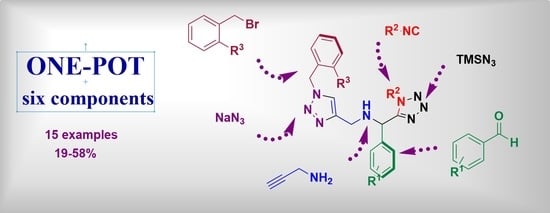
A One-Pot Six-Component Reaction for the Synthesis of 1,5-Disubstituted Tetrazol-1,2,3-Triazole Hybrids and Their Cytotoxic Activity against the MCF-7 Cell Line
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for Compounds 13a–o (GP)
3.1.3. Synthesis and Characterization of Compounds 13a–o
3.2. Cell Line
3.3. Cell Proliferation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Heravi, M.M.; Zadssirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Petri, G.L.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M.V.; Barraja, P.; Montalbano, A. Bioactive pyrrole-based compounds with target selectivity. Eur. J. Med. Chem. 2020, 208, 112783. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Belstein J. Org. Chem. 2011, 7, 442–495. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Ryabukhin, S.V.; Judd, D.B. The Symbiotic Relationship Between Drug Discovery and Organic Chemistry. Chem. Eur. J. 2020, 26, 1196–1237. [Google Scholar] [CrossRef]
- Laraia, L.; Robke, L.; Waldmann, H. Bioactive Compound Collections: From Design to Target Identification. Chem 2018, 4, 705–730. [Google Scholar] [CrossRef]
- Galloway, W.R.J.D.; Isidro-Llobet, A.; Spring, D.A. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010, 1, 80. [Google Scholar] [CrossRef] [Green Version]
- Myznikov, L.V.; Vorona, S.V.; Zevatskii, Y.E. Biologically active compounds and drugs in the tetrazole series. Chem. Heterocycl. Comp. 2021, 57, 224–233. [Google Scholar] [CrossRef]
- Leyva-Ramos, S.; Cardoso-Ortiz, J. Recent Developments in the Synthesis of Tetrazoles and their Pharmacological Relevance. Curr. Org. Chem. 2021, 25, 388–403. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sharma, B.; Mehra, V.; Kumar, V. Recent accomplishments on the synthetic/biological facets of pharmacologically active 1H-1,2,3-triazoles. Eur. J. Med. Chem. 2021, 212, 113069. [Google Scholar] [CrossRef]
- Sahu, A.; Sahu, P.; Agrawal, R. A Recent Review on Drug Modification Using 1,2,3-triazole. Curr. Chem. Biol. 2020, 14, 71–87. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.M.S.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular Hybridization as a Tool for Designing Multitarget Drug Candidates for Complex Diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef]
- Sahil, S.; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar]
- Zhang, J.; Wang, S.; Ba, Y.; Xu, Z. Tetrazole hybrids with potential anticancer activity. Eur. J. Med. Chem. 2019, 178, 341–351. [Google Scholar] [CrossRef]
- Gao, F.; Xiao, J.; Huang, G. Current scenario of tetrazole hybrids for antibacterial activity. Eur. J. Med. Chem. 2019, 184, 111744. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Xu, Z. Tetrazole hybrids and their antifungal activities. Eur. J. Med. Chem. 2019, 170, 225–234. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.I. One-Pot Construction of Bis-Heterocycles through Isocyanide Based Multicomponent Reactions. Chem. Select. 2020, 5, 8040–8061. [Google Scholar]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Morales, C.M.; de Loera, D.; Contreras-Celedón, C.; Cortés-García, C.J.; Chacón-García, L. Synthesis of 1,5-disubstituted tetrazole-1,2,3 triazoles hybrids via Ugi-azide/CuAAC. Synth. Commun. 2019, 49, 2086–2095. [Google Scholar] [CrossRef]
- Cortes-García, C.J.; Islas-Jácome, A.; Rentería-Gómez, A.; Gámez-Montaño, R. Synthesis of 1,5-disubstituted tetrazoles containing a fragment of the anticancer drug imatinib via a microwave-assisted Ugi-azide reaction. Monatsh Chem. 2016, 147, 1277–1290. [Google Scholar] [CrossRef]
- Fakhree, A.A.; Ghasemi, Z.; Rahimi, M.; Shahrisa, A. Enhanced catalytic performance of copper iodide in 1,2,3-triazole-imidazole hybrid synthesis, and evaluation of their anti-cancer activities along with optical properties besides 1H-tetrazole-imidazole hybrids. Appl. Organomet. Chem. 2020, 34, e5773. [Google Scholar] [CrossRef]
- Lei, X.; Lampiri, P.; Patil, P.; Angeli, G.; Neochoritis, C.G.; Dömling, A. A multicomponent tetrazolo indole synthesis. Chem. Commun. 2021, 57, 6652–6655. [Google Scholar] [CrossRef]
- Saha, D.; Kharbanda, A.; Essien, N.; Zhang, L.; Cooper, R.; Basak, D.; Kendrick, S.; Frett, B.; Li, H. Intramolecular cyclization of imidazo [1,2-a] pyridines via a silver mediated/palladium catalyzed C–H activation strategy. Org. Chem. Front. 2019, 6, 2234–2239. [Google Scholar] [CrossRef]
- Ojeda, G.M.; Ranjan, P.; Fedoseev, P.; Amable, L.; Sharma, U.K.; Rivera, D.G.; Van der Eycken, E.V. Combining the Ugi-azide multicomponent reaction and rhodium(III)-catalyzed annulation for the synthesis of tetrazole-isoquinolone/pyridone hybrids. Belstein J. Org. Chem. 2019, 15, 2447–2457. [Google Scholar] [CrossRef]
- Brauch, S.; van Berkel, S.S.; Westermann, B. Higher-order multicomponent reactions: Beyond four reactants. Chem. Soc. Rev. 2013, 42, 4948–4962. [Google Scholar] [CrossRef]
- National Breast Cancer Coalition. Available online: https://www.stopbreastcancer.org/information-center/facts-figures/ (accessed on 10 August 2021).
- Sun, Y.; Zhao, Z.; Yang, Z.; Xu, F.; Lu, H.; Zhu, Z.; Shi, W.; Jiang, J.; Yao, P.; Zhu, H. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataollahi, M.R.; Sharifi, J.; Paknahad, M.R.; Paknahad, A. Breast cancer and associated factors: A review. J. Med. Life 2015, 8, 6–11. [Google Scholar]
- Guo, S.; Zhou, Y.; Dal, B.; Huo, C.; Liu, C.; Zhao, Y. CuI/Et2NH-Catalyzed One-Pot Highly Efficient Synthesis of 1,4-Disubstituted 1,2,3-Triazoles in Green Solvent Glycerol. Synthesis 2018, 50, 2191–2199. [Google Scholar]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [PubMed]
- Boichuk, S.; Galembikova, A.; Sitenkov, A.; Khusnutdinov, R.; Dunaev, P.; Valeeva, E.; Usolova, N. Establishment and characterization of a triple negative basal-like breast cancer cell line with multi-drug resistance. Oncol. Lett. 2017, 14, 5039–5045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaban, S.; Negm, A.; Ashmawy, A.M.; Ahmed, D.M.; Wessjohann, L.A. Combinatorial synthesis, in silico, molecular and biochemical studies of tetrazole-derived organic selenides with increased selectivity against hepatocellular carcinoma. Eur. J. Med. Chem. 2016, 122, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Vanaparthi, S.; Bantu, R.; Jain, N.; Janardhan, S.; Nagarapu, L. Synthesis and anti-proliferative activity of a novel 1,2,3-triazole tethered chalcone acetamide derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 127304. [Google Scholar] [CrossRef]
- Lambert, P.A.; Somers, K.D.; Kohn, E.C.; Perry, R.R. Antiproliferative and antiinvasive effects of carboxyamido-triazole on breast cancer cell lines. Surgery 1997, 122, 372–379. [Google Scholar]






| Entry | Solvent | Yield % |
|---|---|---|
| 1 | MeOH [1M] | 58 1 |
| 2 | Glycerol [1M] | 11 1 |
| 3 | t-BuOH:H2O [0.1M, 1:1 v/v] | 15 1 |
| 4 | TFE [1M] | ND 2 |
| Product | IC50(µM) MCF-7 | Product | IC50(µM) MCF-7 |
|---|---|---|---|
| 13a | 200 | 13i | 200 |
| 13b | 31.63 | 13j | 44.51 |
| 13c | 200 | 13k | 60.77 |
| 13d | 55.48 | 13l | 22.84 |
| 13e | 62.73 | 13m | 200 |
| 13f | 19.76 | 13n | 29.25 |
| 13g | 200 | 13o | 200 |
| 13h | 41.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Morales, C.M.; Araujo-Huitrado, J.G.; López-Hernández, Y.; Contreras-Celedón, C.; Islas-Jácome, A.; Granados-López, A.J.; Solorio-Alvarado, C.R.; López, J.A.; Chacón-García, L.; Cortés-García, C.J. A One-Pot Six-Component Reaction for the Synthesis of 1,5-Disubstituted Tetrazol-1,2,3-Triazole Hybrids and Their Cytotoxic Activity against the MCF-7 Cell Line. Molecules 2021, 26, 6104. https://doi.org/10.3390/molecules26206104
Aguilar-Morales CM, Araujo-Huitrado JG, López-Hernández Y, Contreras-Celedón C, Islas-Jácome A, Granados-López AJ, Solorio-Alvarado CR, López JA, Chacón-García L, Cortés-García CJ. A One-Pot Six-Component Reaction for the Synthesis of 1,5-Disubstituted Tetrazol-1,2,3-Triazole Hybrids and Their Cytotoxic Activity against the MCF-7 Cell Line. Molecules. 2021; 26(20):6104. https://doi.org/10.3390/molecules26206104
Chicago/Turabian StyleAguilar-Morales, Cesia M., Jorge Gustavo Araujo-Huitrado, Yamilé López-Hernández, Claudia Contreras-Celedón, Alejandro Islas-Jácome, Angelica Judith Granados-López, Cesar R. Solorio-Alvarado, Jesús Adrián López, Luis Chacón-García, and Carlos J. Cortés-García. 2021. "A One-Pot Six-Component Reaction for the Synthesis of 1,5-Disubstituted Tetrazol-1,2,3-Triazole Hybrids and Their Cytotoxic Activity against the MCF-7 Cell Line" Molecules 26, no. 20: 6104. https://doi.org/10.3390/molecules26206104
APA StyleAguilar-Morales, C. M., Araujo-Huitrado, J. G., López-Hernández, Y., Contreras-Celedón, C., Islas-Jácome, A., Granados-López, A. J., Solorio-Alvarado, C. R., López, J. A., Chacón-García, L., & Cortés-García, C. J. (2021). A One-Pot Six-Component Reaction for the Synthesis of 1,5-Disubstituted Tetrazol-1,2,3-Triazole Hybrids and Their Cytotoxic Activity against the MCF-7 Cell Line. Molecules, 26(20), 6104. https://doi.org/10.3390/molecules26206104