In Silico Analysis of Fungal and Chloride-Dependent α-Amylases within the Family GH13 with Identification of Possible Secondary Surface-Binding Sites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sequence Logos of α-Amylases from Different GH13 Subfamilies
2.2. Analysis of Chloride Anion Binding
2.3. Analysis of Surface Binding Sites
2.4. Evolutionary Relatedness of α-Amylases from Fungi and Other Taxa
2.5. Conclusions
3. Materials and Methods
3.1. Sequence Collection
3.2. Sequence Analysis
3.3. Comparison of Chloride- and Surface-Binding Sites
3.4. Evolutionary Relationships
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Janecek, S.; Svensson, B.; MacGregor, E.A. α-Amylase: An enzyme specificity found in various families of glycoside hydrolases. Cell. Mol. Life Sci. 2014, 71, 1149–1170. [Google Scholar] [CrossRef]
- Pujadas, G.; Palau, J. Evolution of α-amylases: Architectural features and key residues in the stabilization of the (β/α)(8) scaffold. Mol. Biol. Evol. 2001, 18, 38–54. [Google Scholar] [CrossRef] [Green Version]
- Linden, A.; Wilmanns, M. Adaptation of class-13 α-amylases to diverse living conditions. Chembiochem 2004, 5, 231–239. [Google Scholar] [CrossRef]
- Mehta, D.; Satyanarayana, T. Bacterial and archaeal α-amylases: Diversity and amelioration of the desirable characteristics for industrial applications. Front. Microbiol. 2016, 7, 1129. [Google Scholar] [CrossRef] [Green Version]
- Bozic, N.; Loncar, N.; Slavic, M.S.; Vujcic, Z. Raw starch degrading α-amylases: An unsolved riddle. Amylase 2017, 1, 12–25. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [Green Version]
- Janecek, S.; Gabrisko, M. Remarkable evolutionary relatedness among the enzymes and proteins from the α-amylase family. Cell. Mol. Life Sci. 2016, 73, 2707–2725. [Google Scholar] [CrossRef]
- Blesak, K.; Janecek, S. Sequence fingerprints of enzyme specificities from the glycoside hydrolase family GH57. Extremophiles 2012, 16, 497–506. [Google Scholar] [CrossRef]
- Janecek, S.; Kuchtova, A. In silico identification of catalytic residues and domain fold of the family GH119 sharing the catalytic machinery with the α-amylase family GH57. FEBS Lett. 2012, 586, 3360–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerenyiova, L.; Janecek, S. A detailed in silico analysis of the amylolytic family GH126 and its possible relatedness to family GH76. Carbohydr. Res. 2020, 494, 108082. [Google Scholar] [CrossRef]
- Jespersen, H.M.; MacGregor, E.A.; Henrissat, B.; Sierks, M.R.; Svensson, B. Starch- and glycogen-debranching and branching enzymes: Prediction of structural features of the catalytic (β/α)8-barrel domain and evolutionary relationship to other amylolytic enzymes. J. Protein Chem. 1993, 12, 791–805. [Google Scholar] [CrossRef]
- Svensson, B. Protein engineering in the α-amylase family: Catalytic mechanism, substrate specificity, and stability. Plant Mol. Biol. 1994, 25, 141–157. [Google Scholar] [CrossRef]
- Kuriki, T.; Imanaka, T. The concept of the α-amylase family: Structural similarity and common catalytic mechanism. J. Biosci. Bioeng. 1999, 87, 557–565. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Janecek, S.; Svensson, B. Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochim. Biophys. Acta 2001, 1546, 1–20. [Google Scholar] [CrossRef]
- van der Maarel, M.J.E.C.; van der Veen, B.; Uitdehaag, J.C.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, Y.; Kusunoki, M.; Harada, W.; Kakudo, M. Structure and possible catalytic residues of Taka-amylase A. J. Biochem. 1984, 95, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Uitdehaag, J.C.; Mosi, R.; Kalk, K.H.; van der Veen, B.A.; Dijkhuizen, L.; Withers, S.G.; Dijkstra, B.W. X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the α-amylase family. Nat. Struct. Biol. 1999, 6, 432–436. [Google Scholar] [CrossRef] [Green Version]
- Janecek, S. How many conserved sequence regions are there in the α-amylase family? Biologia 2002, 57 (Suppl. 11), 29–41. [Google Scholar]
- Oslancova, A.; Janecek, S. Oligo-1,6-glucosidase and neopullulanase enzyme subfamilies from the α-amylase family defined by the fifth conserved sequence region. Cell. Mol. Life Sci. 2002, 59, 1945–1959. [Google Scholar] [CrossRef] [PubMed]
- Stam, M.R.; Danchin, E.G.; Rancurel, C.; Coutinho, P.M.; Henrissat, B. Dividing the large glycoside hydrolase family 13 into subfamilies: Towards improved functional annotations of α-amylase-related proteins. Protein Eng. Des. Sel. 2006, 19, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Janecek, S.; Zamocka, B. A new GH13 subfamily represented by the α-amylase from the halophilic archaeon. Haloarcula Hisp. Extrem. 2020, 24, 207–217. [Google Scholar] [CrossRef]
- Janecek, S.; Kuchtova, A.; Petrovicova, S. A novel GH13 subfamily of α-amylases with a pair of tryptophans in the helix α3 of the catalytic TIM-barrel, the LPDlx signature in the conserved sequence region V and a conserved aromatic motif at the C-terminus. Biologia 2015, 70, 1284–1294. [Google Scholar] [CrossRef]
- Sarian, F.D.; Janecek, S.; Pijning, T.; Ihsanawati; Nurachman, Z.; Radjasa, O.K.; Dijkhuizen, L.; Natalia, D.; van der Maarel, M.J.E.C. A new group of glycoside hydrolase family 13 α-amylases with an aberrant catalytic triad. Sci. Rep. 2017, 7, 44230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janecek, S. Sequence similarities and evolutionary relationships of microbial, plant and animal α-amylases. Eur. J. Biochem. 1994, 224, 519–524. [Google Scholar] [CrossRef]
- Janecek, S.; Leveque, E.; Belarbi, A.; Haye, B. Close evolutionary relatedness of α-amylases from Archaea and plants. J. Mol. Evol. 1999, 48, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Da Lage, J.L.; Feller, G.; Janecek, S. Horizontal gene transfer from Eukarya to bacteria and domain shuffling: The α-amylase model. Cell. Mol. Life Sci. 2004, 61, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Majzlova, K.; Pukajova, Z.; Janecek, S. Tracing the evolution of the α-amylase subfamily GH13_36 covering the amylolytic enzymes intermediate between oligo-1,6-glucosidases and neopullulanases. Carbohydr Res. 2013, 367, 48–57. [Google Scholar] [CrossRef]
- van der Kaaij, R.M.; Janecek, S.; van der Maarel, M.J.E.C.; Dijkhuizen, L. Phylogenetic and biochemical characterization of a novel cluster of intracellular fungal α-amylase enzymes. Microbiology 2007, 153, 4003–4015. [Google Scholar] [CrossRef] [Green Version]
- Da Lage, J.L.; Binder, M.; Hua-Van, A.; Janecek, S.; Casane, D. Gene make-up: Rapid and massive intron gains after horizontal transfer of a bacterial α-amylase gene to Basidiomycetes. BMC Evol. Biol. 2013, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Janickova, Z.; Janecek, S. Fungal α-amylases from three GH13 subfamilies: Their sequence-structural features and evolutionary relationships. Int. J. Biol. Macromol. 2020, 159, 763–772. [Google Scholar] [CrossRef]
- Feller, G.; Lonhienne, T.; Deroanne, C.; Libioulle, C.; Van Beeumen, J.; Gerday, C. Purification, characterization, and nucleotide sequence of the thermolabile α-amylase from the antarctic psychrotroph Alteromonas haloplanctis A23. J. Biol. Chem. 1992, 267, 5217–5221. [Google Scholar] [CrossRef]
- Feller, G.; Payan, F.; Theys, F.; Qian, M.; Haser, R.; Gerday, C. Stability and structural analysis of α-amylase from the antarctic psychrophile Alteromonas haloplanctis A23. Eur. J. Biochem. 1994, 222, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Feller, G.; Bussy, O.; Houssier, C.; Gerday, C. Structural and functional aspects of chloride binding to Alteromonas haloplanctis α-amylase. J. Biol. Chem. 1996, 271, 23836–23841. [Google Scholar] [CrossRef] [Green Version]
- Aghajari, N.; Feller, G.; Gerday, C.; Haser, R. Crystal structures of the psychrophilic α-amylase from Alteromonas haloplanctis in its native form and complexed with an inhibitor. Protein Sci. 1998, 7, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghajari, N.; Feller, G.; Gerday, C.; Haser, R. Structural basis of α-amylase activation by chloride. Protein Sci. 2002, 11, 1435–1441. [Google Scholar] [CrossRef]
- Buisson, G.; Duee, E.; Haser, R.; Payan, F. Three dimensional structure of porcine pancreatic α-amylase at 2.9 Å resolution. Role of calcium in structure and activity. EMBO J. 1987, 6, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Haser, R.; Payan, F. Structure and molecular model refinement of pig pancreatic α-amylase at 2.1 Å resolution. J. Mol. Biol. 1993, 231, 785–799. [Google Scholar] [CrossRef]
- Brayer, G.D.; Luo, Y.; Withers, S.G. The structure of human pancreatic α-amylase at 1.8 Å resolution and comparisons with related enzymes. Protein Sci. 1995, 4, 1730–1742. [Google Scholar] [CrossRef]
- Ramasubbu, N.; Paloth, V.; Luo, Y.; Brayer, G.D.; Levine, M.J. Structure of human salivary α-amylase at 1.6 Å resolution: Implications for its role in the oral cavity. Acta Crystallogr. D Biol. Crystallogr. 1996, 52, 435–446. [Google Scholar] [CrossRef]
- Strobl, S.; Maskos, K.; Betz, M.; Wiegand, G.; Huber, R.; Gomis-Rüth, F.X.; Glockshuber, R. Crystal structure of yellow meal worm α-amylase at 1.64 Å resolution. J. Mol. Biol. 1998, 278, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Ajandouz, E.H.; Payan, F.; Nahoum, V. Molecular basis of the effects of chloride ion on the acid-base catalyst in the mechanism of pancreatic α-amylase. Biochemistry 2005, 44, 3194–3201. [Google Scholar] [CrossRef]
- D’Amico, S.; Gerday, C.; Feller, G. Structural similarities and evolutionary relationships in chloride-dependent α-amylases. Gene 2000, 253, 95–105. [Google Scholar] [CrossRef]
- Cockburn, D.; Wilkens, C.; Ruzanski, C.; Andersen, S.; Nielsen, J.W.; Smith, A.M.; Field, R.A.; Willemoës, M.; Hachem, M.A.; Svensson, B. Analysis of surface binding sites (SBSs) in carbohydrate active enzymes with focus on glycoside hydrolase families 13 and 77—A mini-review. Biologia 2014, 69, 705–712. [Google Scholar] [CrossRef]
- Janecek, S.; Marecek, F.; MacGregor, E.A.; Svensson, B. Starch-binding domains as CBM families-history, occurrence, structure, function and evolution. Biotechnol. Adv. 2019, 37, 107451. [Google Scholar] [CrossRef]
- Cockburn, D.; Nielsen, M.M.; Christiansen, C.; Andersen, J.M.; Rannes, J.B.; Blennow, A.; Svensson, B. Surface binding sites in amylase have distinct roles in recognition of starch structure motifs and degradation. Int. J. Biol. Macromol. 2015, 75, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Baroroh, U.; Yusuf, M.; Rachman, S.D.; Ishmayana, S.; Syamsunarno, M.R.A.A.; Levita, J.; Subroto, T. The importance of surface-binding site towards starch-adsorptivity level in α-amylase: A review on structural point of view. Enzyme Res. 2017, 2017, 4086845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroroh, U.; Yusuf, M.; Rachman, S.D.; Ishmayana, S.; Hasan, K.; Subroto, T. Molecular dynamics study to improve the substrate adsorption of Saccharomycopsis fibuligera R64 α-amylase by designing a new surface binding site. Adv. Appl. Bioinform. Chem. 2019, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wilkens, C.; Svensson, B.; Møller, M.S. Functional roles of starch binding domains and surface binding sites in enzymes involved in starch biosynthesis. Front. Plant. Sci. 2018, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Vujicic-Zagar, A.; Dijkstra, B.W. Monoclinic crystal form of Aspergillus niger α-amylase in complex with maltose at 1.8 angstroms resolution. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Lyhne-Iversen, L.; Hobley, T.J.; Kaasgaard, S.G.; Harris, P. Structure of Bacillus halmapalus α-amylase crystallized with and without the substrate analogue acarbose and maltose. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Bozic, N.; Rozeboom, H.J.; Loncar, N.; Slavic, M.S.; Janssen, D.B.; Vujcic, Z. Characterization of the starch surface binding site on Bacillus paralicheniformis α-amylase. Int. J. Biol. Macromol. 2020, 165, 1529–1539. [Google Scholar] [CrossRef]
- Tan, T.C.; Mijts, B.N.; Swaminathan, K.; Patel, B.K.; Divne, C. Crystal structure of the polyextremophilic α-amylase AmyB from Halothermothrix orenii: Details of a productive enzyme-substrate complex and an N domain with a role in binding raw starch. J. Mol. Biol. 2008, 378, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Haser, R.; Payan, F. Carbohydrate binding sites in a pancreatic α-amylase-substrate complex, derived from X-ray structure analysis at 2.1 Å resolution. Protein Sci. 1995, 4, 747–755. [Google Scholar] [CrossRef]
- Ragunath, C.; Manuel, S.G.A.; Kasinathan, C.; Ramasubbu, N. Structure-function relationships in human salivary α-amylase: Role of aromatic residues in a secondary binding site. Biologia 2008, 63, 1028–1034. [Google Scholar] [CrossRef]
- Pytelkova, J.; Lepsik, M.; Sanda, M.; Talacko, P.; Maresova, L.; Mares, M. Enzymatic activity and immunoreactivity of Aca s 4, an α-amylase allergen from the storage mite Acarus siro. BMC Biochem. 2012, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.L.; Hart, B.J.; Lynch, N.R.; Thomas, W.R.; Smith, W. Molecular characterization of the group 4 house dust mite allergen from Dermatophagoides pteronyssinus and its amylase homologue from Euroglyphus maynei. Int. Arch. Allergy Immunol. 1999, 120, 100–107. [Google Scholar] [CrossRef]
- Oliveira-Neto, O.B.; Batista, J.A.; Rigden, D.J.; Franco, O.L.; Falcao, R.; Fragoso, R.R.; Mello, L.V.; dos Santos, R.C.; Grossi-de-Sa, M.F. Molecular cloning of α-amylases from cotton boll weevil, Anthonomus grandis and structural relations to plant inhibitors: An approach to insect resistance. J. Protein Chem. 2003, 22, 77–87. [Google Scholar] [CrossRef]
- Yamada, T.; Ikeda, M.; Kobayashi, M.; Hattori, K. Cloning and expression of a cDNA encoding larval α-amylase of azuki bean weevil, Callosobruchus chinensis. J. Insect Biotechnol. Sericol. 2003, 72, 139–148. [Google Scholar] [CrossRef]
- Grossi de Sa, M.F.; Chrispeels, M.J. Molecular cloning of bruchid (Zabrotes subfasciatus) α-amylase cDNA and interactions of the expressed enzyme with bean amylase inhibitors. Insect Biochem. Mol. Biol. 1997, 27, 271–281. [Google Scholar] [CrossRef]
- Coronado, M.A.; Vargas, C.; Mellado, E.; Tegos, G.; Drainas, C.; Nieto, J.N.J.; Ventosa, A. The α-amylase gene amyH of the moderate halophile Halomonas meridiana: Cloning and molecular characterization. Microbiology 2000, 146, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Na, H.K.; Jhon, D.Y.; Yoo, O.J.; Chun, S.B.; Wui, I.S. Cloning, sequencing and expression of the amylase isozyme gene from Pseudomonas sp. KFCC 10818. Biotechnol. Lett. 1996, 18, 169–174. [Google Scholar] [CrossRef]
- Yang, C.H.; Liu, W.H. Cloning and characterization of a maltotriose-producing α-amylase gene from Thermobifida fusca. J. Ind. Microbiol. Biotechnol. 2007, 34, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Petricek, M.; Tichy, P.; Kuncova, M. Characterization of the α-amylase-encoding gene from Thermomonospora curvata. Gene 1992, 112, 77–83. [Google Scholar] [CrossRef]
- Matsubara, T.; Ben Ammar, Y.; Anindyawati, T.; Yamamoto, S.; Ito, K.; Iizuka, M.; Minamiura, N. Molecular cloning and determination of the nucleotide sequence of raw starch digesting α-amylase from Aspergillus awamori KT-11. J. Biochem. Mol. Biol. 2004, 37, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhoury, A.M.; Woloshuk, C.P. Amy1, the α-amylase gene of Aspergillus flavus: Involvement in aflatoxin biosynthesis in maize kernels. Phytopathology 1999, 89, 908–914. [Google Scholar] [CrossRef] [Green Version]
- Nagamine, K.; Murashima, K.; Kato, T.; Shimoi, H.; Ito, K. Mode of α-amylase production by the shochu koji mold Aspergillus kawachii. Biosci. Biotechnol. Biochem. 2003, 67, 2194–2202. [Google Scholar] [CrossRef]
- Toda, H.; Kondo, K.; Narita, K. The complete amino acid sequence of Taka-amylase A. Proc. Jpn. Acad. 1982, B58, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, I.; Tamura, G.; Ishikawa, T.; Hara, S. Cloning of the α-amylase cDNA of Aspergillus shirousamii and its expression in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 1992, 56, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Champreda, V.; Kanokratana, P.; Sriprang, R.; Tanapongpipat, S.; Eurwilaichitr, L. Purification, biochemical characterization, and gene cloning of a new extracellular thermotolerant and glucose tolerant maltooligosaccharide-forming α-amylase from an endophytic ascomycete Fusicoccum sp. BCC4124. Biosci. Biotechnol. Biochem. 2007, 71, 2010–2020. [Google Scholar] [CrossRef]
- Steyn, A.J.; Marmur, J.; Pretorius, I.S. Cloning, sequence analysis and expression in yeasts of a cDNA containing a Lipomyces kononenkoae α-amylase-encoding gene. Gene 1995, 166, 65–71. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, J.H.; Kim, D.; Day, D.F.; Robyt, J.F.; Park, K.H.; Moon, T.W. Cloning and expression of Lipomyces starkeyi α-amylase in Escherichia coli and determination of some of its properties. FEMS Microbiol. Lett. 2004, 233, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Ben Abdelmalek, I.; Urdaci, M.C.; Ben Ali, M.; Denayrolles, M.; Chaignepain, S.; Limam, F.; Bejar, S.; Marzouki, M.N. Structural investigation and homology modeling studies of native and truncated forms of α-amylases from Sclerotinia sclerotiorum. J. Microbiol. Biotechnol. 2009, 19, 1306–1318. [Google Scholar] [CrossRef]
- Hostinova, E.; Janecek, S.; Gasperik, J. Gene sequence, bioinformatics and enzymatic characterization of α-amylase from Saccharomycopsis fibuligera KZ. Protein J. 2010, 29, 355–564. [Google Scholar] [CrossRef]
- Roth, C.; Moroz, O.V.; Turkenburg, J.P.; Blagova, E.; Waterman, J.; Ariza, A.; Ming, L.; Tianqi, S.; Andersen, C.; Davies, G.J.; et al. Structural and functional characterization of three novel fungal amylases with enhanced stability and pH tolerance. Int. J. Mol. Sci. 2019, 20, 4902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorimachi, K.; Le Gal-Coëffet, M.F.; Williamson, G.; Archer, D.B.; Williamson, M.P. Solution structure of the granular starch binding domain of Aspergillus niger glucoamylase bound to β-cyclodextrin. Structure 1997, 5, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Janecek, S.; Sevcik, J. The evolution of starch-binding domain. FEBS Lett. 1999, 456, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Janecek, S.; Svensson, B.; MacGregor, E.A. Relation between domain evolution, specificity, and taxonomy of the α-amylase family members containing a C-terminal starch-binding domain. Eur. J. Biochem. 2003, 270, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, S.A.; Shafreen, R.B.; Priyanga, A.; Shiburaj, S.; Pandian, S.K. A highly divergent α-amylase from Streptomyces spp.: An evolutionary perspective. Int. J. Biol. Macromol. 2020, 163, 2415–2428. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.A.; Shafreen, R.B.; Balaji, K.; Ibrahim, K.S.; Shiburaj, S.; Gayathri, V.; Pandian, S.K. Cloning, expression, homology modelling and molecular dynamics simulation of four domain-containing α-amylase from Streptomyces griseus. J. Biomol. Struct. Dyn. 2021, 39, 2152–2163. [Google Scholar] [CrossRef]
- Yuuki, T.; Nomura, T.; Tezuka, H.; Tsuboi, A.; Yamagata, H.; Tsukagoshi, N.; Udaka, S. Complete nucleotide sequence of a gene coding for heat- and pH-stable α-amylase of Bacillus licheniformis: Comparison of the amino acid sequences of three bacterial liquefying α-amylases deduced from the DNA sequences. J. Biochem. 1985, 98, 1147–1156. [Google Scholar] [CrossRef]
- Marion, C.L.; Rappleye, C.A.; Engle, J.T.; Goldman, W.E. An α-(1,4)-amylase is essential for α-(1,3)-glucan production and virulence in Histoplasma capsulatum. Mol. Microbiol. 2006, 62, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.; Sepulveda, V.E.; Goldman, W.E.; San-Blas, G.; Nino-Vega, G.A. Expression of Paracoccidioides brasiliensis AMY1 in a Histoplasma capsulatum amy1 mutant, relates an α-(1,4)-amylase to cell wall α-(1,3)-glucan synthesis. PLoS ONE 2012, 7, e50201. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Li, S.; Kaminskyj, S.G. Characterization of Aspergillus nidulans α-glucan synthesis: Roles for two synthases and two amylases. Mol. Microbiol. 2014, 91, 579–595. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and biosynthesis of cell wall α-1,3-glucan in fungi. J. Fungi 2017, 3, 63. [Google Scholar] [CrossRef]
- Miyazawa, K.; Yoshimi, A.; Kasahara, S.; Sugahara, A.; Koizumi, A.; Yano, S.; Kimura, S.; Iwata, T.; Sano, M.; Abe, K. Molecular mass and localization of α-1,3-glucan in cell wall control the degree of hyphal aggregation in liquid culture of Aspergillus nidulans. Front. Microbiol. 2018, 9, 2623. [Google Scholar] [CrossRef] [PubMed]
- Ramli, A.N.; Azhar, M.A.; Shamsir, M.S.; Rabu, A.; Murad, A.M.; Mahadi, N.M.; Illias, R.M. Sequence and structural investigation of a novel psychrophilic α-amylase from Glaciozyma antarctica PI12 for cold-adaptation analysis. J. Mol. Model. 2013, 19, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Jones., R.A.; Jermiin, L.S.; Easteal, S.; Patel, B.K.; Beacham, I.R. Amylase and 16S rRNA genes from a hyperthermophilic archaebacterium. J. Appl. Microbiol. 1999, 86, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Du, M.; Cheng, B.; Wang, L.; Liu, X.; Ma, C.; Yang, C.; Xu, P. Close relationship of a novel Flavobacteriaceae α-amylase with archaeal α-amylases and good potentials for industrial applications. Biotechnol. Biofuels. 2014, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagomori, B.Y.; Dos Santos, F.C.; Barbosa-Tessmann, I.P. Recombinant expression, purification, and characterization of an α-amylase from Massilia timonae. 3 Biotech. 2021, 11, 13. [Google Scholar] [CrossRef]
- Yi, Z.; Fang, Y.; He, K.; Liu, D.; Luo, H.; Zhao, D.; He, H.; Jin, Y.; Zhao, H. Directly mining a fungal thermostable α-amylase from Chinese Nong-flavor liquor starter. Microb. Cell Fact. 2018, 17, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Yi, Z.; Fang, Y.; Jin, Y.; He, K.; Xiao, Y.; Zhao, D.; Luo, H.; He, H.; Sun, Q.; et al. Biochemical and synergistic properties of a novel α-amylase from Chinese nong-flavor Daqu. Microb. Cell Fact. 2021, 20, 80. [Google Scholar] [CrossRef]
- Chen, W.; Xie, T.; Shao, Y.; Chen, F. Phylogenomic relationships between amylolytic enzymes from 85 strains of fungi. PLoS ONE 2012, 7, e49679. [Google Scholar] [CrossRef] [Green Version]
- Sumitani, J.; Tottori, T.; Kawaguchi, T.; Arai, M. New type of starch-binding domain: The direct repeat motif in the C-terminal region of Bacillus sp. no. 195 α-amylase contributes to starch binding and raw starch degrading. Biochem. J. 2000, 350, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Chang, J.C.; Chen, J.P. Cloning and nucleotide sequence of an extracellular α-amylase gene from Aeromonas hydrophila MCC-1. J. Gen. Microbiol. 1993, 139, 3215–3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Lage, J.L.; Van Wormhoudt, A.; Cariou, M.L. Diversity and evolution of the α-amylase genes in animals. Biologia 2002, 57 (Suppl. 11), 181–189. [Google Scholar]
- Saltzmann, K.D.; Saltzmann, K.A.; Neal, J.J.; Scharf, M.E.; Bennett, G.W. Characterization of BGTG-1, a tergal gland-secreted α-amylase, from the German cockroach, Blattella germanica (L.). Insect Mol. Biol. 2006, 15, 425–433. [Google Scholar] [CrossRef]
- Grossman, G.L.; Campos, Y.; Severson, D.W.; James, A.A. Evidence for two distinct members of the amylase gene family in the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 1997, 27, 769–781. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Zhu, G.; Hayashi, M.; Shimomura, N.; Yamaguchi, T.; Aimi, T. Differential expression of three α-amylase genes from the basidiomycetous fungus Pholiota microspora. Mycoscience 2017, 58, 188–191. [Google Scholar] [CrossRef]
- Benson, B.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [Green Version]
- The UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [Green Version]
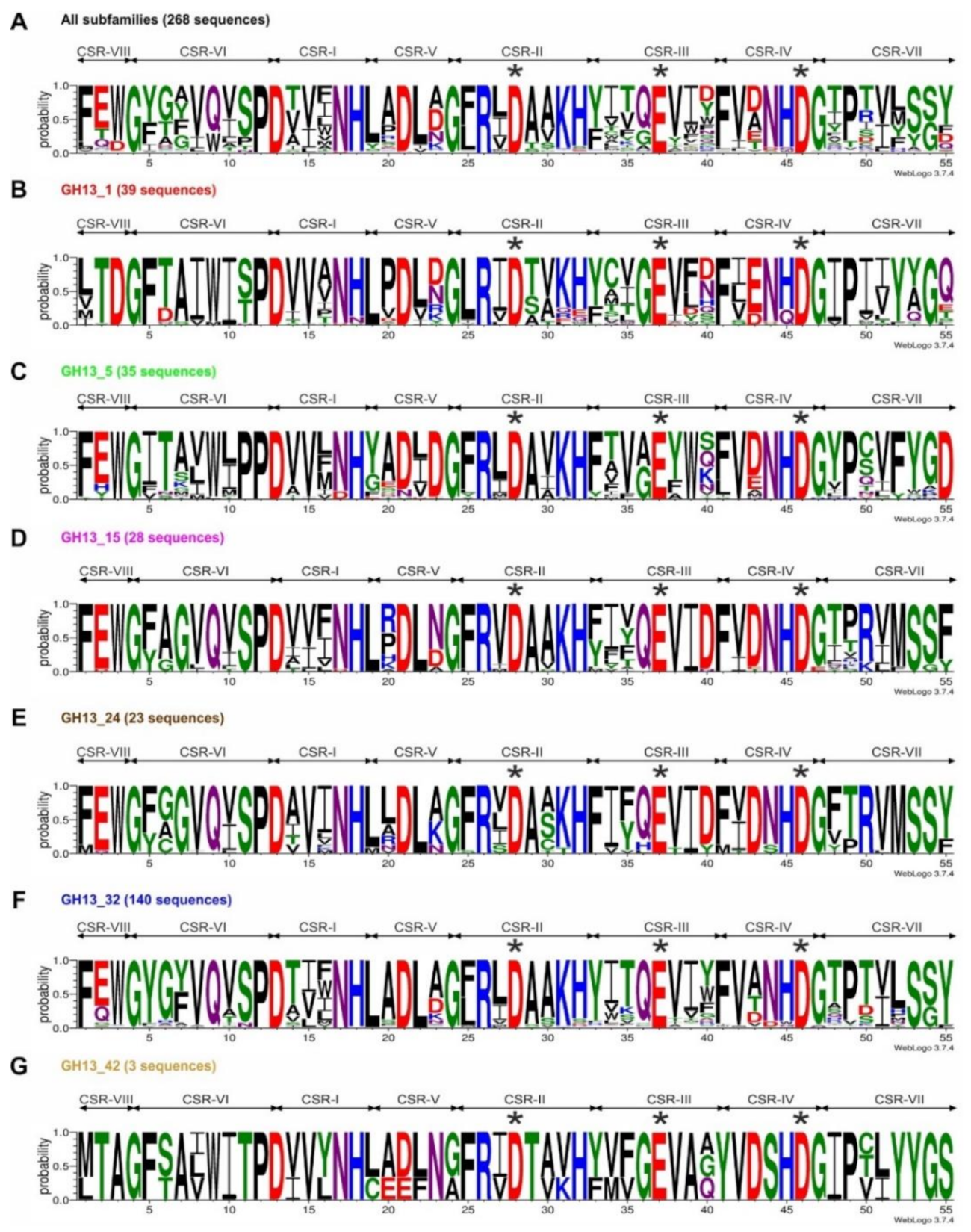
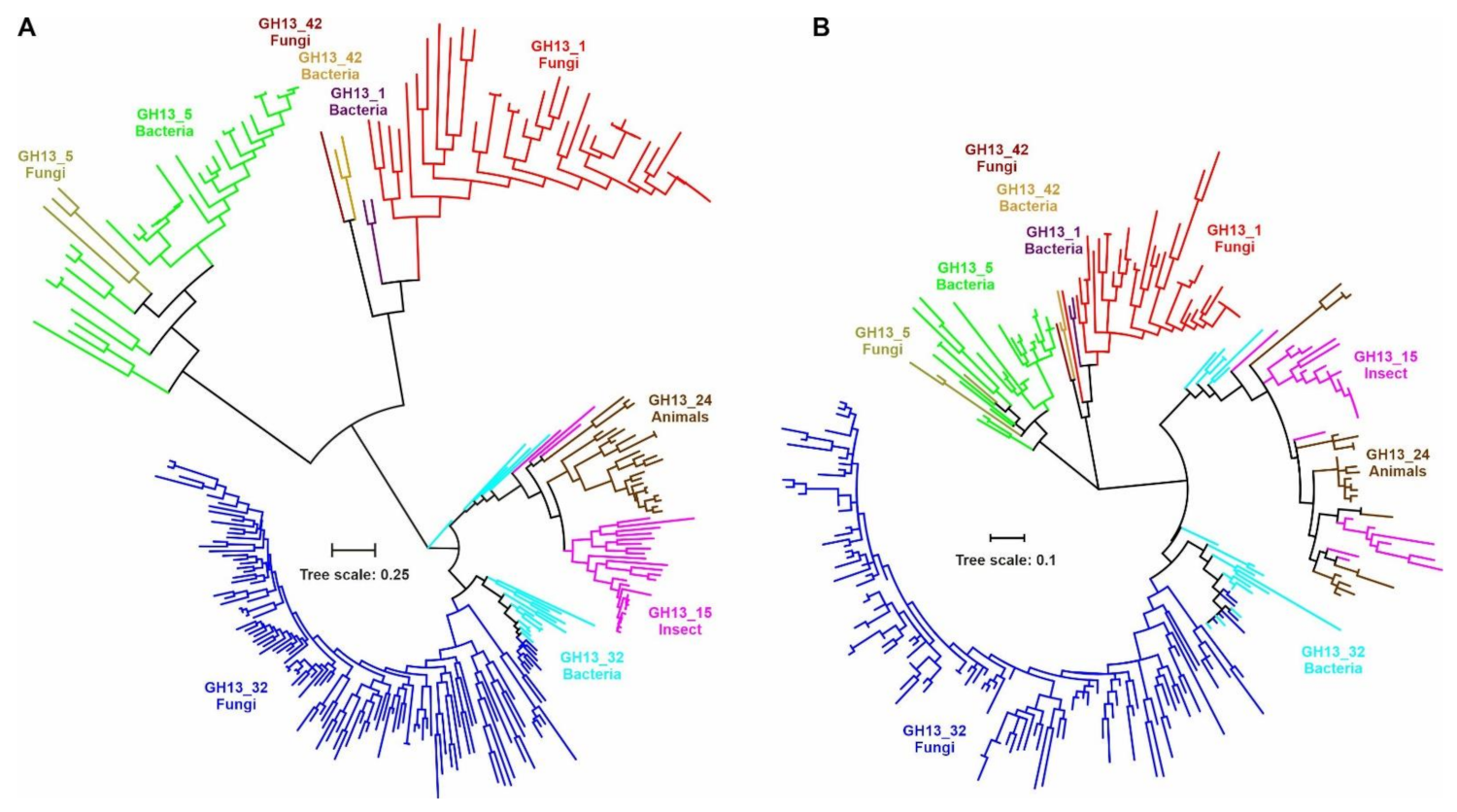
| Subfamily | GH13_1 | GH13_5 | GH13_15 | GH13_24 | GH13_32 | GH13_42 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | E | P | E | P | E | P | E | P | E | P | E | |
| 2 | 37 | 32 | 3 | 0 | 28 | 0 | 23 | 20 | 120 | 2 | 1 | |
| Total | 39 | 35 | 28 | 23 | 140 | 3 | ||||||
| α-Amylase | I | I |
|---|---|---|
| AAA85446_Paenibacillus_polymyxa | G | R |
| CAA49465_Thermoactinomyces_vulgaris | G | A |
| BAD06003_Aspergillus_awamori | Y | D |
| BAD06002_Aspergillus_awamori | Y | W |
| AAF14264_Aspergillus_flavus | Y | W |
| BAD01051_Aspergillus_kawachii | Y | W |
| BAA22993_Aspergillus_kawachii | Y | D |
| EAA64850_Aspergillus_nidulans | Y | Y |
| AAF17100_Aspergillus_nidulans | S | T |
| P56271_Aspergillus_niger | Y | D |
| CAK44871_Aspergillus_niger | Y | W |
| CAK40249_Aspergillus_niger | V | Y |
| CAK41088_Aspergillus_niger | S | Y |
| CAA31218_Aspergillus_oryzae | Y | W |
| BAA01255_Aspergillus_shirousami | Y | W |
| AEB80431_Aspergillus_tubingensis | Y | D |
| BAA12010_Cryptococcus_sp_S_2 | Y | Q |
| ABG48762_Fusicoccum_sp_BCC4124 | Y | W |
| AAO12212_Lipomyces_kononenkoae | D | V |
| AAC49622_Lipomyces_spencermartinsiae | Y | W |
| AAN75021_Lipomyces_starkeyi | Y | W |
| AFD54462_Malbranchea_cinnamomea | T | D |
| ABF72529_Ophiostoma_floccosum | S | S |
| EPS26265_Penicillium_oxalicum | S | N |
| ABO42285_Phanerochaete_chrysosporium | S | E |
| BAW15173_Pholiota_microspora | K | Q |
| BAF98616_Pichia_burtonii | T | S |
| AGJ52081_Rhizomucor_pusillus | T | M |
| ADL28123_Rhizopus_oryzae | T | M |
| ADD80242_Saccharomycopsis_fibuligera | S | S |
| ABS76467_Saitozyma_flava | Y | Q |
| CAB11471_Schizosaccharomyces_pombe | S | H |
| CAB40006_Schizosaccharomyces_pombe | S | Q |
| CAA34162_Schwanniomyces_occidentalis | Y | S |
| CAA51912_Schwanniomyces_occidentalis | Y | D |
| ACN82436_Sclerotinia_sclerotiorum | Y | Y |
| CAA03110_Thermomyces_lanuginosus | S | K |
| BAG69580_Trichoderma_viride | N | K |
| CAJ21046_Valsaria_rubricosa | N | V |
| α-Amylase | I | I | II | III | I | I | I | I | II | II | III | III |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AWX66236_Alicyclobacillus_sp_18711 | T | N | W | Y | F | Y | D | I | W | W | W | G |
| AAQ01675_Alkalimonas_amylolytica | S | Y | N | V | F | V | I | F | W | - | N | - |
| CAL14744_Anoxybacillus_flavithermus | W | W | W | Y | F | Y | P | I | W | W | W | G |
| AEW07376_Bacillus_acidicola | W | W | W | Y | F | Y | P | I | W | W | W | G |
| AAA22191_Bacillus_amyloliquefaciens | R | W | W | Y | F | Y | T | T | W | W | W | G |
| ABY86223_Bacillus_cereus | W | W | W | Y | F | Y | P | T | W | W | W | G |
| CAD26699_Bacillus_halmapalus | W | W | W | Y | F | Y | E | I | W | W | W | G |
| AAA22226_Bacillus_licheniformis | R | W | W | Y | F | Y | T | T | W | W | W | G |
| AEM05860_Bacillus_licheniformis | R | W | W | Y | F | Y | T | T | W | W | W | G |
| AAK00598_Bacillus_megaterium | W | W | W | Y | F | Y | P | T | W | W | W | G |
| AGN35141_Bacillus_paralicheniformis | R | W | W | Y | F | Y | T | T | W | W | W | G |
| AAR68734_Bacillus_sp | W | W | W | Y | F | Y | S | I | W | W | W | G |
| AAA22231_Bacillus_sp_707 | W | W | W | Y | F | Y | E | I | W | W | W | G |
| BAF03567_Bacillus_sp_JAMB_204 | R | W | W | Y | F | Y | S | T | Y | Y | W | G |
| CAC39917_Bacillus_sp_KSM_K38 | W | W | W | Y | F | Y | D | I | W | W | W | G |
| AAB18785_Bacillus_sp_MK_716 | W | W | W | Y | F | Y | P | I | W | W | W | G |
| AAA63900_Bacillus_sp_TS_23 | W | W | W | Y | F | Y | P | I | W | W | W | G |
| ABW87262_Bacillus_sp_YX_YX1 | R | W | W | Y | F | Y | T | T | W | W | W | G |
| AAF00567_Cytophaga_sp | R | Y | W | Y | F | Y | P | T | W | W | W | G |
| AAC74994_Escherichia_coli | T | E | W | V | F | V | P | A | W | H | W | G |
| AFZ41193_Exiguobacterium_sp_DAU5 | W | W | W | Y | F | Y | N | T | W | W | S | G |
| AAA22235_Geobacillus_stearothermophilus | W | W | W | Y | F | Y | P | I | W | W | W | G |
| ABX83871_Geobacillus_thermodenitrificans | W | R | W | R | F | R | P | I | W | W | F | K |
| AFC87833_Geobacillus_thermoleovorans | W | W | W | Y | F | Y | P | I | W | W | W | G |
| ACL70573_Halothermothrix_orenii | W | Y | S | V | F | V | Y | W | W | W | W | W |
| CAQ30277_Nostoc_sp_PCC_7119 | W | W | W | Y | F | Y | P | A | Y | Y | D | G |
| AAA27110_Salmonella_typhimurium | T | E | W | V | F | V | P | A | W | H | W | G |
| BAA24178_Streptococcus_equinus | R | Y | W | L | Y | L | E | I | W | Y | N | G |
| AAA97431_Streptococcus_equinus | R | Y | W | L | Y | L | E | I | W | Y | N | G |
| AAN59233_Streptococcus_mutans | R | W | W | I | Y | I | E | I | W | Y | S | G |
| CCD30600_uncultured_bacterium | R | Y | K | I | F | I | Y | Y | W | W | Y | R |
| ALP73597_Vibrio_alginolyticus | W | W | W | Y | F | Y | W | A | W | W | W | G |
| ABK62854_Histoplasma_capsulatum | R | Y | F | Y | L | Y | D | V | W | F | W | G |
| ABS11196_Paracoccidioides_brasiliensis | R | Y | F | Y | F | Y | E | L | W | Y | W | G |
| BAW15172_Pholiota_microspora | R | W | N | Y | F | Y | D | N | W | W | W | G |
| α-Amylase | I | I | II | II | III | IV |
|---|---|---|---|---|---|---|
| ABL09312_Acarus_siro | T | W | F | W | W | N |
| BAB85635_Anguilla_japonica | Y | W | W | W | W | W |
| AAL37207_Crassostrea_gigas | W | W | Y | W | W | W |
| AAL37183_Crassostrea_gigas | W | W | F | W | W | W |
| AAD38942_Dermatophagoides_pteronyssinus | W | W | F | W | W | N |
| AAD38943_Euroglyphus_maynei | W | W | F | W | W | N |
| AAC60246_Gallus_gallus | Y | W | W | W | W | W |
| ABO26610_Haliotis_discus_discus | Y | W | F | W | W | T |
| BAM74656_Haliotis_discus_hannai | Y | W | F | W | W | T |
| AAA51724_Homo_sapiens (pancreas) | Y | W | W | W | W | W |
| AAH63129_Homo_sapiens (saliva) | Y | W | W | W | W | W |
| AAA37221_Mus_musculus | Y | W | W | W | W | F |
| AAA37230_Mus_musculus | Y | W | W | W | W | W |
| H2N0D4_Oryzias_latipes | Y | W | W | W | W | L |
| CAA68065_Pecten_maximus | N | W | F | W | W | W |
| CAA54524_Penaeus_vannamei | Y | W | R | W | W | F |
| CAB65552_Penaeus_vannamei | Y | W | R | W | W | F |
| AAF65827_Pseudopleuronectes_americanus | Y | W | W | W | W | C |
| AAA40725_Rattus_norvegicus | Y | W | W | W | W | W |
| AAH88228_Rattus_norvegicus | Y | W | W | W | W | F |
| P83053_Struthio_camelus | Y | W | W | W | W | W |
| AAF02828_Sus_scrofa | Y | W | W | W | W | W |
| CAC87125_Tetraodon_nigroviridis | Y | W | W | W | W | L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janíčková, Z.; Janeček, Š. In Silico Analysis of Fungal and Chloride-Dependent α-Amylases within the Family GH13 with Identification of Possible Secondary Surface-Binding Sites. Molecules 2021, 26, 5704. https://doi.org/10.3390/molecules26185704
Janíčková Z, Janeček Š. In Silico Analysis of Fungal and Chloride-Dependent α-Amylases within the Family GH13 with Identification of Possible Secondary Surface-Binding Sites. Molecules. 2021; 26(18):5704. https://doi.org/10.3390/molecules26185704
Chicago/Turabian StyleJaníčková, Zuzana, and Štefan Janeček. 2021. "In Silico Analysis of Fungal and Chloride-Dependent α-Amylases within the Family GH13 with Identification of Possible Secondary Surface-Binding Sites" Molecules 26, no. 18: 5704. https://doi.org/10.3390/molecules26185704
APA StyleJaníčková, Z., & Janeček, Š. (2021). In Silico Analysis of Fungal and Chloride-Dependent α-Amylases within the Family GH13 with Identification of Possible Secondary Surface-Binding Sites. Molecules, 26(18), 5704. https://doi.org/10.3390/molecules26185704







