Garcinia Biflavonoid 1 Improves Lipid Metabolism in HepG2 Cells via Regulating PPARα
Abstract
:1. Introduction
2. Results
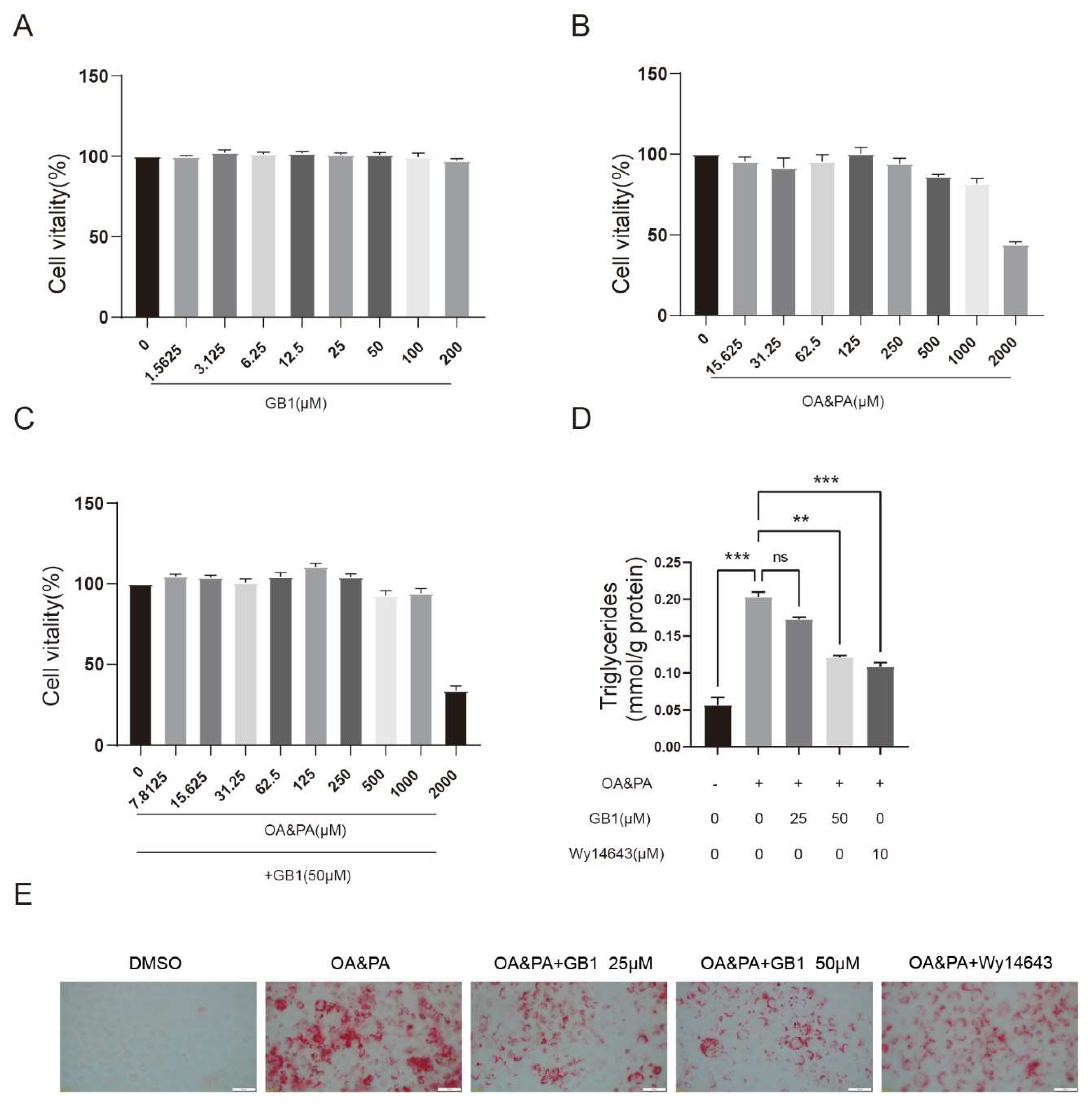
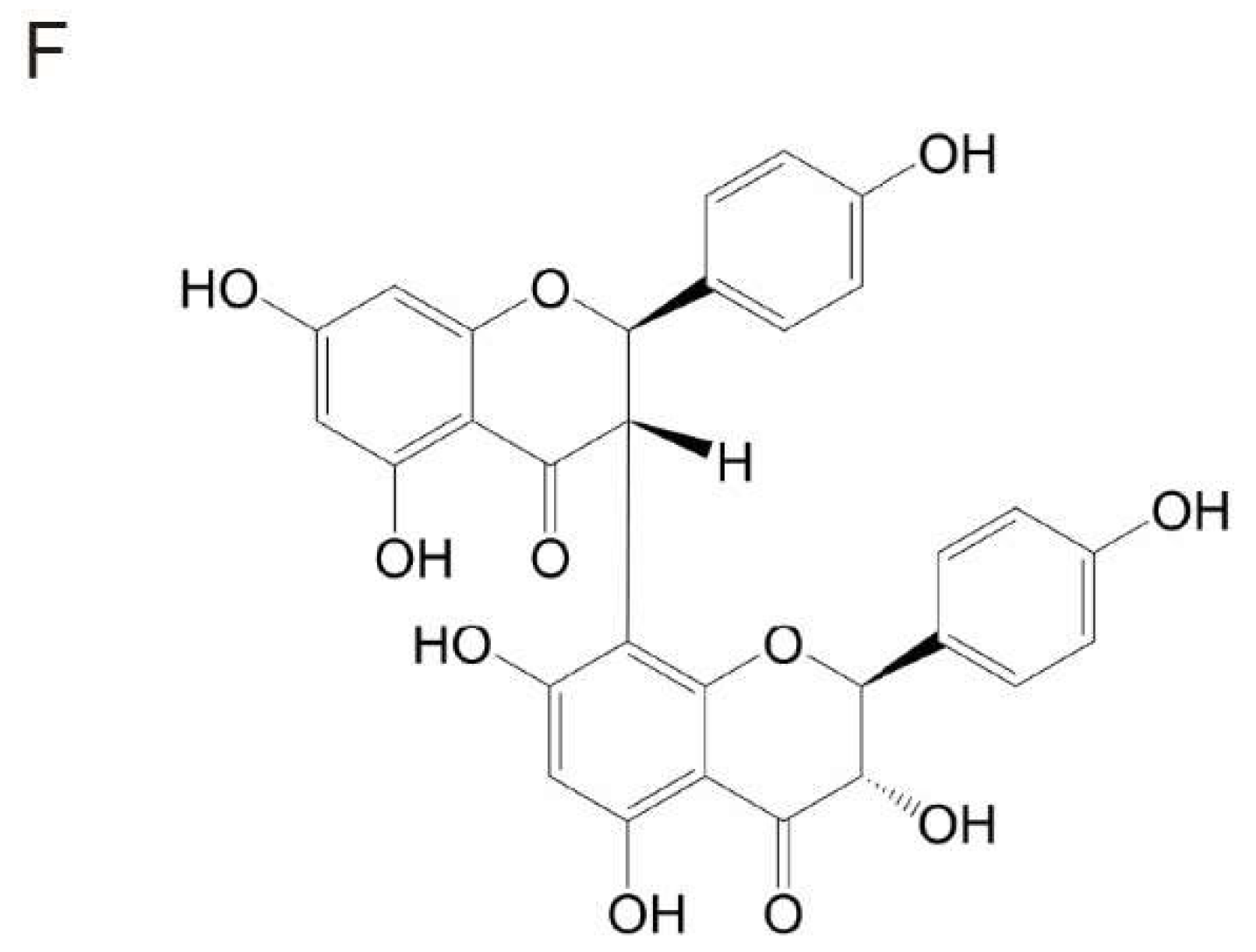
2.1. GB1 Effectively Ameliorates the Lipid Deposition in Cells
2.2. GB1 Regulates Lipid Metabolism by Facilitating Lipid Oxidization and Decreasing the Level of Fatty Acids
2.3. GB1 Improves OA&PA Induced ROS Burden
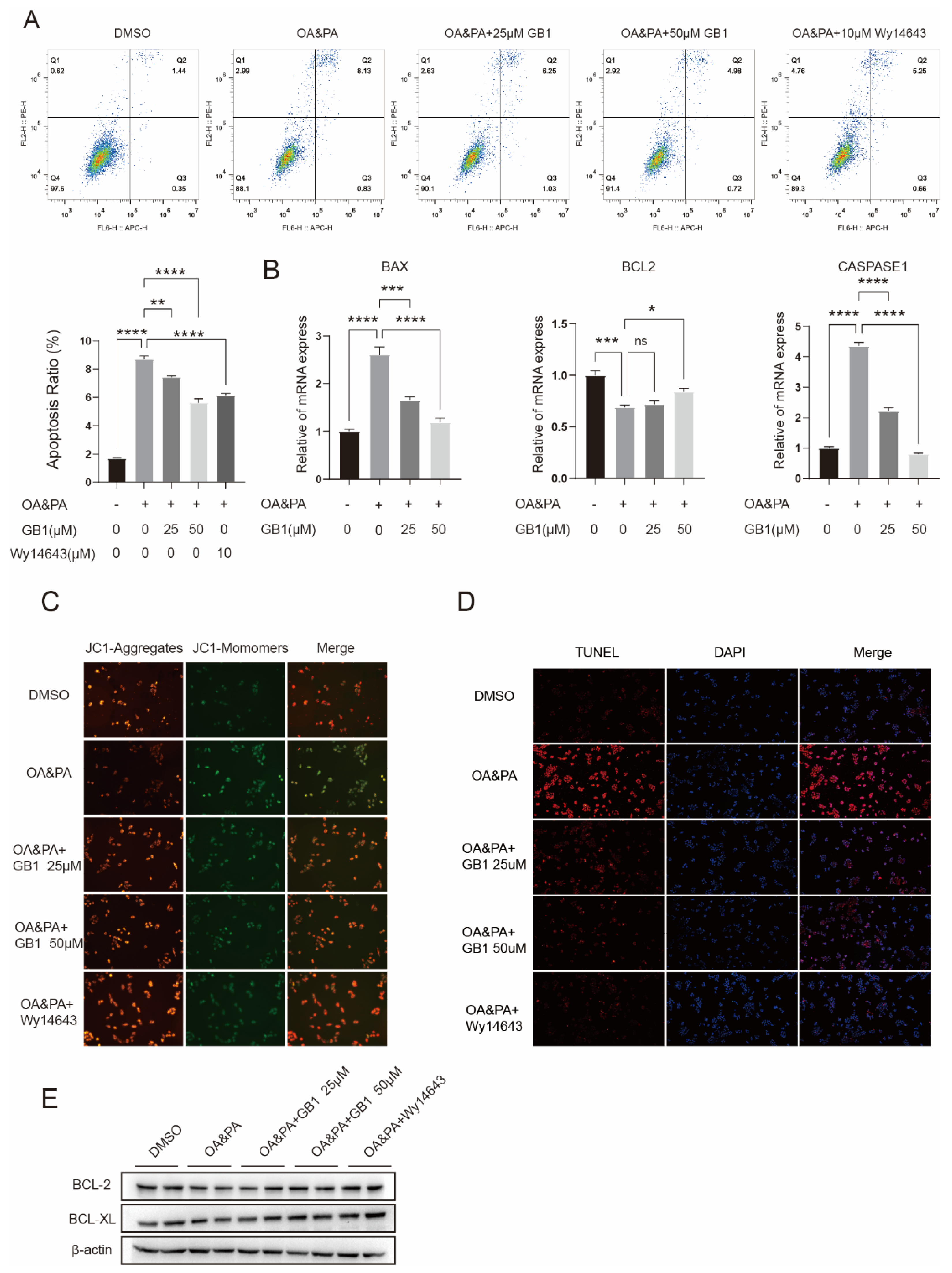
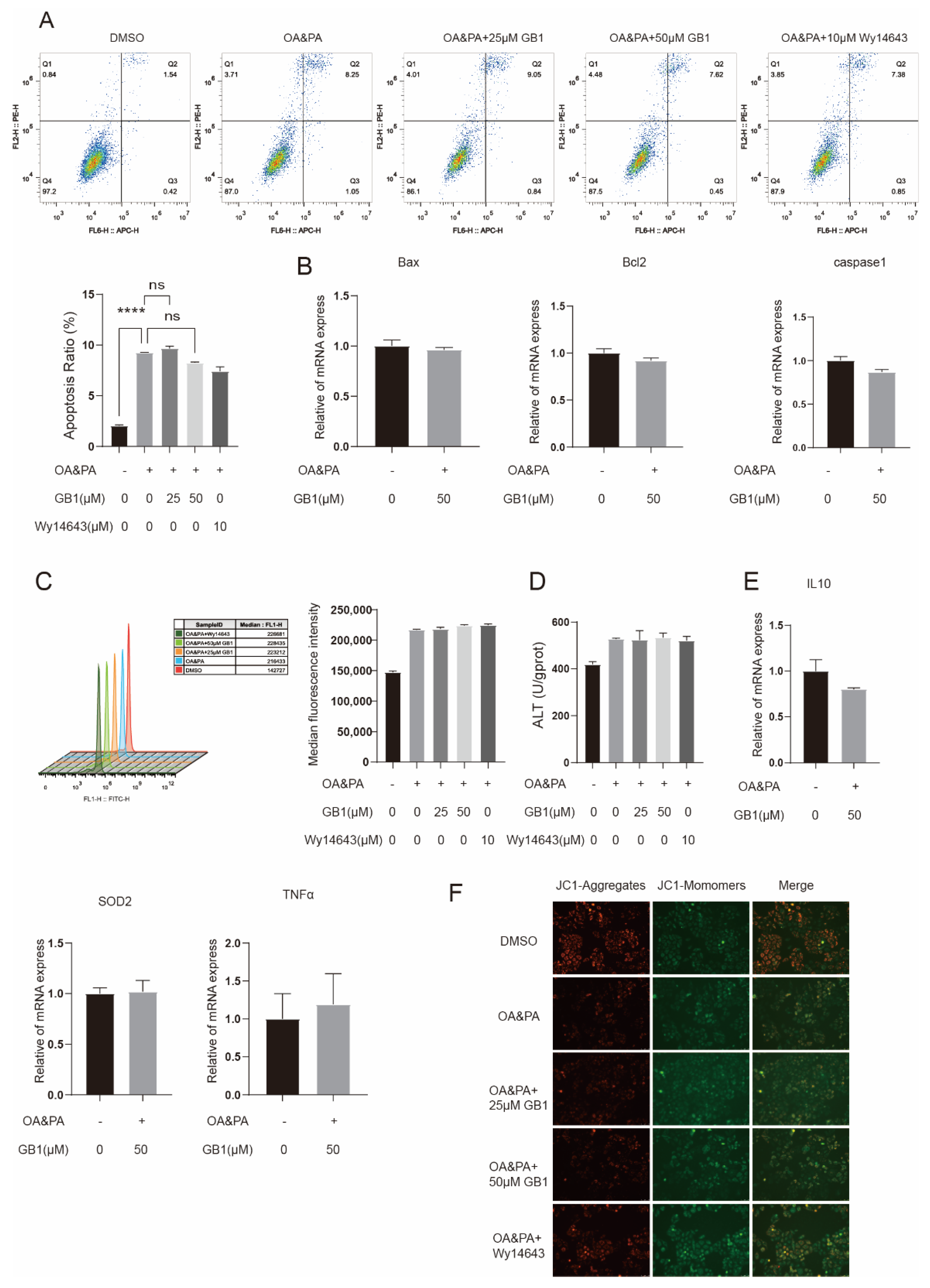
2.4. GB1 Reduces OA&PA Induced Apoptosis
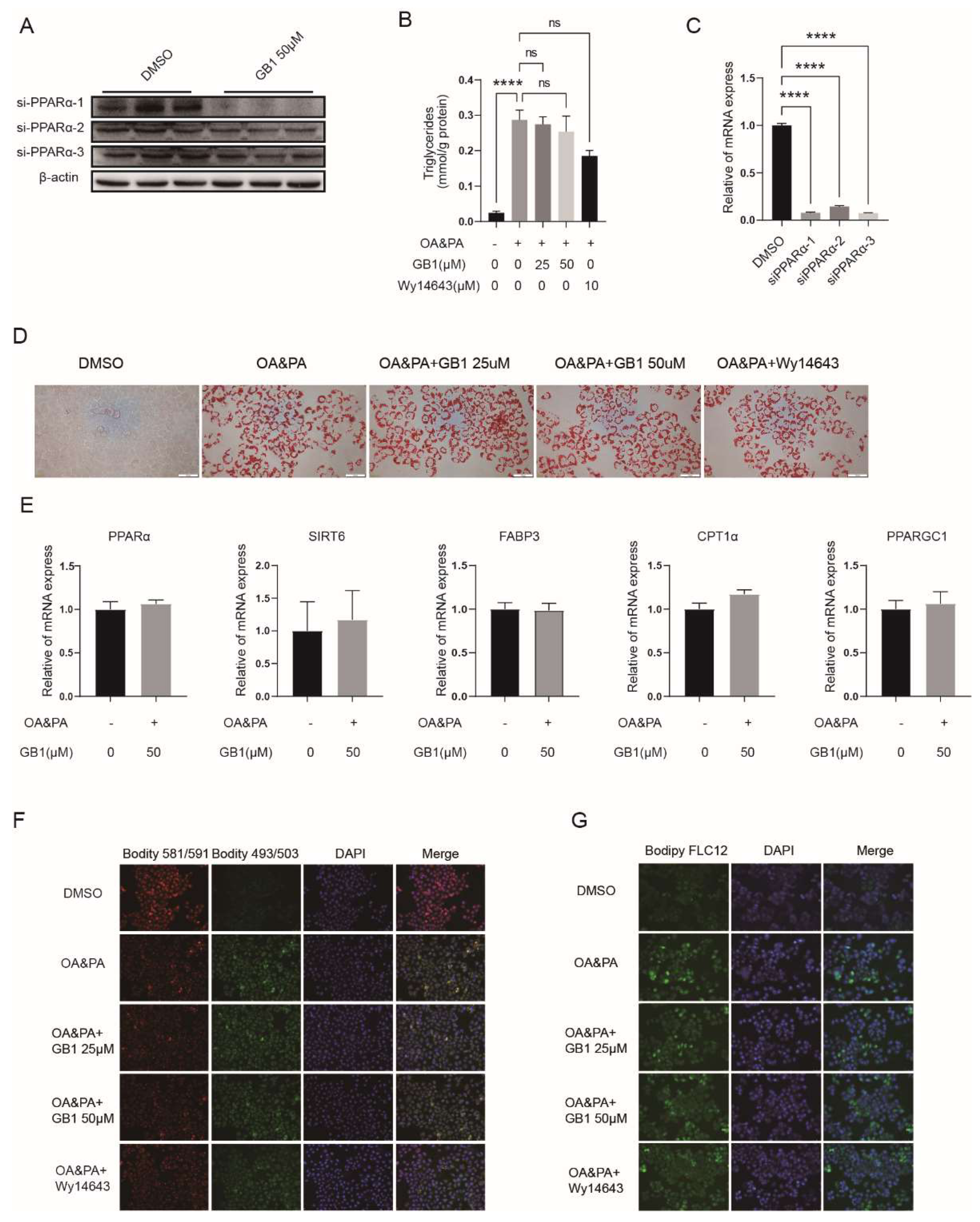
2.5. PPARα Is a Candidate Target of GB1
2.6. GB1 Fails to Improve Inflammation and Apoptosis in PPARα-Deficient Cells
3. Discussion
4. Materials and Methods
4.1. Cell Activity
4.2. Western Blot
4.3. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.4. Immunofluorescence Staining
4.5. Levels of Lipid Oxidization and Free Fatty Acids
4.6. Flow Cytometry for Cell Apoptosis and ROS Generation
4.7. Mitochondrial Membrane Potential (MMP)
4.8. TUNEL Staining
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhu, J.Z.; Dai, Y.N.; Wang, Y.M.; Zhou, Q.Y.; Yu, C.H.; Li, Y.M. Prevalence of Nonalcoholic Fatty Liver Disease and Economy. Dig. Dis. Sci. 2015, 60, 3194–3202. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Tessari, P.; Coracina, A.; Cosma, A.; Tiengo, A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.L.; Chen, H.; Wang, C.L.; Liang, L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Non-alcoholic fatty liver disease. BMC Med. 2017, 15, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Polizzi, A.; Fouche, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Regnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef] [Green Version]
- Naiman, S.; Huynh, F.K.; Gil, R.; Glick, Y.; Shahar, Y.; Touitou, N.; Nahum, L.; Avivi, M.Y.; Roichman, A.; Kanfi, Y.; et al. SIRT6 Promotes Hepatic Beta-Oxidation via Activation of PPARalpha. Cell Rep. 2019, 29, 4127–4143.e8. [Google Scholar] [CrossRef] [Green Version]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [Green Version]
- Adaramoye, O.A.; Adeyemi, E.O. Hypoglycaemic and hypolipidaemic effects of fractions from kolaviron, a biflavonoid complex from Garcinia Kola in streptozotocin-induced diabetes mellitus rats. J. Pharm. Pharmacol. 2006, 58, 121–128. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Awogbindin, I.; Okusaga, J.O. Effect of Kolaviron, a Biflavonoid Complex from Garcinia kola Seeds, on Ethanol-Induced Oxidative Stress in Liver of Adult Wistar Rats. J. Med. Food 2009, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Adaramoye, O.A.; Adeyemi, E.O. Hepatoprotection of D-Galactosamine-Induced Toxicity in Mice by Purified Fractions from Garcinia kola Seeds. Basic Clin. Pharmacol. Toxicol. 2006, 98, 135–141. [Google Scholar] [CrossRef]
- Farombi, E.O.; Tahnteng, J.G.; Agboola, A.O.; Nwankwo, J.O.; Emerole, G.O. Chemoprevention of 2-Acetylamino-uoreneinduced Hepatotoxicity and Lipid Peroxidation in Rats by KolavironÐA Garcinia kola Seed Extract. Food Chem. Toxicol. 2000, 38, 535–541. [Google Scholar] [CrossRef]
- He, X.; Yang, F.; Huang, X. Proceedings of Chemistry, Pharmacology, Pharmacokinetics and Synthesis of Biflavonoids. Molecules 2021, 26, 6088. [Google Scholar] [CrossRef]
- Iwu, M.M.; Igboko, O.A.; Okunji, C.O.; Tempesta, M.S. Antidiabetic and aldose reductase activities of biflavanones of Garcinia kola. J. Pharm. Pharmacol. 1990, 42, 290–292. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, C.; Lu, X.; Deng, C.; Xu, Q.; Guo, W.; Wu, Q.; Wang, Q.; Liu, C.; Huang, X.; et al. GB1a Ameliorates Ulcerative Colitis via Regulation of the NF-kappaB and Nrf2 Signaling Pathways in an Experimental Model. Front. Med. 2021, 8, 654867. [Google Scholar]
- Reeves, H.L.; Zaki, M.Y.; Day, C.P. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig. Dis. Sci. 2016, 61, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.; Verrijken, A.; Caron, S.; Prawitt, J.; Paumelle, R.; Derudas, B.; Lefebvre, P.; Taskinen, M.R.; Van Hul, W.; Mertens, I.; et al. PPARalpha gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef] [PubMed]
- Adaramoye, O.; Nwaneri, V.; Anyanwu, K.C.; Farombi, E.; Emerole, G. Possible anti-atherogenic effect of kolaviron (a Garcinia kola seed extract) in hypercholesterolaemic rats. Clin. Exp. Pharmacol. Physiol. 2005, 32, 40–46. [Google Scholar] [CrossRef]
- Ayepola, O.R.; Cerf, M.E.; Brooks, N.L.; Oguntibeju, O.O. Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes-induced nephrotoxic rats. Phytomedicine 2014, 21, 1785–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erukainure, O.L.; Salau, V.F.; Chukwuma, C.I.; Islam, M.S. Kolaviron: A Biflavonoid with Numerous Health Benefits. Curr. Pharm. Des. 2021, 27, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Patsouris, D.; Mandard, S.; Voshol, P.J.; Escher, P.; Tan, N.S.; Havekes, L.M.; Koenig, W.; März, W.; Tafuri, S.; Wahli, W.; et al. PPARα governs glycerol metabolism. J. Clin. Investig. 2004, 114, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Peroxisome proliferator–activated receptor α mediates the adaptive response to fasting. J. Clin. Investig. 1999, 103, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Wagner, M.; Xiao, R.; Kim, K.H.; Feng, D.; Lazar, M.A.; Moore, D.D. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 2014, 516, 112–115. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [Green Version]


| Primer | Sequences |
|---|---|
| SIRT6 | F:CCCACGGAGTCTGGACCAT |
| R:CTCTGCCAGTTTGTCCCTG | |
| PPARα | F:ATGGTGGACACGGAAAGCC |
| R:CGATGGATTGCGAAATCTCTTGG | |
| β-actin | F:CATGTACGTTGTATCCAGGC |
| R:CTCCTTAATGTCACGCAGAT | |
| ACADM | F:ACAGGGGTTCAGACTGTATT |
| R:TCCTCCGTTGGTTATCCACAT | |
| CPT1α | F:TCCAGTTGGCTTATCGTGGTG |
| R:TCCAGAGTCCGATTGATTGC | |
| FABP3 | F:GGCACCTGGAAGCTAGTGG |
| R:CTGCCTGGTAGCAAAACCC | |
| PPARGC1 | F:TCTGATCTTATGGATGACAT |
| R:CCAAGTCGTTCACATCTATTA | |
| MGLL | F:ATGCCAGAGGAAAGTTCCCC |
| R:CGTCTGCATTGACCAGGTG | |
| HMOX1 | F:AGACTGCGTTCCTGCTCAAC |
| R:AAGCCCTACACAACTGTCG | |
| IL10 | F:GACTTTAAGGGTTACTGGGTTG |
| R:TCACATGCGCCTGATGTCTG | |
| Bax | F:CCCGGAGGTCTTTTTCCGAG |
| R:CCAGCCCATGATGGTTCTGAT | |
| CASPASE 1 | F:TTTCCGCAAGGTTCGATTTCA |
| R:GGCATCTGCGCTCTACCATC | |
| BCL-2 | F:GGTGGGGTCATGTGTGTGG |
| R:CGGTTCAGGTACTCATCATCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-X.; Yang, F.; He, X.-Q.; Li, T.; Sun, Y.-Z.; Song, J.-P.; Huang, X.-A.; Guo, W.-F. Garcinia Biflavonoid 1 Improves Lipid Metabolism in HepG2 Cells via Regulating PPARα. Molecules 2022, 27, 1978. https://doi.org/10.3390/molecules27061978
Chen H-X, Yang F, He X-Q, Li T, Sun Y-Z, Song J-P, Huang X-A, Guo W-F. Garcinia Biflavonoid 1 Improves Lipid Metabolism in HepG2 Cells via Regulating PPARα. Molecules. 2022; 27(6):1978. https://doi.org/10.3390/molecules27061978
Chicago/Turabian StyleChen, Hai-Xin, Fan Yang, Xin-Qian He, Ting Li, Yong-Zhi Sun, Jian-Ping Song, Xin-An Huang, and Wen-Feng Guo. 2022. "Garcinia Biflavonoid 1 Improves Lipid Metabolism in HepG2 Cells via Regulating PPARα" Molecules 27, no. 6: 1978. https://doi.org/10.3390/molecules27061978
APA StyleChen, H.-X., Yang, F., He, X.-Q., Li, T., Sun, Y.-Z., Song, J.-P., Huang, X.-A., & Guo, W.-F. (2022). Garcinia Biflavonoid 1 Improves Lipid Metabolism in HepG2 Cells via Regulating PPARα. Molecules, 27(6), 1978. https://doi.org/10.3390/molecules27061978






