α is an immune system mediator in response to internal or external pathogens [24]. Plasma samples were examined after treatment with different doses of NaAsO2 alone or in combination with MLT for the assessment of TNF-α. There was no statistical difference in the TNF-α levels of the control and MLT groups. A significant dose-dependent increase in the TNF-α levels of the 1/10, 1/3, and ½ LD50 NaAsO2 groups (all p < 0.0001) was observed, in contrast to the control and MLT groups. However, MLT treatment prevented the increase in NaAsO2-induced TNF-α levels compared to the ½ LD50 NaAsO2 group. A significant decrease in TNF-α levels in the MLT + NaAsO2 group was observed. These findings indicate that exposure to MLT can help reverse the increase in TNF-α levels caused by NaAsO2 (Figure 1).
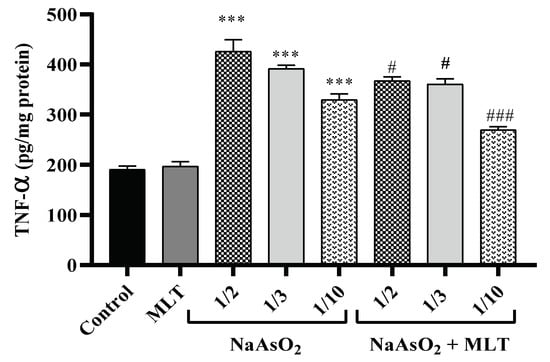
Figure 1.
Assessment of pro-inflammatory TNF-α. Abbreviations: TNF-α: tumor necrosis factor-alpha; MLT; melatonin, NaAsO2; sodium arsenite; Results are expressed as mean ± SEM for five animals in each group (n = 5). *** p < 0.0001 (Vs. control or melatonin) ### p < 0.0001; # p < 0.05 (Vs. ½ LD50 NaAsO2).
2.5. High Mobility Group Box 1 Levels
High mobility group box 1 (HMGB1), a conserved nuclear protein and late mediator of inflammatory pathways, has a regulatory role in cellular damage during inflammation [25]. Heart and lung samples were examined for the level of HMGB1. No statistically significant difference was found between the HMGB1 levels of the control and the MLT groups. Compared to the control and MLT groups, a significant increase in HMGB1 levels in the ½ LD50 NaAsO2 group (both p < 0.0001) was observed. However, compared to the ½ LD50 NaAsO2 group, MLT treatment prevented the increase in NaAsO2-induced HMGB1 levels in both heart and lung samples. A significant decrease in levels of HMGB1 was observed in the MLT + NaAsO2 group. These results show that exposure to MLT will help reverse high levels of HMGB1 induced by NaAsO2 (Figure 2).
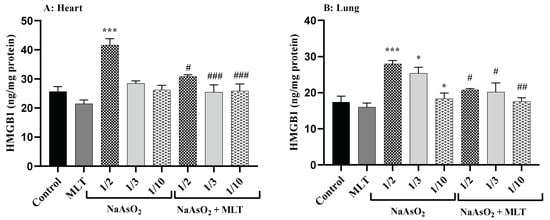
Figure 2.
Assessment of High Mobility Group Box 1 (A) High Mobility Group Box 1 levels in heart; (B) High Mobility Group Box 1 levels in lung Abbreviations: HMGB1: High Mobility Group Box 1; MLT; melatonin, NaAsO2; sodium arsenite; Results are expressed as mean ± SEM for five animals in each group (n = 5). *** p < 0.0001; * p < 0.05 (Vs. control or melatonin). ### p < 0.0001; ## p < 0.001; # p < 0.05 (Vs. ½ LD50 NaAsO2).
2.6. 8-Hydroxy-2-deoxyguanosine Levels
8-hydroxy-2-deoxy guanosine (8-OHdG) is a DNA oxidation product that establishes as an oxidative stress marker. Environmental factors affect the hydroxylation of guanosine [26]. Plasma samples were examined after treatment with different doses of NaAsO2 alone or combined with MLT to assess 8OHdG. There was no statistical difference between the 8OHdG levels of the control and the MLT groups. Significant increases in 8OHdG levels in the 1/2 and 1/3 LD50 NaAsO2 groups (both p < 0.0001) were observed, in contrast to the control or MLT group. However, MLT treatment prevented the increase in NaAsO2-induced 8OHdG levels compared to the ½ LD50 NaAsO2 group. A significant decrease in 8OHdG levels in the MLT + NaAsO2 group was observed. These findings indicate that exposure to MLT can help reverse oxidative DNA damage caused by NaAsO2 (Figure 3).
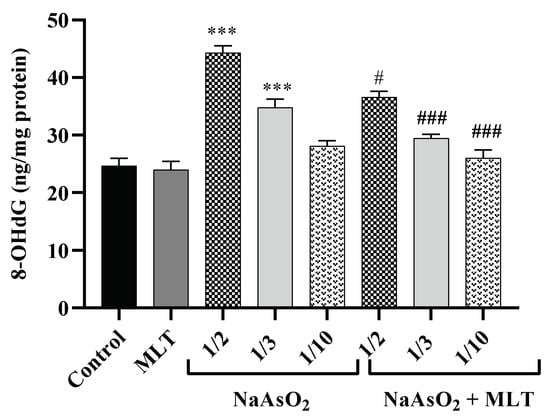
Figure 3.
Assessment of 8-hydroxy-2-deoxyguanosine Abbreviations: 8OHdG: 8-hydroxy-2-deoxyguanosine; MLT; melatonin, NaAsO2; sodium arsenite; Results are expressed as mean ± SEM for five animals in each group (n = 5). *** p < 0.0001 (Vs. control or melatonin) ### p < 0.0001; # p < 0.05 (Vs. ½ LD50 NaAsO2).
2.7. Lactate Levels
Lactate synthesis increases during impaired cellular function and elevation of anaerobic reactions. Elevated lactate level is an indicator of mitochondrial damage and tissue hypoperfusion [27]. Heart and lung samples were examined after treatment with different doses of NaAsO2 alone or with MLT to assess lactate. We found different lactate levels in heart and lung samples. There was no statistical difference between the lactate levels of the control and the MLT groups.
In contrast to the control or MLT group, a significant increase in lactate levels in the 1/10, 1/3, and ½ LD50 NaAsO2 groups was observed. However, MLT treatment prevented the increase in NaAsO2-induced lactate level in both heart and lung samples compared to the 1/10 LD50 NaAsO2 and ½ LD50 NaAsO2 groups (all p < 0.0001). A significant decrease in lactate levels in the MLT + NaAsO2 group was observed. These findings indicate that exposure to MLT can help reverse increased lactate levels caused by NaAsO2 (Table 3).

Table 3.
Assessment of lactate.
2.8. Real-Time Reverse Transcription PCR for Gene Expression
In the presence of ½ (fold change 0.09) and 1/3 (fold change 0.23) LD50 of NaAsO2 (both p < 0.0001), the mRNA expression level of the anti-aging KL gene in the heart samples was significantly downregulated compared to the control (fold change 1.02) or MLT (fold change 1.02) groups (both p < 0.0001). On the other hand, MLT treatment tended to reduce the effects of NaAsO2, as demonstrated by the higher mRNA expression levels of KL genes in all treatment groups (all p < 0.0001) compared to the ½ LD50 NaAsO2 group. In the ½, 1/3, and 1/10 LD50 NaAsO2 + MLT treatment groups, the mRNA KL gene expression level increased compared to the ½ LD50 NaAsO2 group (All p < 0.0001). TERT is a telomerase catalytic subunit that, along with the telomerase RNA component (TERC), forms the fundamental unit of the telomerase complex. In normal cells, TERT gene expression is inactive or very low. In comparison to the control (fold change 1.00) and MLT (fold change 0.92) groups, we observed that exposure to ½ (fold change 2.54) and 1/3 (fold change 2.30) LD50 NaAsO2 (both p < 0.001) significantly doubled TERT gene mRNA expression. On the other hand, MLT exposure tended to minimize and normalize TERT overexpression in the treatment groups. The ½ and 1/10 LD50 NaAsO2 + MLT treatment groups had significantly decreased mRNA TERT gene expression (both p < 0.001). TRADD is a human adaptor protein responsible for programmed cell death and is encoded by the TRADD gene. Similar to TERT, in comparison to the control (1.03-fold) and MLT (1.02-fold) groups, we observed that exposure to ½ (1.81-fold) and 1/3 (1.56-fold) LD50 NaAsO2 (both p < 0.05) significantly increased TRADD gene mRNA expression. When compared to the ½ LD50 NaAsO2 group, MLT treatment reduced TRADD gene mRNA expression by 1.16 and 1.09-fold in the ½ and 1/10 LD50 NaAsO2 + MLT treatment groups (both p < 0.05) (Figure 4).
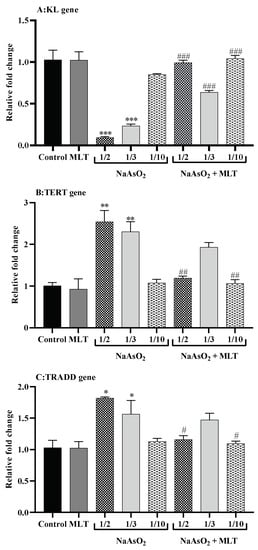
Figure 4.
Quantitative real-time reverse transcription PCR for gene expression (A) Telomerase reverse transcriptase (TERT) gene expression; (B) Type 1 associated death domain tumor necrosis factor receptor (TRADD) gene expression; (C) Klotho related (KL) gene expression. Abbreviations: MLT; melatonin, NaAsO2; sodium arsenite; Results are expressed as mean ± SEM. Samples were analyzed in triplicate. *** p < 0.0001; ** p < 0.001; * p < 0.05 (Vs. control or melatonin) ### p < 0.0001; ## p < 0.001; # p < 0.05 (Vs. ½ LD50 NaAsO2).
2.9. Histological Evaluations of Heart and Lung Tissues
Representative results of the histological analysis of heart and lung tissues retrieved from rats treated with melatonin and NaAsO2 in 1/2, 1/3, 1/10 doses are presented in Figure 5. Any changes, including congestion, acute inflammatory response, necrosis, hemorrhage, or hyperemia, have been comparatively assessed in the different groups. Micrographs of heart and lung tissue from the 1/3 and 1/10 doses were normal without any histopathological changes. Histopathological evaluation of the group injected with the 1/2 dose of arsenic showed myocardial cell necrosis. However, in the 1/2 dose group, which also received melatonin, less necrosis of the heart tissue was apparent. When compared to the group that received melatonin and 1/2 arsenic in the lung, the group receiving only 1/2 arsenic showed more hyperemia, edema, and inflammatory cell infiltration.
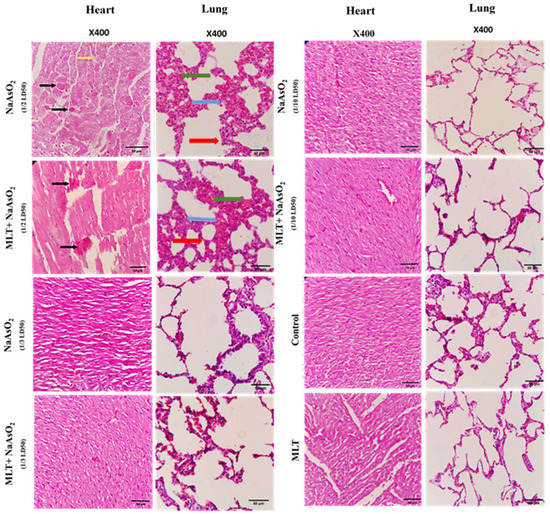
Figure 5.
Hematoxylin–eosin-stained sections of tissues retrieved from animals treated with melatonin and arsenic in 1/2, 1/3, 1/10 doses, obtained at sacrifice after 30 days’ post-treatment. Black arrow refers to myocardial necrosis, Red arrow refers to inflammatory cells, Blue refers to Edema, Green arrow refers to hyperemia.
3. Discussion
NaAsO2 is widely used in agriculture because of its antifungal, antibacterial, herbicidal, and rodenticidal properties. As a result of the widespread use of arsenic-based herbicides, pesticides, and livestock antibiotics [28]. On the other hand, MLT has shown anti-inflammatory, anti-oxidant, and anti-free radical effects [29]. Scavenging reactive oxygen and nitrogen species and boosting anti-oxidant defense prevents tissue damage and blocks transcriptional factors of pro-inflammatory cytokines [30]. MLT helps minimize arsenic-induced oxidative stress, as shown in this study. Exposure to the ½, 1/3, and 1/10 LD50 NaAsO2 caused an increase in the total As tissue concentration in both heart and lung tissues in a dose-dependent manner. MLT treatment, on the other hand, was found to lower As tissue concentration. MDA is a lipid peroxidation marker that plays a key role in the rise of oxidative stress by increasing ROS and early-stage inflammatory markers like TNF-α [31,32].
In contrast to the control and MLT groups, we found that NaAsO2 exposure caused a significant dose-dependent increase in MDA and, subsequently, ROS levels. MLT treatment was shown to reverse NaAsO2-induced MDA and ROS levels in both heart and lung samples. Our results are similar to those of Taysi et al., who identified that MLT could reverse oxidative injury caused by irradiation [33]. HMGB1 is an inflammatory cytokine that is released during the delayed phase of inflammation. We found the highest level of HMGB1 in both heart and lung tissues exposed to 1/2 LD50 NaAsO2, even after tissue extraction on the 30th day of analysis. MLT treatment, on the other hand, resulted in lower levels of HMGB1.
Similarly, plasma samples exposed to 1/2, 1/3, and 1/10 LD50 NaAsO2 showed elevated levels of TNF-α and 8OHdG, which was prevented by MLT, suggesting its role as a protector against oxidative DNA damage [34]. High lactate levels are used as a biomarker for tissue hypoperfusion and mitochondrial insufficiency [35]. After 30 days, there was an increase in lactate levels in both heart and lung tissues, indicating that NaAsO2 has a detrimental effect on mitochondria. On the other hand, MLT was found to defend against this effect, reducing lactate levels in both tissues.
The protective effect of MLT was also confirmed by assessing KL, TERT, and TRADD mRNA expression levels. KL has been identified as a gene that plays a role in the aging process [36]. In rats, arsenite reduces circulating and renal KL and causes tubular damage [37]. In the heart tissue exposed to NaAsO2, we discovered a dose-dependent significant decrease (p < 0.0001) in the expression of mRNA KL, which was recovered after MLT treatment (p < 0.0001). Our results were similar to those of a study conducted on KL mutant mice, a genetic model of aging, in which MLT minimized oxidative stress and memory loss associated with KL deficiency [38]. The counteracting effect of MLT on aging was further confirmed in our study by the mRNA TERT gene expression level. In telomeres, which are strongly conserved and susceptible to age-dependent gradual attrition [39], NaAsO2 effectively doubled TERT gene mRNA expression in a dose-dependent manner. At the same time, MLT appeared to reduce and normalize TERT overexpression in the treatment groups, indicating that MLT counteracts NaAsO2-induced heart tissue aging. TRADD is a cell death gene that contributes to tissue aging by activating NF-кB and inducing apoptosis [40,41]. The apoptosis-inducing effect of NaAsO2 on heart tissues was confirmed by a significant increase in TRADD gene mRNA expression, which was decreased by MLT treatment. This indicates that MLT treatment restores regular TRADD gene mRNA expression and thus prevents heart tissue injury (Figure 5).
In this study, the IP administration of both agents was preferred over the oral route to avoid the possible modification and degradation in the gastrointestinal tract. Additionally, IP administration provides faster and more reliable absorption than oral, ensuring optimal bioavailability of both small and large agents in rodent experimental studies [42].
Several studies and reviews indicate that melatonin produces significantly higher protective effects than the classic anti-oxidants [43]. In these studies, melatonin was found to be more effective than vitamin E [44,45,46], Beta-carotene [47], and Ascorbic acid [48,49]. Furthermore, in clinical settings, including chronic diseases such as rheumatoid arthritis [50], blood hypertension [51], and ischemia [52], beneficial anti-oxidant effects of melatonin in comparison to vitamins have been reported. Similarly, we found that exposure to NaAsO2 causes oxidative stress in the lungs and heart tissues dose-dependently in drug and chemical toxicity. At the high ½ LD50 NaAsO2 exposure level, TNF-α and 8OHdG in the plasma and MDA, ROS, and HMGB1 in the heart and lung tissues were elevated. These facts indicate that NaAsO2 exposure triggered oxidative stress in pulmonary and heart tissues by producing MDA and ROS and increasing the circulation of pro-inflammatory cytokines such as TNF-α. Tissue hypoperfusion, mitochondrial insufficiency, and inflammation may be caused by high tissue lactate and HMGB1 levels. Increased plasma 8OHdG levels suggest that NaAsO2 causes DNA damage.
According to the histological analysis, dose 1/2 of NaAsO2 in the heart tissues of rats caused cell necrosis correlated with the results for TERT and TRADD gene mRNA expression, showing a significant increase in the apoptosis-inducing effects of arsenic on heart tissues (Figure 4 and Figure 5). However, with the combined use of MLT and NaAsO2, necrosis clearance was much more potent and faster in MLT presence than in its absence. This clearance is evidenced by the decreased mRNA expression levels of the TERT and TRADD genes. Analogously, MLT attenuated the toxic effects of arsenic in histologically examined lung specimens, being correlated with the results obtained from inflammatory and oxidative stress biomarkers and the decrease in the score of hyperemia, edema, and inflammatory cells (Table 4). Furthermore, NaAsO2 exposure resulted in lower KL mRNA expression and higher TERT and TRADD mRNA expression, indicating a risk of heart tissue aging and cell death.

Table 4.
Histopathological scoring.
On the other hand, MLT treatment reduced MDA, ROS, HMGB1, and lactate levels in lung and heart tissues, as well as TNF-α and 8OHdG levels in plasma. It also boosted the mRNA expression of the KL gene while suppressing the mRNA expression of the TERT and TRADD genes. This indicates that MLT protects lung and heart tissues by reversing the risk of oxidative stress-induced aging triggered by NaAsO2. However, more research is needed to explore the protective role of MLT against arsenic-induced aging in other tissues such as the liver and kidney and the efficacy of MLT in lowering tissue arsenic levels.
4. Materials and Methods
4.1. Chemicals
MLT (CAS number 73-31-4), NaAsO2 (CAS number 7784-46-5), Suprapur HNO3 (Merck, Darmstadt, Germany), and all other chemicals used in this study were purchased from Sigma-Aldrich (GmbH, Munich, Germany). The Lactate isolation kit and ELISA kit for high mobility group box 1 (HMGB1) analysis were purchased from ZellBio GmbH (Ulm, Munich, Germany), and the TNF-α analysis kit was obtained from Diaclone (Besancon Cedex, France). Thermo Scientific Revert Aid first strand cDNA Synthesis Kit was obtained from Thermo Fisher (Vilnius, Lithuania).
4.2. Ethical Approval
Animal handling, care, killing, and other procedures involving animals were conducted in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Biochemical and laboratory research and experiments were performed as per Good Laboratory Practices. The Research Committee at the National Institute for Medical Research Development (NIMAD) provided ethical approval for this study involving the use and treatment of animals under a code number: IR.NIMAD.REC.1397.542.
4.3. Study Design
In this study, we used male mice, not females, since the cyclic variations in the level of female hormones can influence toxicological studies. Thus, healthy and disease-free adult 30–35 g male NMRI mice aged 7 to 8 weeks were obtained from the animal house of the Faculty of Pharmacy of Tehran University of Medical Sciences, Tehran, Iran. They were kept under controlled environmental conditions, including room temperature of 20 to 25 °C, 50 to 55% relative humidity, and 12-h light and dark period. They had free access to a standard diet and water. The mice were divided into eight groups of six mice each. Group 1 received a standard normal diet. Group 2 received intraperitoneal (IP) injection of 10 mg/kg/day MLT, Group 3, 4 and 5 received 1.5 (1/10 LD50), 5 (1/3 LD50) and 7.5 (1/2 LD50) mg/kg NaAsO2, respectively. Groups 6, 7, and 8 were injected intraperitoneally with 1.5 (1/10 LD50), 5 (1/3 LD50), and 7.5 (1/2 LD50) mg/kg of NaAsO2 along with 10 mg/kg/day MLT, respectively during the last ten days of the experiment. After one month of treatment, IP injections of 100 mg/kg of ketamine and 10 mg/kg xylazine were used to kill the mice. Blood samples were collected in EDTA and serum separator tubes. The hearts and lungs were immediately removed and frozen at −80 °C for biochemical analysis. The tissue was collected from the heart and lung specimens for pathological examination. After washing with sterile phosphate buffer (pH 7.4), they were kept in 10 mL 10% formalin (Figure 6).
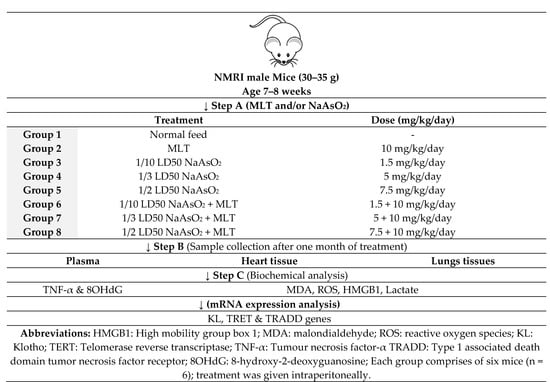
Figure 6.
Study design.
4.4. Assessment of Total Tissue Arsenic Using an Inductively Coupled Plasma Mass Spectrometer
The total arsenic in sample tissues was determined using an Inductively Coupled Plasma Mass Spectrometer (ICP-MS) (Perkin Elmer ELAN 6100 DRC-e). The instrument parameters are mentioned in the supplementary Table S1. Two mL phosphate buffer was added to 0.20 g of each sample, and the tissues were thoroughly homogenized. The homogenized tissues were transferred to glass test tubes and dried on a sand bath at 120 °C (approximately overnight). After the samples had reached room temperature, each tube was filled with 0.5 mL concentrated HNO3 and heated for two days. Temperatures did not exceed 120 °C during the heating process. Then 0.5 mL concentrated HNO3 was added to each test tube until white ash remained. After the digestion was completed and the solutions had cooled, 1 mL 10% HNO3 was added to each glass tube and sonicated for 30 min at 50 °C. The contents of the glass tubes were transferred to a 5 mL volumetric flask and calibrated to the sign with 10% HNO3. 10% HNO3 was used to make standard solutions with concentrations of 1, 5, 10, and 15 µg/L of As, and Germanium standard solution (5 g/L) was used as an internal standard solution [53].
4.5. Assessment of Oxidative Stress Biomarkers
Thiobarbituric acid reactive substance (TBARS) assay was used to measure lipid peroxidation (LPO) activity by calculating levels of malondialdehyde (MDA) in heart and lung tissues. Heart or lung tissue samples of 0.15 g each were homogenized in phosphate buffer saline (PBS), blended with 800 mL of trichloroacetic acid, and centrifuged for 40 min at 3000 g. Then, 150 mL of 1% w/v Thiobarbituric acid was added to the 600 mL supernatant and placed in boiling water for 15 minutes. Finally, 400 mL of n-butanol was added, and a spectrophotometer was used to report absorbance at 532 nm [54].
4.6. Assessment of Reactive Oxygen Species
ROS levels in heart and lung samples were calculated according to the previously described protocol [55]; 100 mg of each fresh heart and lung tissue sample were homogenized with an extraction buffer solution containing HEPES (5 mM), KCl (20 mM), EDTA, (1 mM), and sucrose (0.25 M), pH = 7.4 into each vial phosphate buffer and centrifuged at 10000 g for 10 min at 4 °C after adding the DL-dithiothreitol (DTT, 50 µM). In the next step, the supernatants of the heart and lung tissue samples were mixed with 80 µL of assay buffer and 5 µL of DCFH-DA (5 µM) for 15 min in 37 °C; 2′,7′-dichlorofluorescein diacetate (DCF-DA) is a cell-penetrating fluorogenic agent that converts into 2′,7′-dichlorodihydrofluorescein (DCFH). DCFH was then esterase-catalyzed intracellularly and ROS-oxidized into 2′,7′-dichlorofluorescein (DCF). Finally, the DCF absorbance was measured every 5 min by a multi-mode microplate spectrophotometer reader (BioTek®, SynergyTM HT, Winooski, VT, USA) for 60 min with excitation and emission spectra of 488 nm and 525 nm, respectively. For standardizing data obtained from the ROS assay, each group of diluted tissue samples was used for determining the protein level. In summary, 10 µL Bradford reagent was added to 100 µL of diluted samples, and after incubation at room temperature for 15 min, the absorbance of each sample was read at 595 nm.
4.7. Assessment of Pro-Inflammatory Cytokines
Pro-inflammatory cytokine levels were evaluated, i.e., TNF-α in plasma and HMGB1 in heart and lung samples. These are recognized as the primary mediator of sepsis in the early and late phases [56]. HMGB1 level was performed for the heart and lung tissues, which were kept at −80 °C. An amount of 100 mg of each animal’s isolated heart and lung tissues was isolated and homogenized in phosphate buffer saline (PBS). The TNF-α and HMGB1 levels were quantified according to the manufacturer’s protocol. Absorbance was estimated at 450 nm. The TNF-α and HMGB1 levels were recorded in pg/mg protein and ng/mg protein, respectively.
4.8. Assessment of DNA Damage
Eight-hydroxy-2-deoxyguanosine (8OHdG) is a product of oxidative damage to DNA. It is an essential biomarker for DNA damage. Plasma levels of 8-hydroxy-2-deoxy guanosine (8OHdG) were determined according to the 8OHdG ELISA kit (ZellBio GmbH [Ulm, Germany]).
4.9. Assessment of Lactate Level
Tissue sample lactate levels were measured using a colorimetric lactate assay kit according to the provided protocol (ZellBio GmbH [Ulm, Germany]). In brief, 10 mL of perchloric acid 8% was homogenized with 100 mg of heart and lung samples. The standard curve was applied and recorded as mmol/mg of tissue protein to measure lactate levels.
4.10. Quantitative Real-Time Reverse Transcription PCR for Gene Expression
To detect the function of MLT and NaAsO2 in gene expression at the level of mRNA, quantitative real-time reverse transcription PCR (qRT-PCR) was used. Three genes associated with different molecular pathways, TERT, type 1 associated death domain tumor necrosis factor receptor (TRADD), and KL (related to Klotho activity), were evaluated in this respect. Tissue RNA was extracted using the RNX-Plus solution (SinaClon, Iran) protocol from frozen heart samples. RNA purity was spectrophotometrically calculated by Thermo Scientific NanoDrop (Thermo Scientific, Boston, MA, USA). The RNase-Free DNase I Kit was used to remove genomic DNA, then one microgram of extracted RNA was reverse transcribed to cDNA. Target genes have been normalized with the Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene as an internal control. The LightCycler® 96 System (Roche, Indianapolis, IN, USA) was used for qRT-PCR analysis. The SYBR green master mix was used, and qRT-PCR was performed under conditions, i.e., one cycle at 94 °C for 10 min, 40 cycles at 95 °C for 15 s, and annealing temperature for 30 and then 72 °C for 25 s. The results were reported according to the Pfaffle Method [57]. The specific primers used in the present study are shown in Table 5.

Table 5.
The primers of genes used for RT-PCR.
4.11. Histological Ex Vivo Evaluations
Heart and lung tissue samples were harvested after sacrificing the animals at day 30, while tissue samples were stored in 10 mL of 0.9% NaCl solution. The specimens were fixed in 10% neutral buffered formalin (NBF, PH. 7.26) for 48 h, embedded in paraffin, and cut into 5 µm sections. The sections were stained with hematoxylin-eosin and evaluated by an independent examiner using light microscopy (Olympus BX51; Olympus, Tokyo, Japan).
4.12. Statistical Analysis
The results were presented as mean ± standard error of the mean (SEM). Statistical significance was assessed using a one-way analysis of variance (ANOVA) with statistical significance at p < 0.05.
5. Conclusions
We conclude that MLT treatment can help reduce NaAsO2-induced oxidative stress-mediated aging. NaAsO2 causes oxidative stress, inflammation, DNA damage, and cell death in lung and heart tissues by raising MDA, ROS, lactate, HMGB1, TNF-α, and 8OHdG levels, which may lead to tissue aging and injury, as evidenced by decreased mRNA expression levels of KL induced by NaAsO2, and increased expression of the TERT and TRADD genes (Figure 7). MLT treatment, on the other hand, reduced the dose-dependent toxicity of NaAsO2. MLT has been shown to protect the lungs and heart from NaAsO2-induced tissue injury and aging by reducing the risk of oxidative stress, inflammation, DNA damage, and normalizing the mRNA expression levels of the KL, TERT, and TRADD genes.
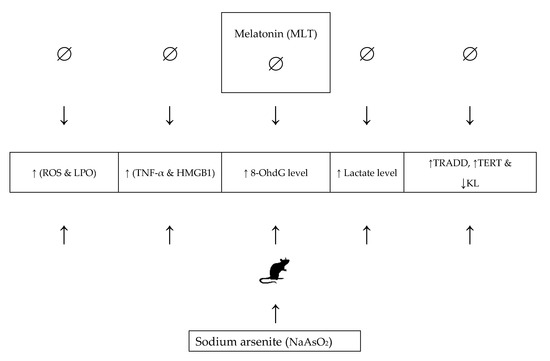
Figure 7.
The role and mechanism of melatonin in alleviating NaAsO2-induced aging. Abbreviations: ROS: reactive oxygen species; LPO: lipid peroxidation; HMGB1: high mobility group box 1; TRADD: Tumour necrosis factor-α (TNF-α) receptor type 1-associated death domain; TERT: telomerase reverse transcriptase; KL: Klotho.
Supplementary Materials
The following are available online. Table S1: Instrument parameters for inductively coupled plasma mass spectrometer (ICP-MS).
Author Contributions
Conception and design of the study: M.A., M.B. and T.D.; Acquisition of data: M.B., T.D., M.K., S.M.-N., R.F. (Ramtin Farhadi), M.R., Z.B., H.H.-A., M.G. and R.F. (Roham Foroumadi); Analysis and/or interpretation of data: M.R., Z.B., H.H.-A., S.M.D.; Drafting the article: M.A., M.B., T.D., M.K., S.M.-N., S.M.D.; Revision and approval of the submitted version: M.B., T.D., M.K., S.M.-N., R.F. (Ramtin Farhadi), M.R., Z.B., H.H.-A., R.F. (Roham Foroumadi), S.M.D., M.G. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
Elite Researcher Grant Committee supported the research described in this manuscript under award number 972793 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran, received by Maryam Baeeri.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of NIMAD involving the use and treatment of animals under a code number: IR.NIMAD.REC.1397.542.
Acknowledgments
Authors acknowledge the Iran National Science Foundation (INSF) support as a non-specific seat award directed to MA. The authors thank the help of the labs’ staff.
Conflicts of Interest
There is no conflict of interest among authors.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Ng, J.C.; Wang, J.; Shraim, A. A Global Health Problem Caused by Arsenic from Natural Sources. Chemosphere 2003, 52, 1353–1359. [Google Scholar] [CrossRef]
- Wu, J. Challenges for Safe and Healthy Drinking Water in China. Curr. Environ. Health Rep. 2020, 7, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Metals in Cosmetics: Implications for Human Health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Ratnaike, R.N. Acute and Chronic Arsenic Toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kessel, M.; Liu, S.X.; Xu, A.; Santella, R.; Hei, T.K. Arsenic Induces Oxidative DNA Damage in Mammalian Cells. Mol. Cell Biochem. 2002, 234–235, 301–308. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms Underlying the Activation of TERT Transcription and Telomerase Activity in Human Cancer: Old Actors and New Players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akincilar, S.C.; Unal, B.; Tergaonkar, V. Reactivation of Telomerase in Cancer. Cell. Mol. Life Sci. CMLS 2016, 73, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, D.; Collotta, A.; Carfi, M.; Bowe, G.; Vahter, M.; Hartung, T.; Gribaldo, L. Arsenic Induces Telomerase Expression and Maintains Telomere Length in Human Cord Blood Cells. Toxicology 2009, 260, 132–141. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Patel, K.K.; Dehari, D.; Agrawal, A.K.; Singh, S. Melatonin and Its Ubiquitous Anticancer Effects. Mol. Cell. Biochem. 2019, 462, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Anti-oxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Gutiérrez, S.; López-Vicente, M.; Lostalé, F.; Fuentes-Broto, L.; Martínez-Ballarín, E.; García, J.J. Protective Effect of Melatonin Against Mitomycin C-Induced Genotoxic Damage in Peripheral Blood of Rats. J. Biomed. Biotechnol. 2009, 2009, 791432. [Google Scholar] [CrossRef]
- Nooshinfar, E.; Safaroghli-Azar, A.; Bashash, D.; Akbari, M.E. Melatonin, an Inhibitory Agent in Breast Cancer. Breast Cancer 2017, 24, 42–51. [Google Scholar] [CrossRef]
- Leon-Blanco, M.M.; Guerrero, J.M.; Reiter, R.J.; Calvo, J.R.; Pozo, D. Melatonin Inhibits Telomerase Activity in the MCF-7 Tumor Cell Line both In Vivo and In Vitro. J. Pineal. Res. 2003, 35, 204–211. [Google Scholar] [CrossRef]
- Martínez-Campa, C.M.; Alonso-González, C.; Mediavilla, M.D.; Cos, S.; González, A.; Sanchez-Barcelo, E.J. Melatonin Down-Regulates hTERT Expression Induced by Either Natural Estrogens (17beta-estradiol) or Metalloestrogens (cadmium) in MCF-7 Human Breast Cancer Cells. Cancer Lett. 2008, 268, 272–277. [Google Scholar] [CrossRef]
- Pant, H.H.; Rao, M.V. Evaluation of In Vitro Anti-Genotoxic Potential of Melatonin against Arsenic and Fluoride in Human Blood Cultures. Ecotoxicol. Environ. Saf. 2010, 73, 1333–1337. [Google Scholar] [CrossRef]
- Lin, A.M.; Feng, S.F.; Chao, P.L.; Yang, C.H. Melatonin Inhibits Arsenite-Induced Peripheral Neurotoxicity. J. Pineal. Res. 2009, 46, 64–70. [Google Scholar] [CrossRef]
- Lin, A.M.; Fang, S.F.; Chao, P.L.; Yang, C.H. Melatonin Attenuates Arsenite-Induced Apoptosis in Rat Brain: Involvement of Mitochondrial and Endoplasmic Reticulum Pathways and Aggregation of Alpha-Synuclein. J. Pineal. Res. 2007, 43, 163–171. [Google Scholar] [CrossRef]
- Teng, Y.C.; Tai, Y.I.; Huang, H.J.; Lin, A.M. Melatonin Ameliorates Arsenite-Induced Neurotoxicity: Involvement of Autophagy and Mitochondria. Mol. Neurobiol. 2015, 52, 1015–1022. [Google Scholar] [CrossRef]
- Durappanavar, P.N.; Nadoor, P.; Waghe, P.; Pavithra, B.H.; Jayaramu, G.M. Melatonin Ameliorates Neuropharmacological and Neurobiochemical Alterations Induced by Subchronic Exposure to Arsenic in Wistar Rats. Biol. Trace. Elem. Res. 2019, 190, 124–139. [Google Scholar] [CrossRef]
- Pal, S.; Chatterjee, A.K. Prospective Protective Role of Melatonin against Arsenic-Induced Metabolic Toxicity in Wistar Rats. Toxicology 2005, 208, 25–33. [Google Scholar] [CrossRef]
- Uygur, R.; Aktas, C.; Caglar, V.; Uygur, E.; Erdogan, H.; Ozen, O.A. Protective Effects of Melatonin against Arsenic-Induced Apoptosis and Oxidative Stress in Rat Testes. Toxicol. Ind. Health 2016, 32, 848–859. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species-Sources, Functions, Oxidative Damage. Pol. Merkur. Lekarski. 2020, 48, 124–127. [Google Scholar]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-A Signaling in Macrophages. Crit. Rev.™ Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Vijayakumar, E.C.; Bhatt, L.K.; Prabhavalkar, K.S. High Mobility Group Box-1 (HMGB1): A Potential Target in Therapeutics. Curr. Drug Targets 2019, 20, 1474–1485. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Kraut, J.A.; Madias, N.E. Lactic Acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczack, T.; Scheckel, K.; Guerinot, M.L. Understanding Arsenic Dynamics in Agronomic Systems to Predict and Prevent Uptake by Crop Plants. Sci. Total Environ. 2017, 581–582, 209–220. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.; Pellegrini, J.N. A Review of Recent Studies on Malondialdehyde as Toxic Molecule and Biological Marker of Oxidative Stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Saravanan, A.; Elangovan, A.; Ramesh, S.; Annamalai, S.; Namachivayam, A.; Abel, P.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; et al. An Appraisal on Molecular and Biochemical Signalling Cascades during Arsenic-Induced Hepatotoxicity. Life Sci. 2020, 260, 118438. [Google Scholar]
- Taysi, S.; Koc, M.; Büyükokuroğlu, M.E.; Altinkaynak, K.; Sahin, Y.N. Melatonin Reduces Lipid Peroxidation and Nitric Oxide during Irradiation-Induced Oxidative Injury in the Rat Liver. J. Pineal. Res. 2003, 34, 173–177. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, J.; Postelnicu, R.; Mukherjee, V. Lactate: Where Are We Now? Crit. Care Clin. 2020, 36, 115–124. [Google Scholar] [CrossRef]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging and the Failing Kidney. Front. Endocrinol 2020, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Sosa, C.; Guillén, N.; Lucea, S.; Sorribas, V. Effects of Oral Exposure to Arsenite on Arsenic Metabolism and Transport in Rat Kidney. Toxicol. Lett. 2020, 333, 4–12. [Google Scholar] [CrossRef]
- Shin, E.J.; Chung, Y.H.; Le, H.L.; Jeong, J.H.; Dang, D.K.; Nam, Y.; Wie, M.B.; Nah, S.Y.; Nabeshima, Y.; Nabeshima, T.; et al. Melatonin Attenuates Memory Impairment Induced by Klotho Gene Deficiency Via Interactive Signaling between MT2 Receptor, ERK, and Nrf2-Related Anti-oxidant Potential. Int. J. Neuropsychopharm. 2014, 18, 1–14. [Google Scholar]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yuan, W.; Lin, Z. Functional Roles in Cell Signaling of Adaptor Protein TRADD from a Structural Perspective. Comput. Struct. Biotechnol. J. 2020, 18, 2867–2876. [Google Scholar] [CrossRef]
- Pobezinskaya, Y.L.; Liu, Z. The Role of TRADD in Death Receptor Signaling. Cell Cycle 2012, 11, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Matzneller, P.; Kussmann, M.; Eberl, S.; Maier-Salamon, A.; Jäger, W.; Bauer, M.; Langer, O.; Zeitlinger, M.; Poeppl, W. Pharmacokinetics of the P-Gp Inhibitor Tariquidar in Rats after Intravenous, Oral and Intraperitoneal Administration. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal. Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Baydas, G.; Canatan, H.; Turkoglu, A. Comparative Analysis of the Protective Effects of Melatonin and Vitamin E on Streptozocin-Induced Diabetes Mellitus. J. Pineal. Res. 2002, 32, 225–230. [Google Scholar] [CrossRef]
- Wahab, M.H.A.; Akoul, E.-S.E.; Abdel-Aziz, A.-A.H. Modulatory Effects of Melatonin and Vitamin E on Doxorubicin-Induced Cardiotoxicity in Ehrlich Ascites Carcinoma-Bearing Mice. Tumor. J. 2000, 86, 157–162. [Google Scholar] [CrossRef]
- Montilla, P.; Cruz, A.; Padillo, F.J.; Túnez, I.; Gascon, F.; Muñoz, M.C.; Gómez, M.; Pera, C. Melatonin Versus Vitamin E as Protective Treatment against Oxidative Stress after Extra-Hepatic Bile Duct Ligation in Rats. J. Pineal. Res. 2001, 31, 138–144. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Han, B.; Liu, M.; Yeh, C.; Casida, J.E. Phosphine-Induced Oxidative Damage in Rats: Attenuation by Melatonin. Free Radic. Biol. Med. 2000, 28, 636–642. [Google Scholar] [CrossRef]
- Gultekin, F.; Delibas, N.; Yasar, S.; Kilinc, I. In Vivo Changes in Anti-oxidant Systems and Protective Role of Melatonin and a Combination of Vitamin C and Vitamin E on Oxidative Damage in Erythrocytes Induced by Chlorpyrifos-Ethyl in Rats. Arch. Toxicol. 2001, 75, 88–96. [Google Scholar] [CrossRef]
- Rosales-Corral, S.; Tan, D.X.; Reiter, R.J.; Valdivia-Velázquez, M.; Martínez-Barboza, G.; Acosta-Martínez, J.P.; Ortiz, G.G. Orally Administered Melatonin Reduces Oxidative Stress and Proinflammatory Cytokines Induced by Amyloid-β Peptide in Rat Brain: A Comparative, In Vivo Study Versus Vitamin C and E. J. Pineal. Res. 2003, 35, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Stone, T.W.; Darlington, L.G. Inflammatory Status and Kynurenine Metabolism in Rheumatoid Arthritis Treated with Melatonin. Br. J. Clin. Pharmacol. 2007, 64, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Kędziora-Kornatowska, K.; Szewczyk-Golec, K.; Czuczejko, J.; Pawluk, H.; van Marke de Lumen, K.; Kozakiewicz, M.; Bartosz, G.; Kedziora, J. Antioxidative Effects of Melatonin Administration in Elderly Primary Essential Hypertension Patients. J. Pineal. Res. 2008, 45, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuokam, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative Stress Impairs Oocyte Quality and Melatonin Protects Oocytes from Free Radical Damage and Improves Fertilization Rate. J. Pineal. Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Hodjat, M.; Rahimifard, M.; Nigjeh, M.N.; Azizi, M.; Baeeri, M.; Bayrami, Z.; Gholami, M.; Hassani, S.; Abdollahi, M. Assessment of Arsenic-Induced Modifications in the DNA Methylation of Insulin-Related Genes in Rat Pancreatic Islets. Ecotoxicol. Environ. Saf. 2020, 201, 110802. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Browne, R. The Analysis of Free Radicals, Lipid Peroxides, Anti-oxidant Enzymes and Compounds Related to Oxidative Stress as Applied to the Clinical Chemistry Laboratory. Adv. Exp. Med. Biol. 1994, 366, 43–58. [Google Scholar]
- Baeeri, M.; Rahimifard, M.; Daghighi, S.M.; Khan, F.; Salami, S.A.; Moini-Nodeh, S.; Haghi-Aminjan, H.; Bayrami, Z.; Rezaee, F.; Abdollahi, M. Cannabinoids as Anti-ROS in Aged Pancreatic Islet Cells. Life Sci. 2020, 256, 117969. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, Y.-Q. The Regulatory Role of High-Mobility Group Protein 1 in Sepsis-Related Immunity. Front. Immunol. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).