Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors
Abstract
1. Introduction
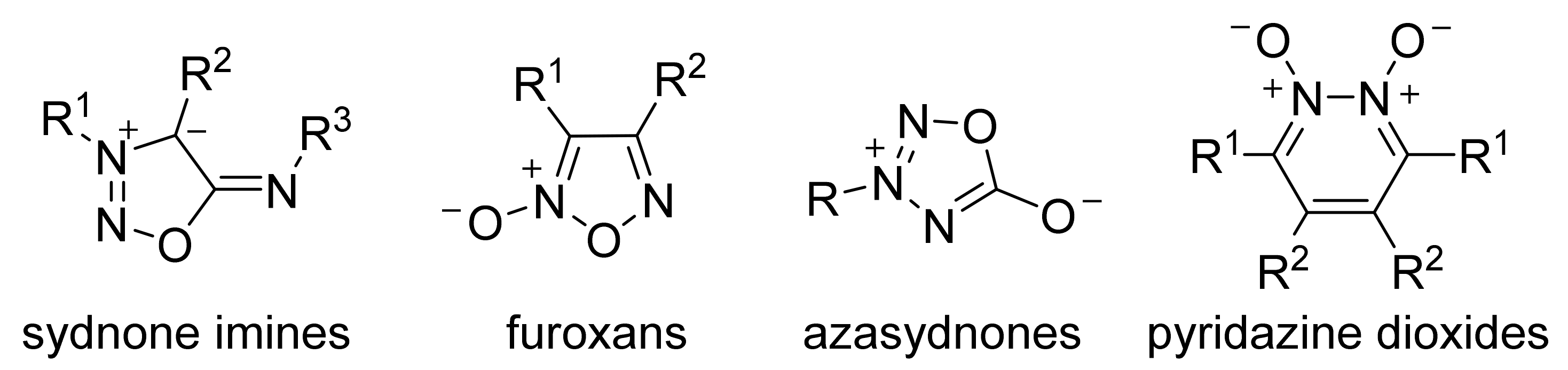
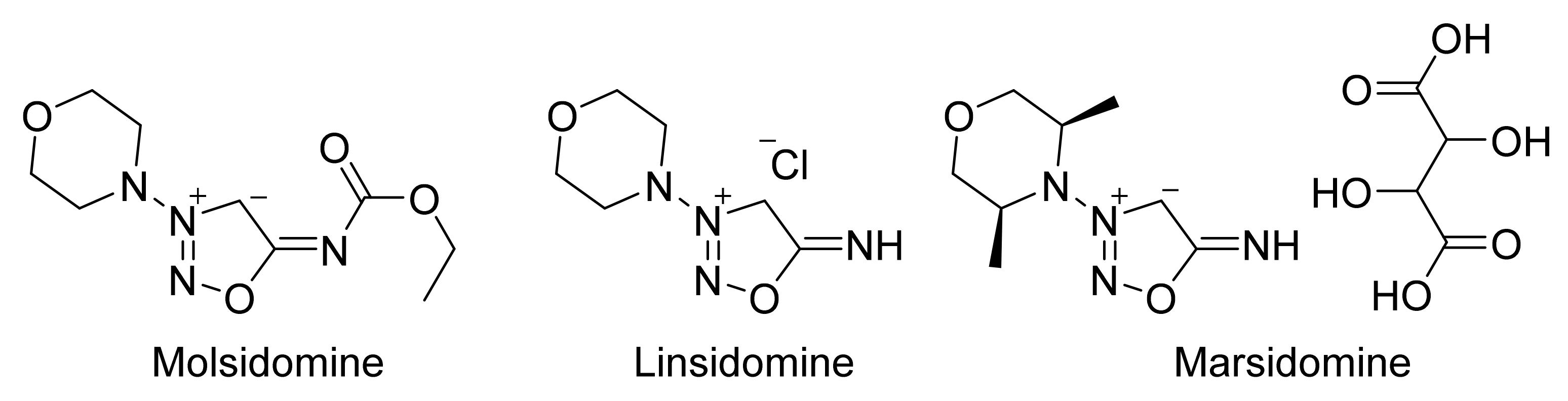
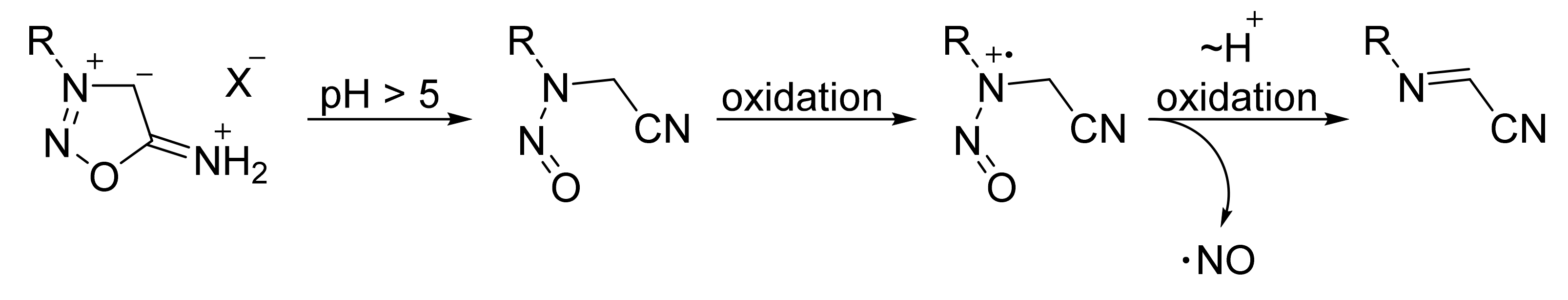
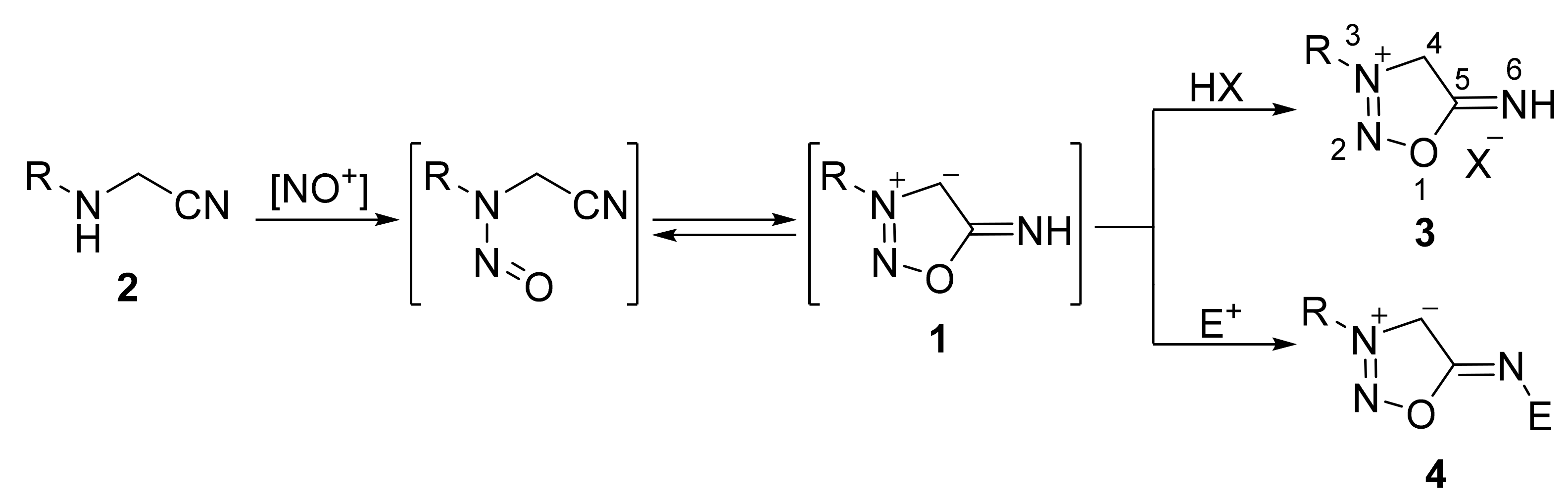
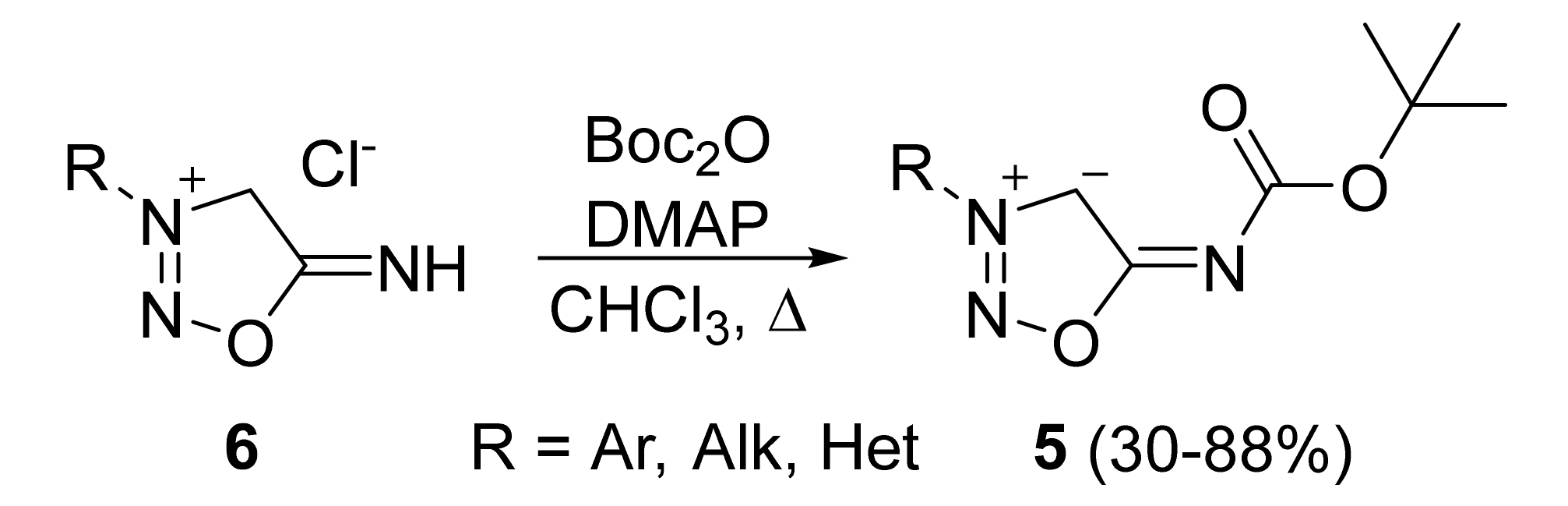
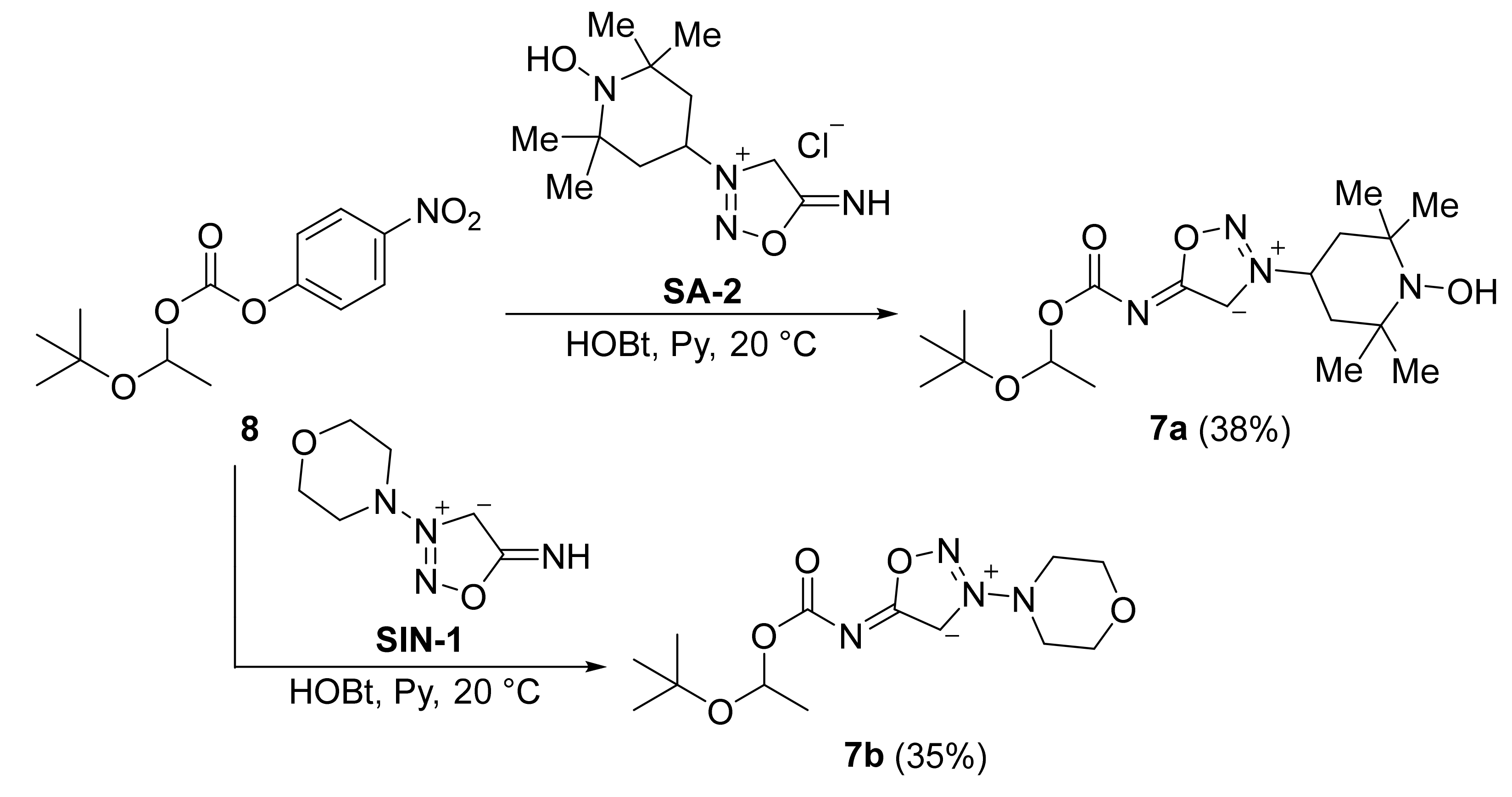
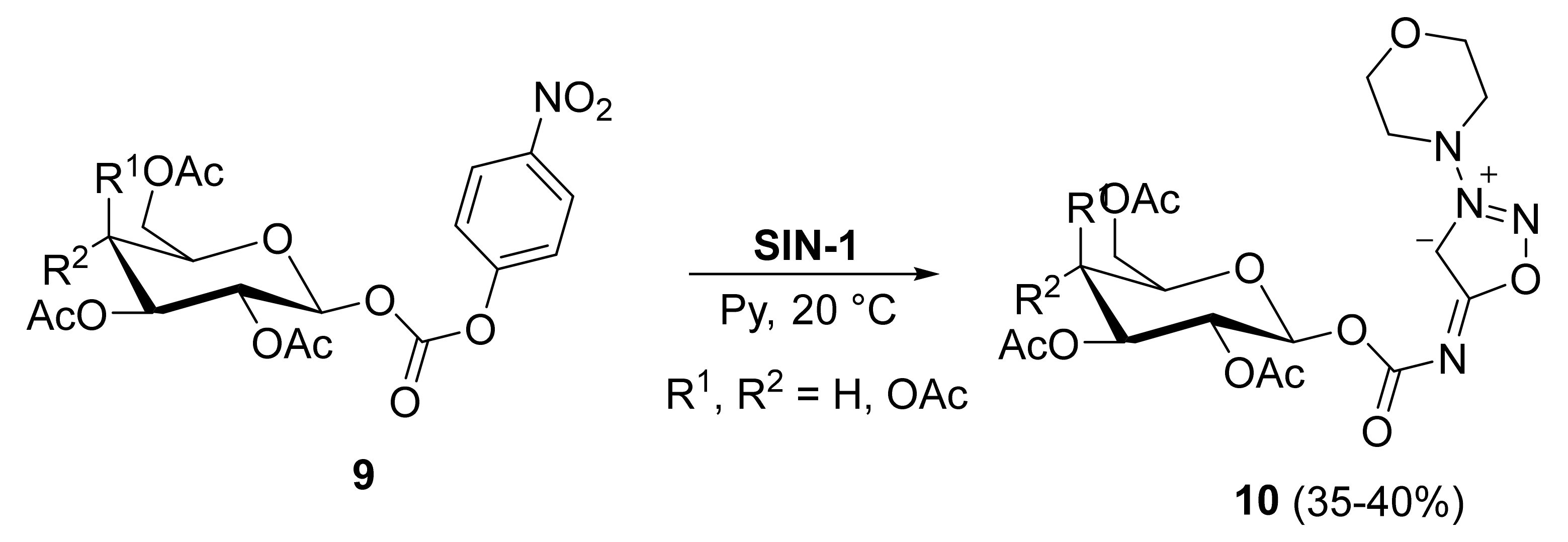
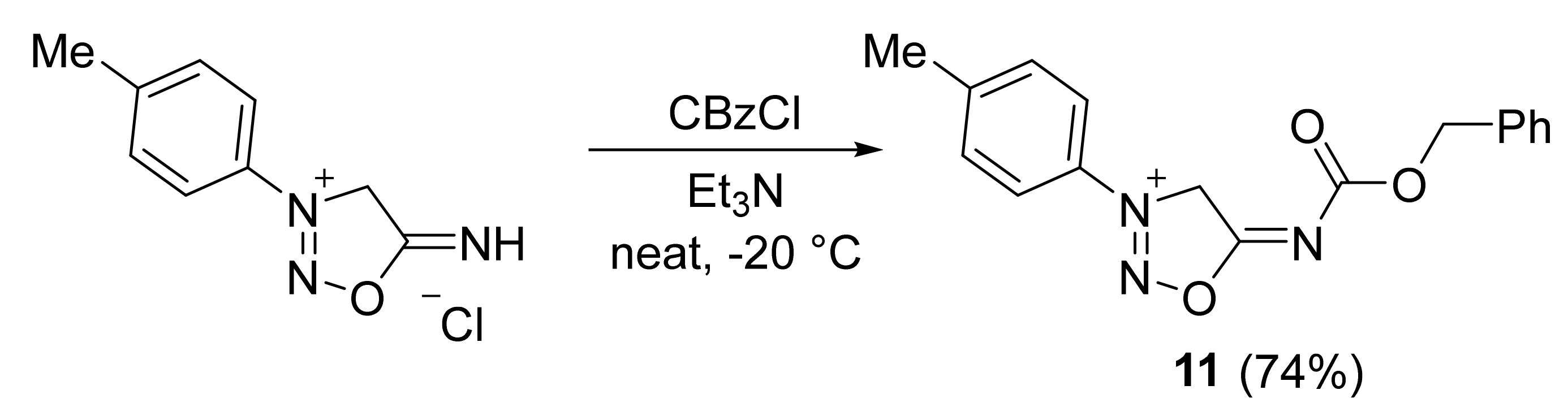
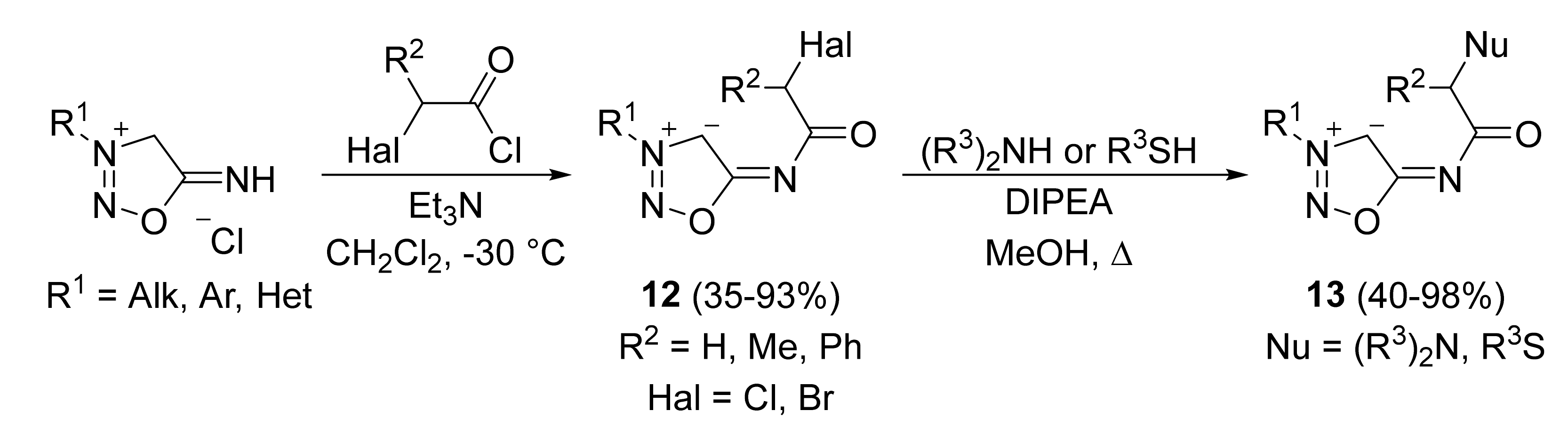
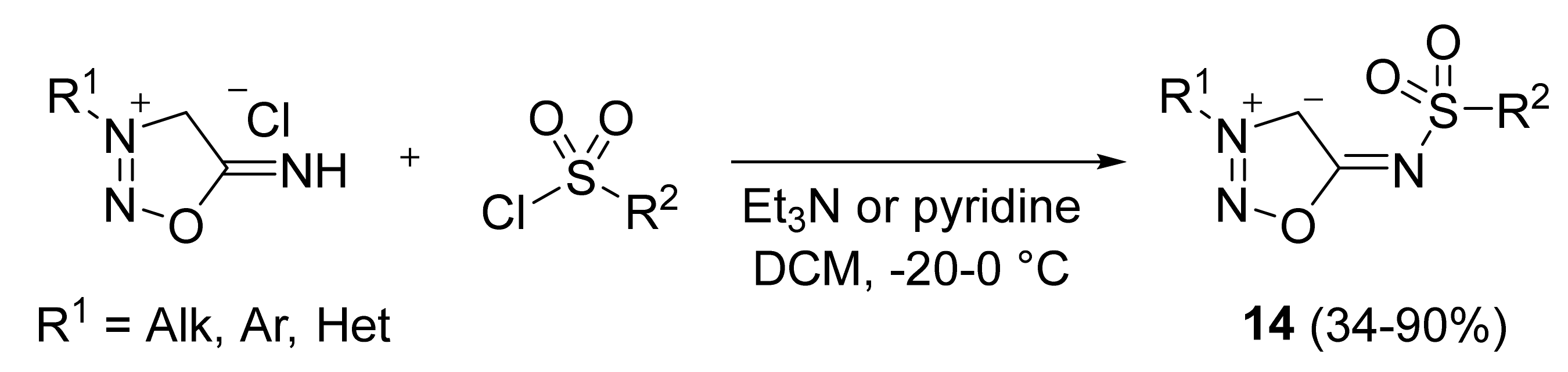
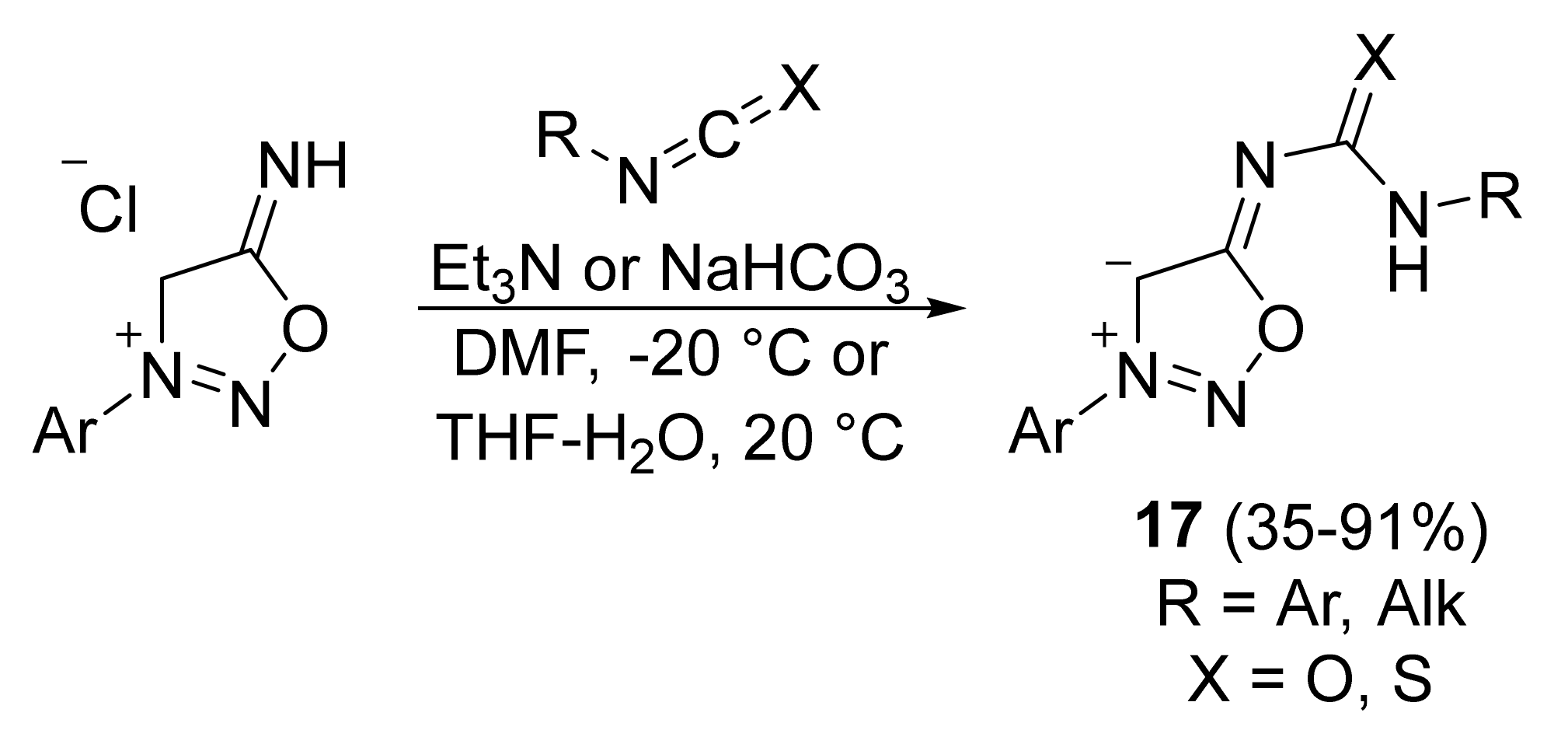
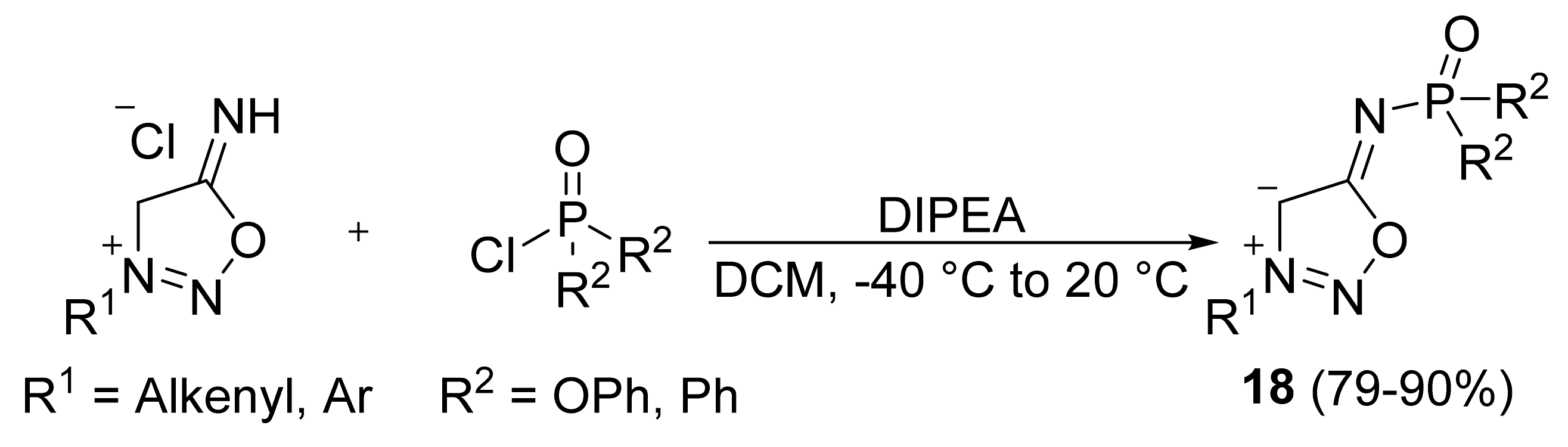
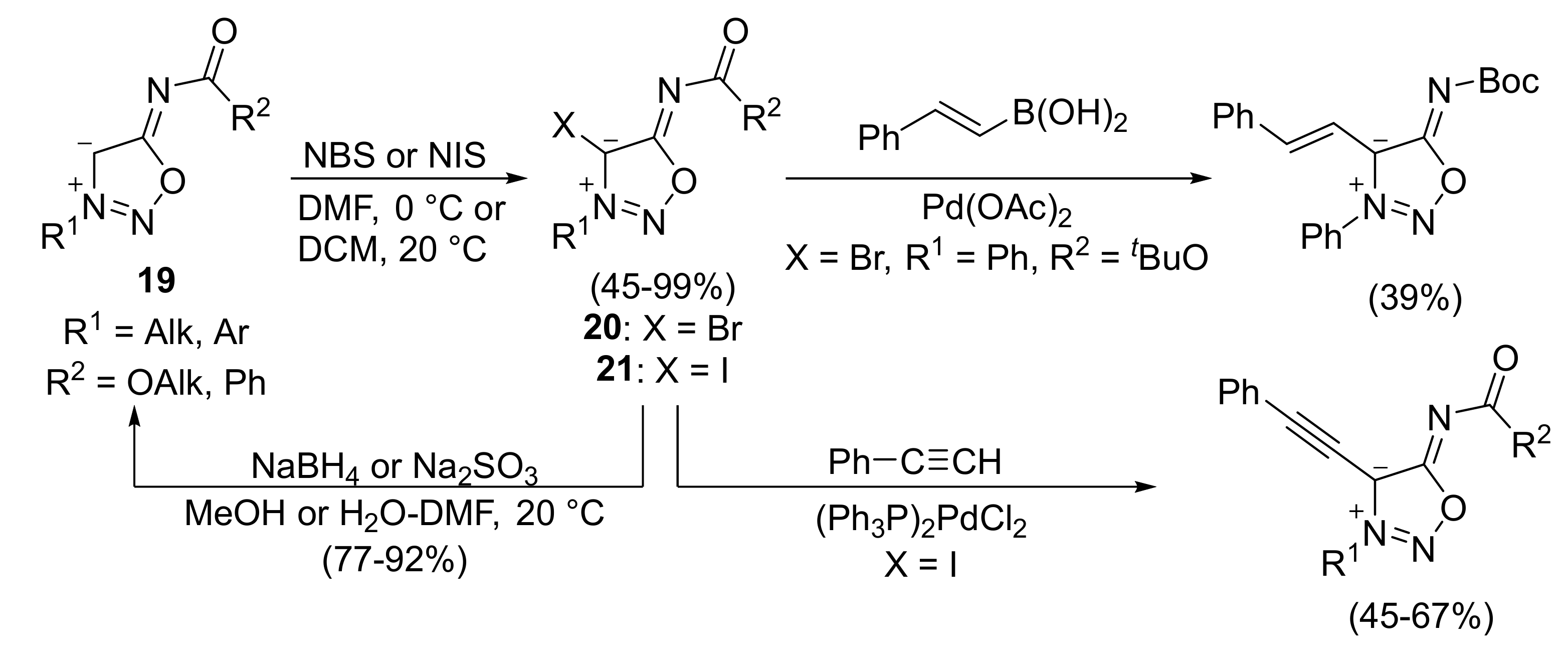
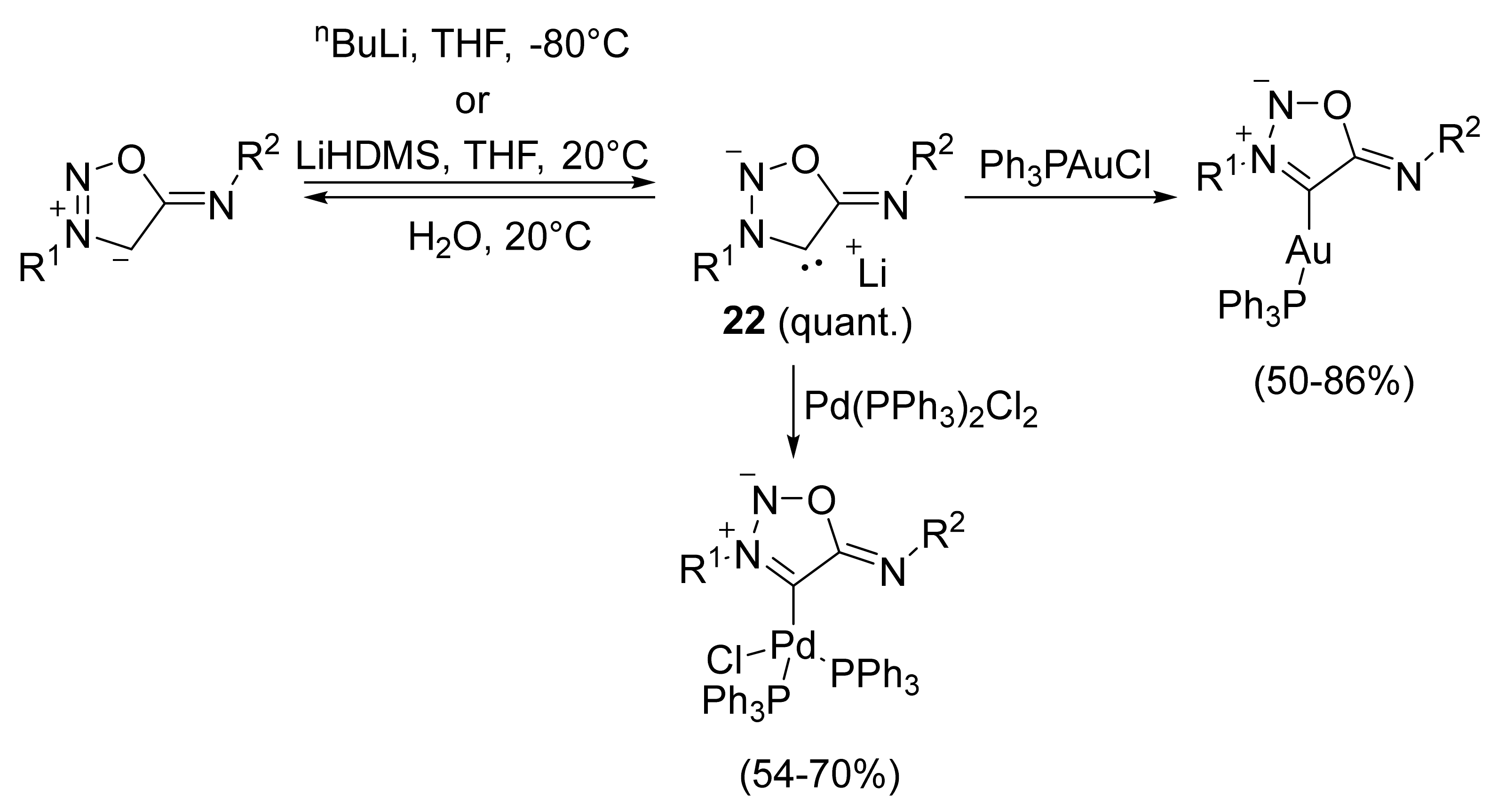
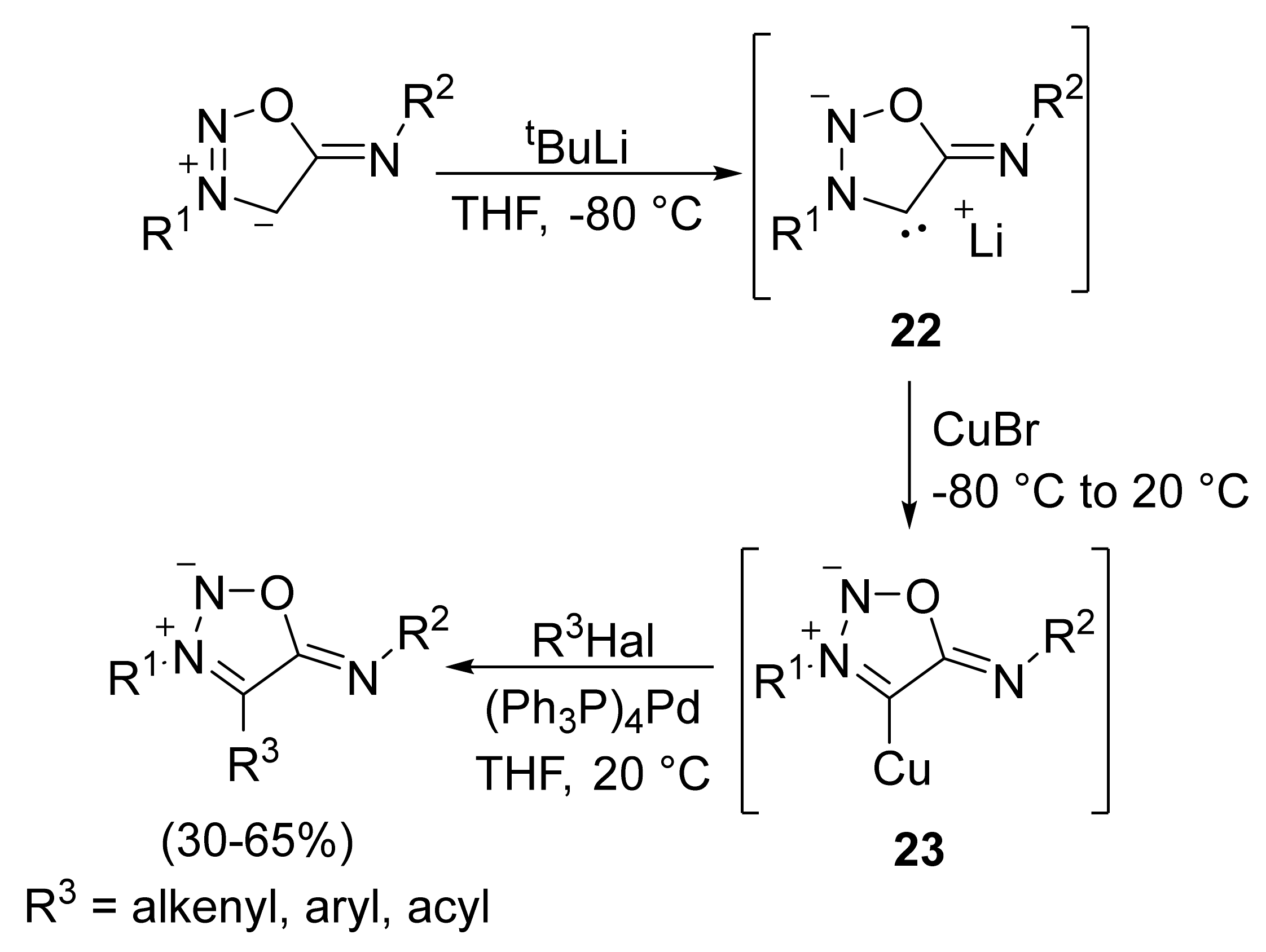
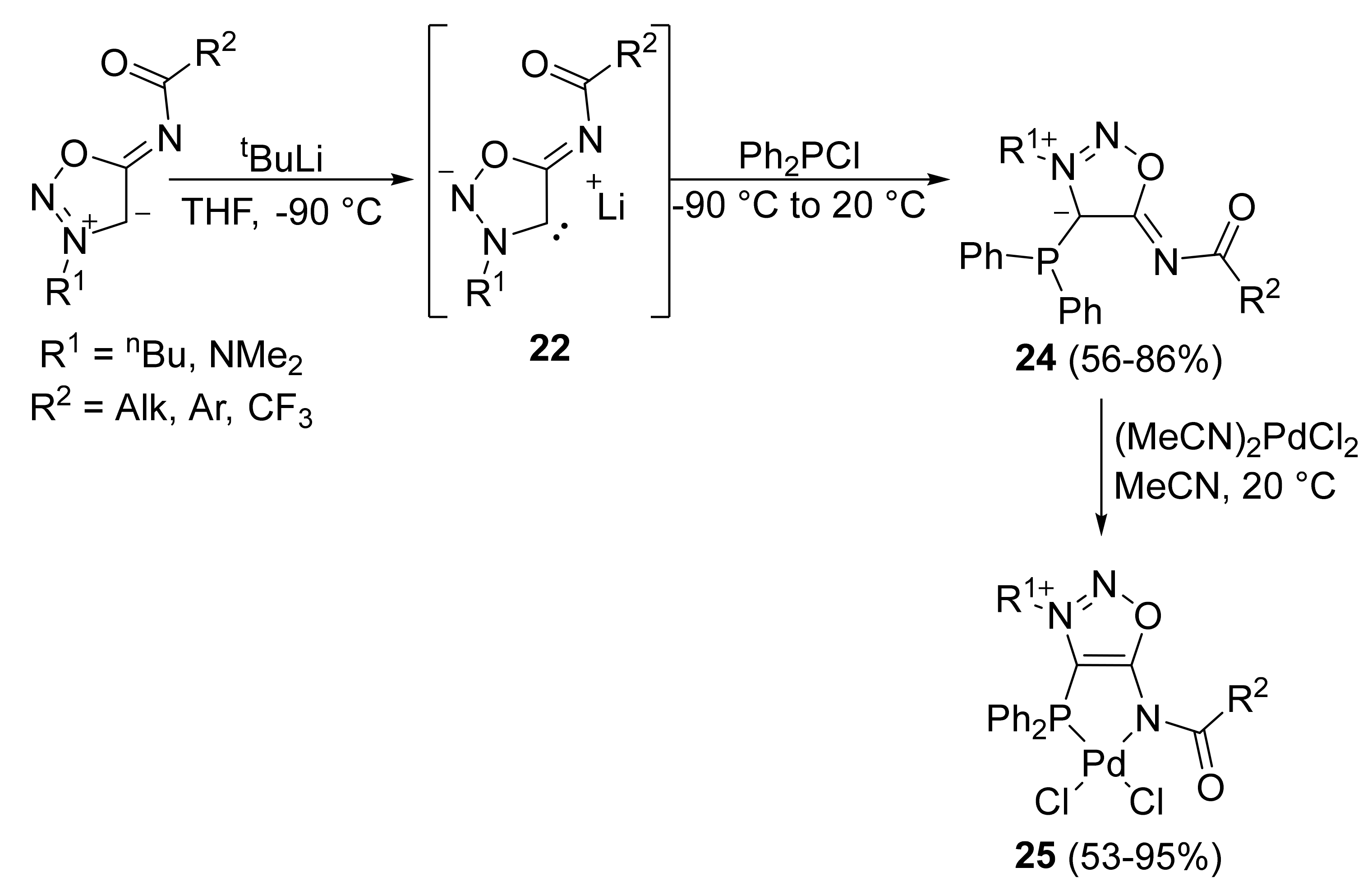
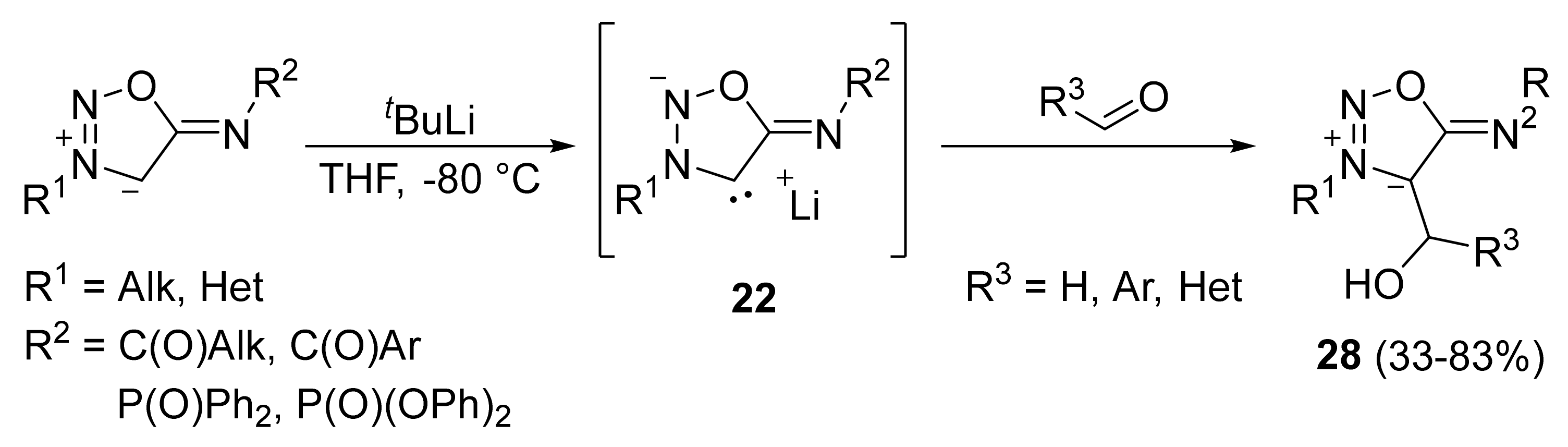
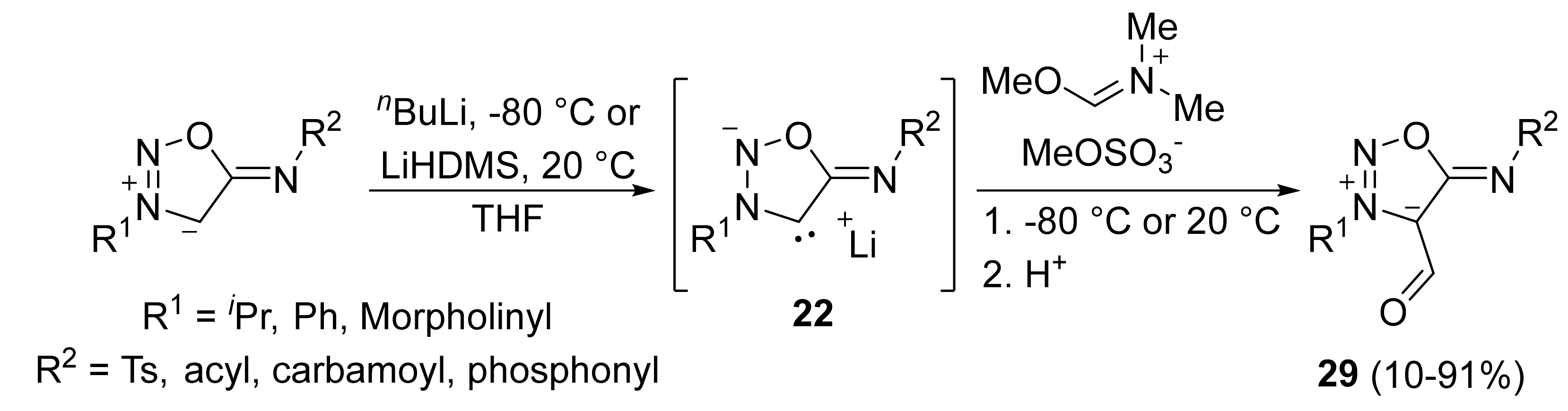
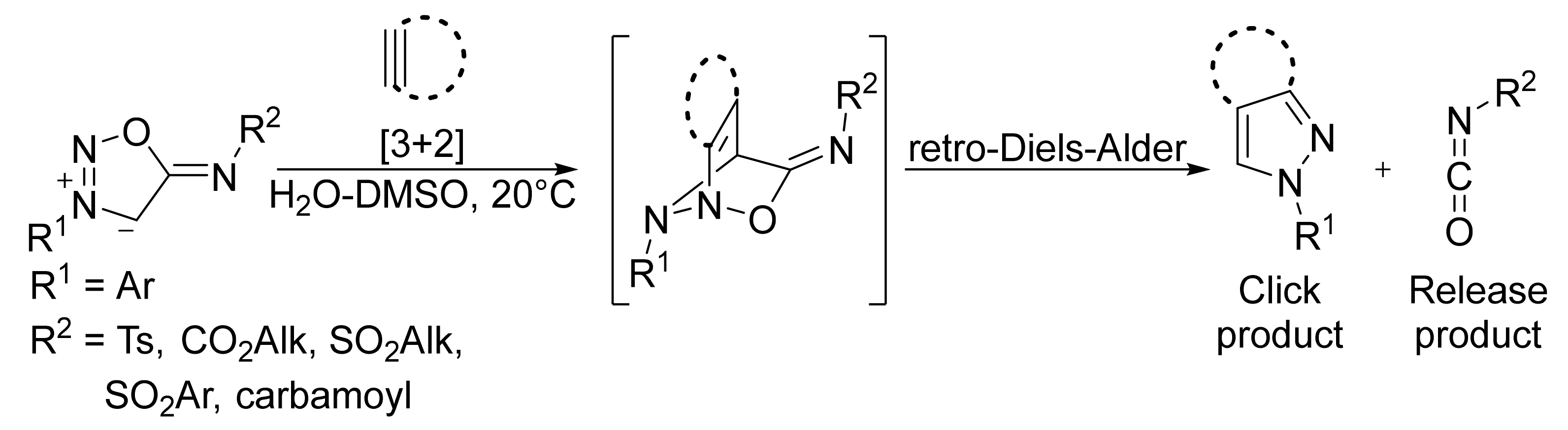
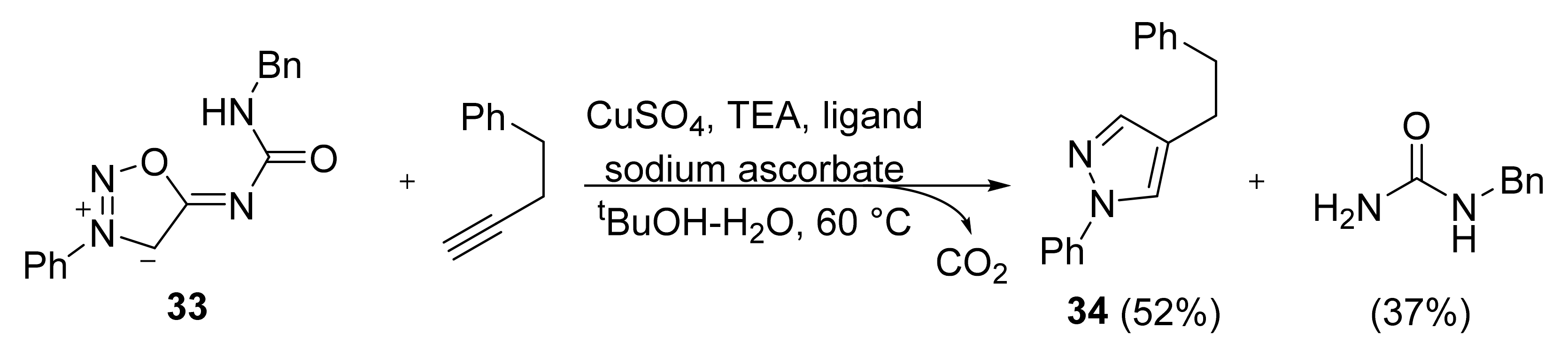
2. Sydnone Imines
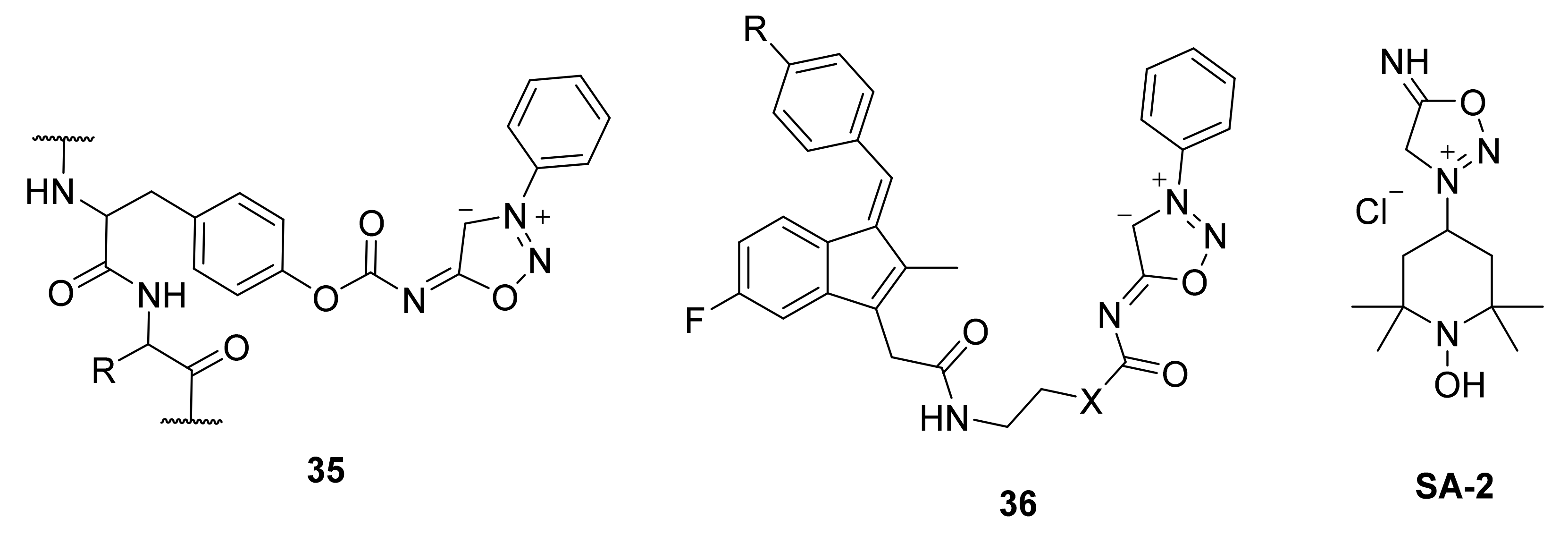
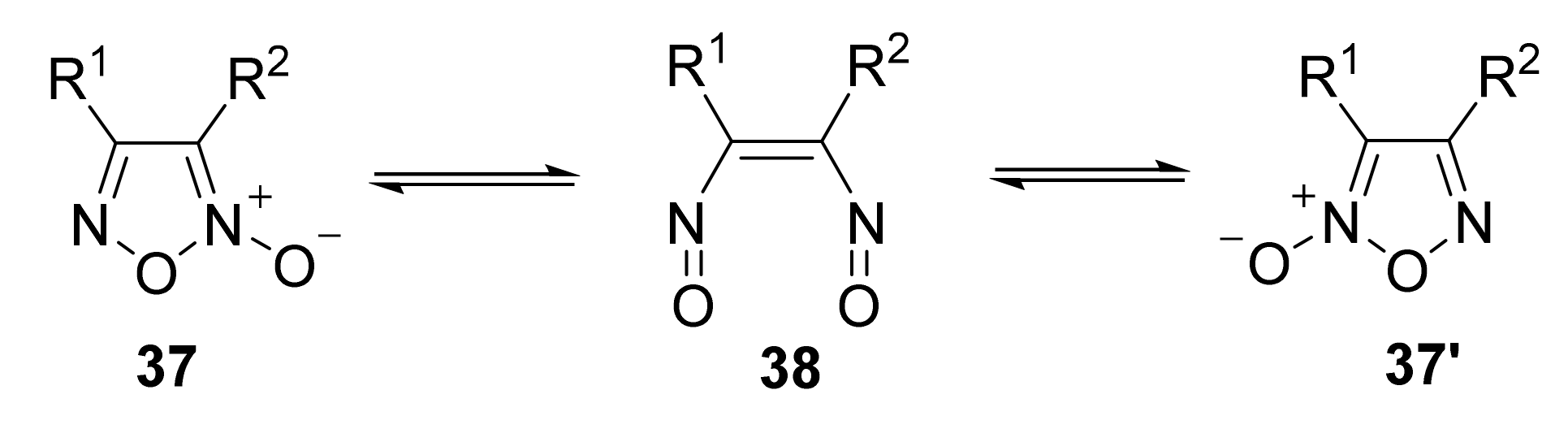
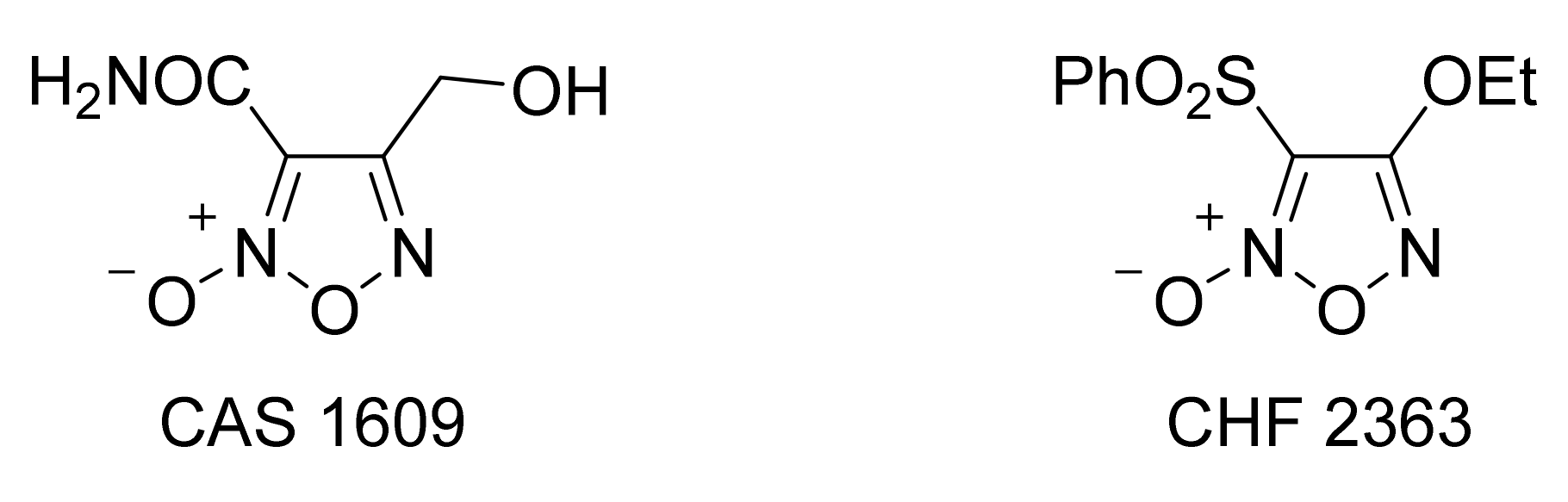
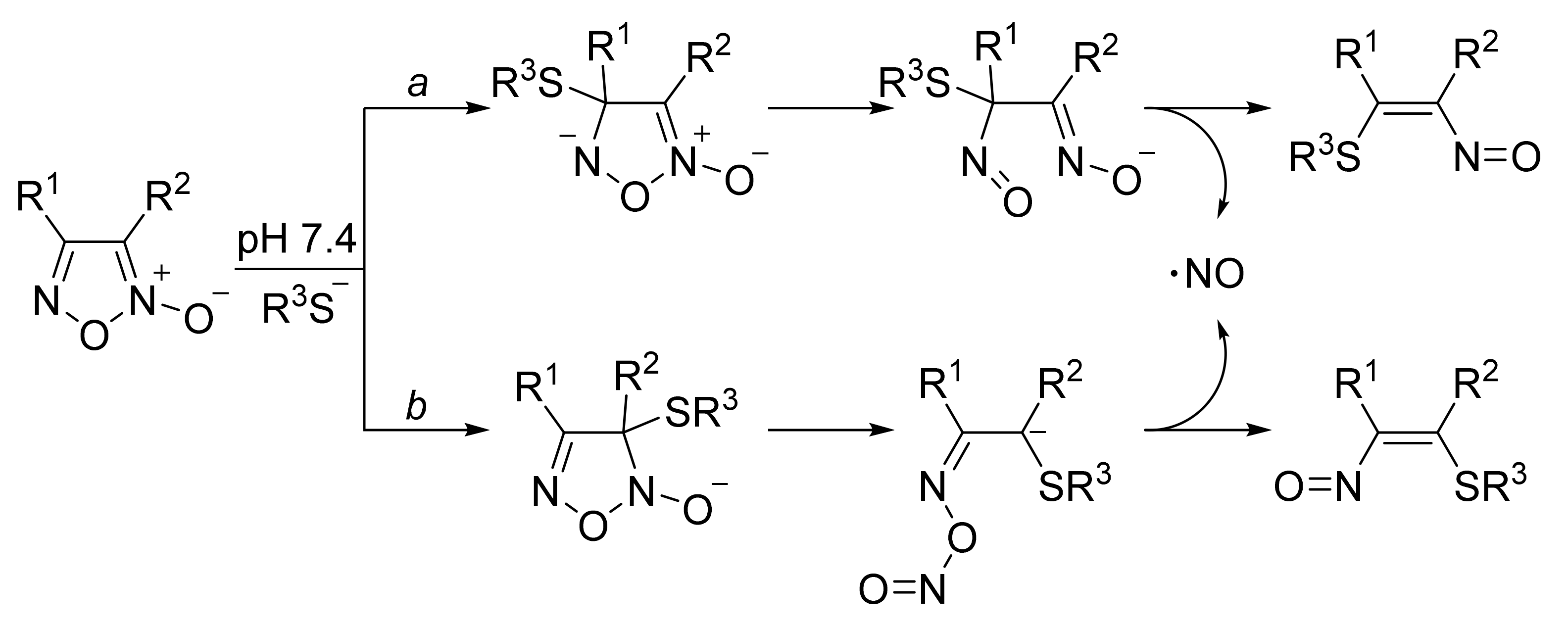
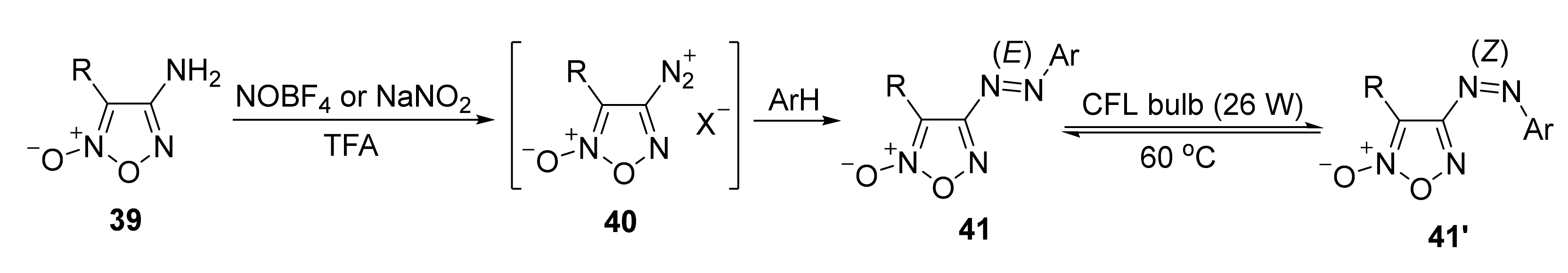
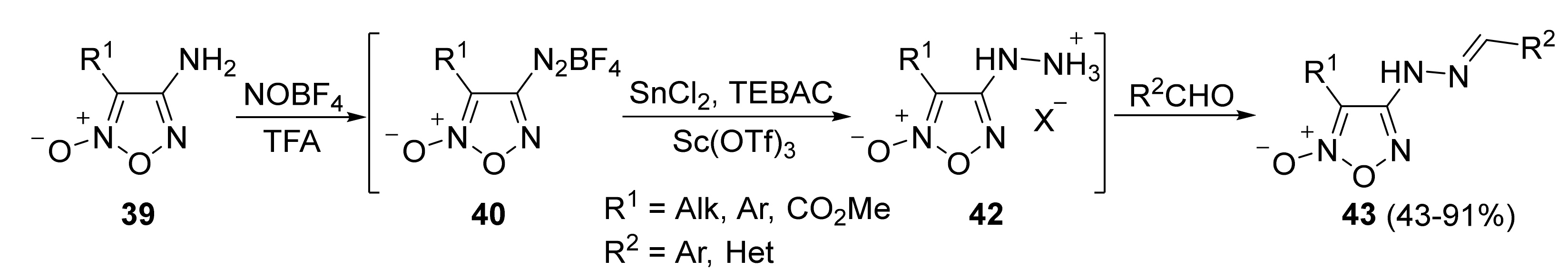
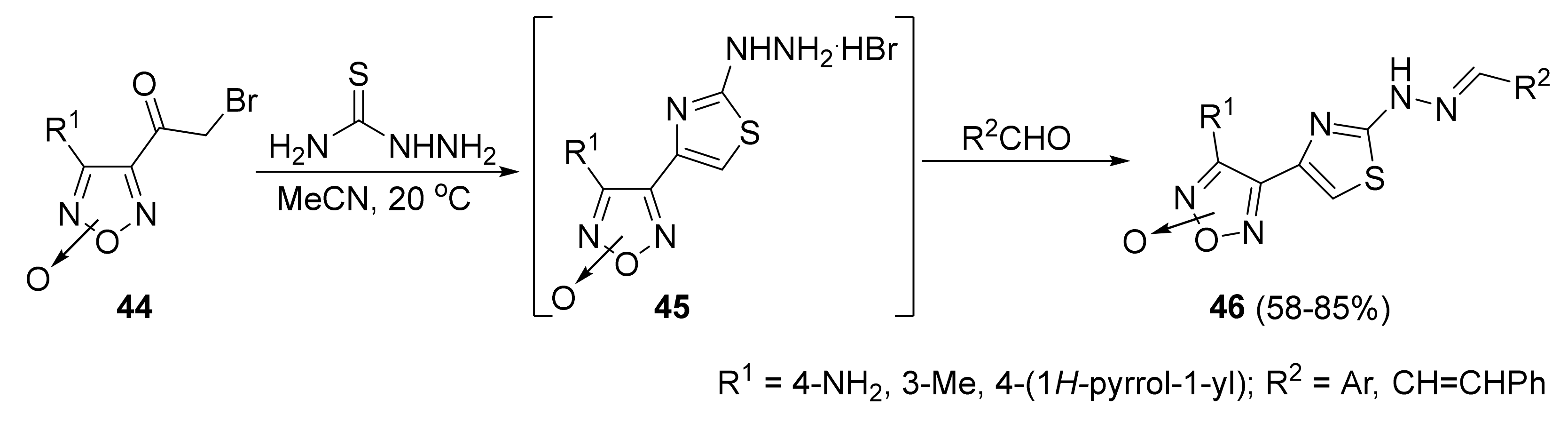
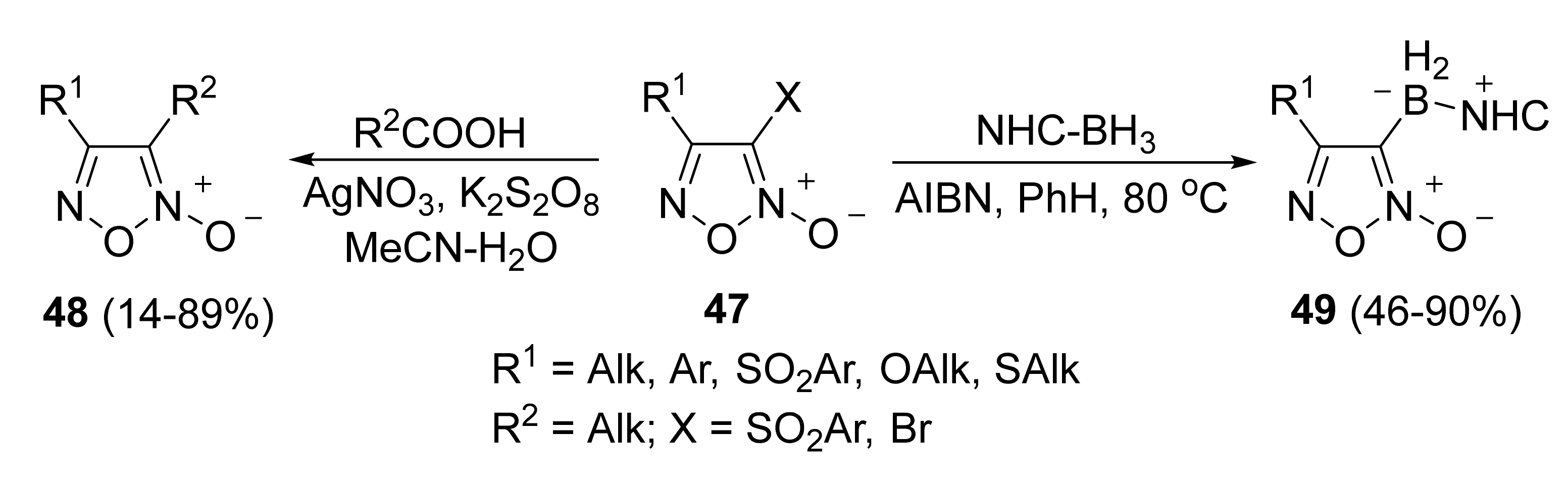
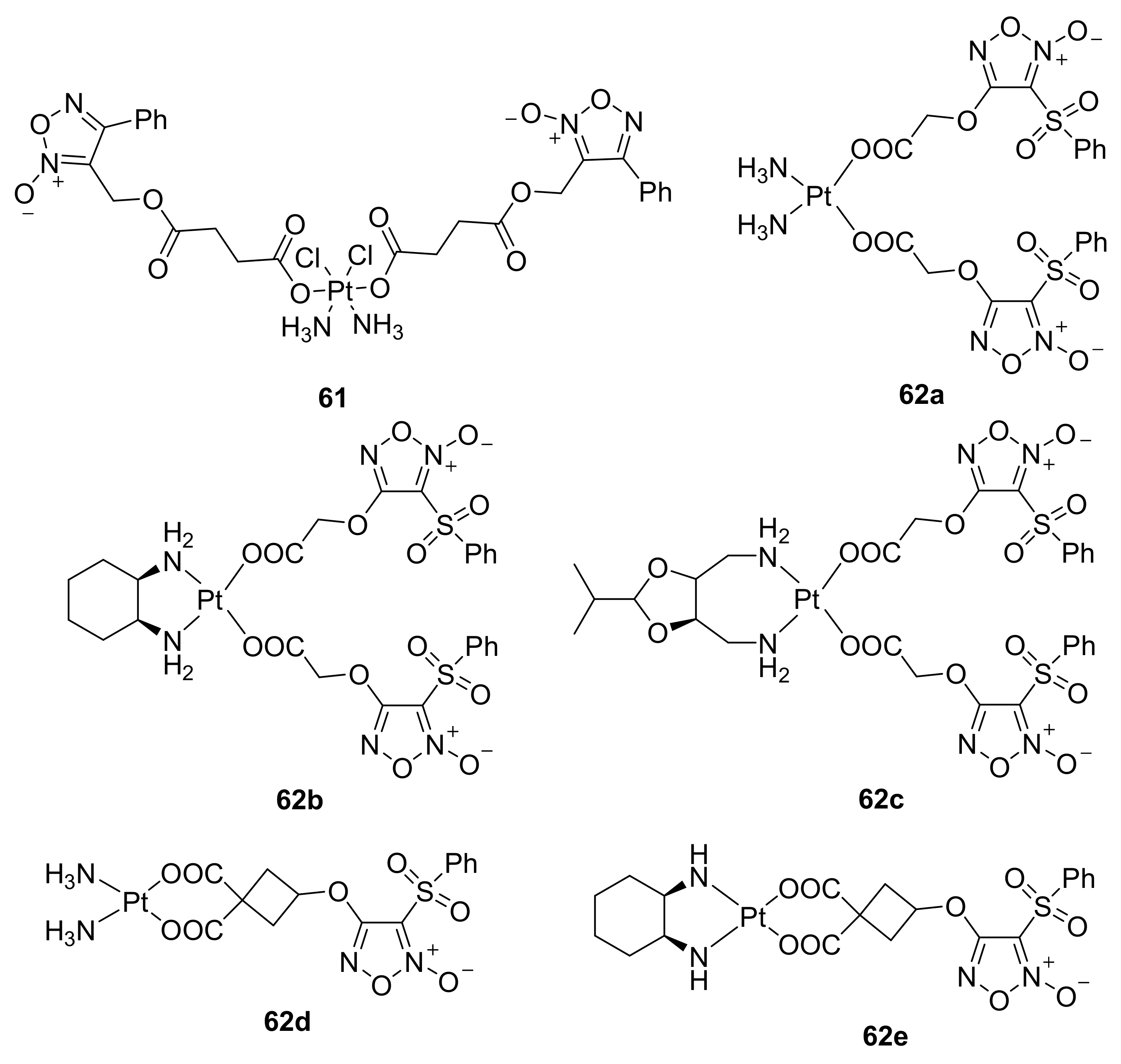
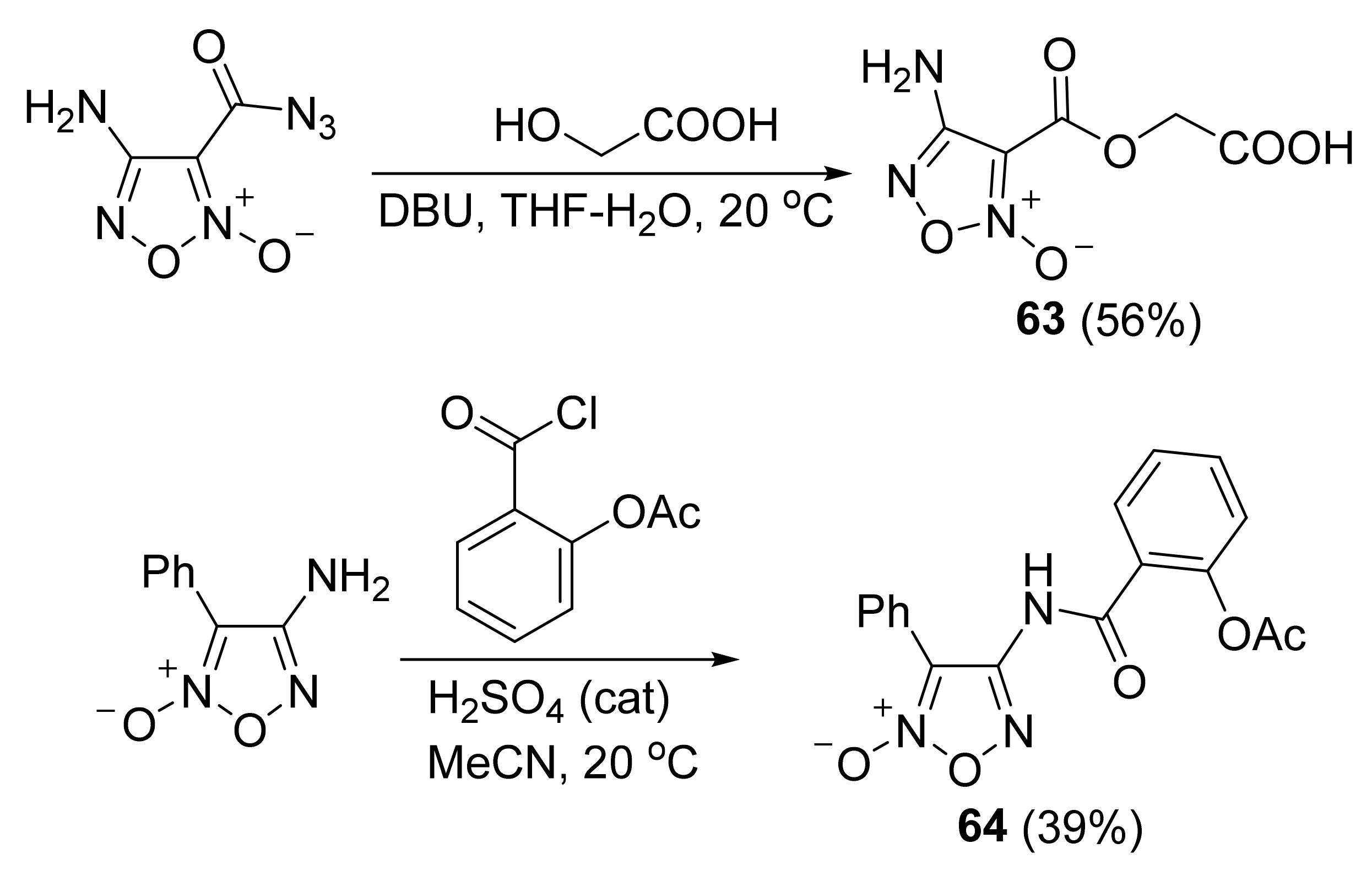
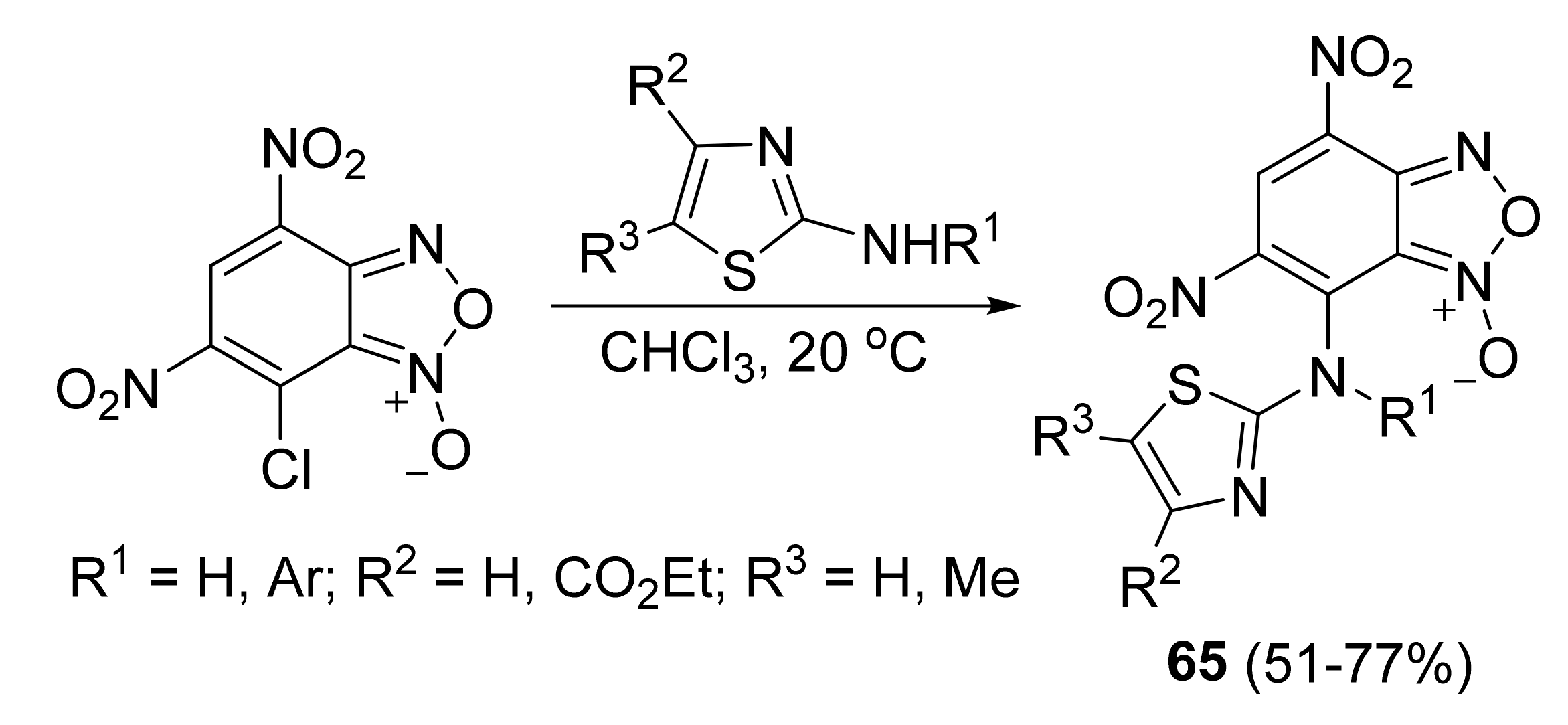
3. Furoxans
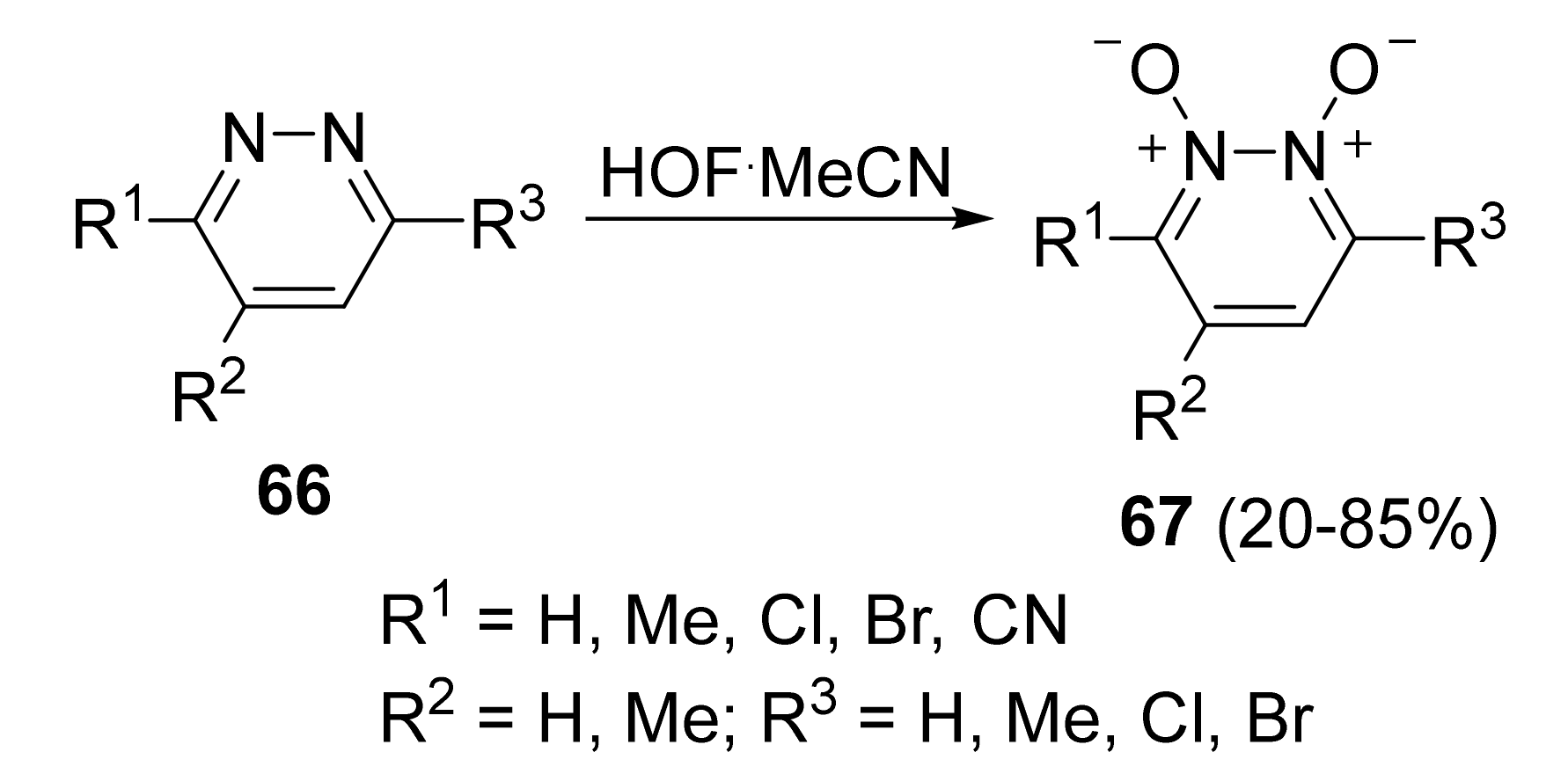
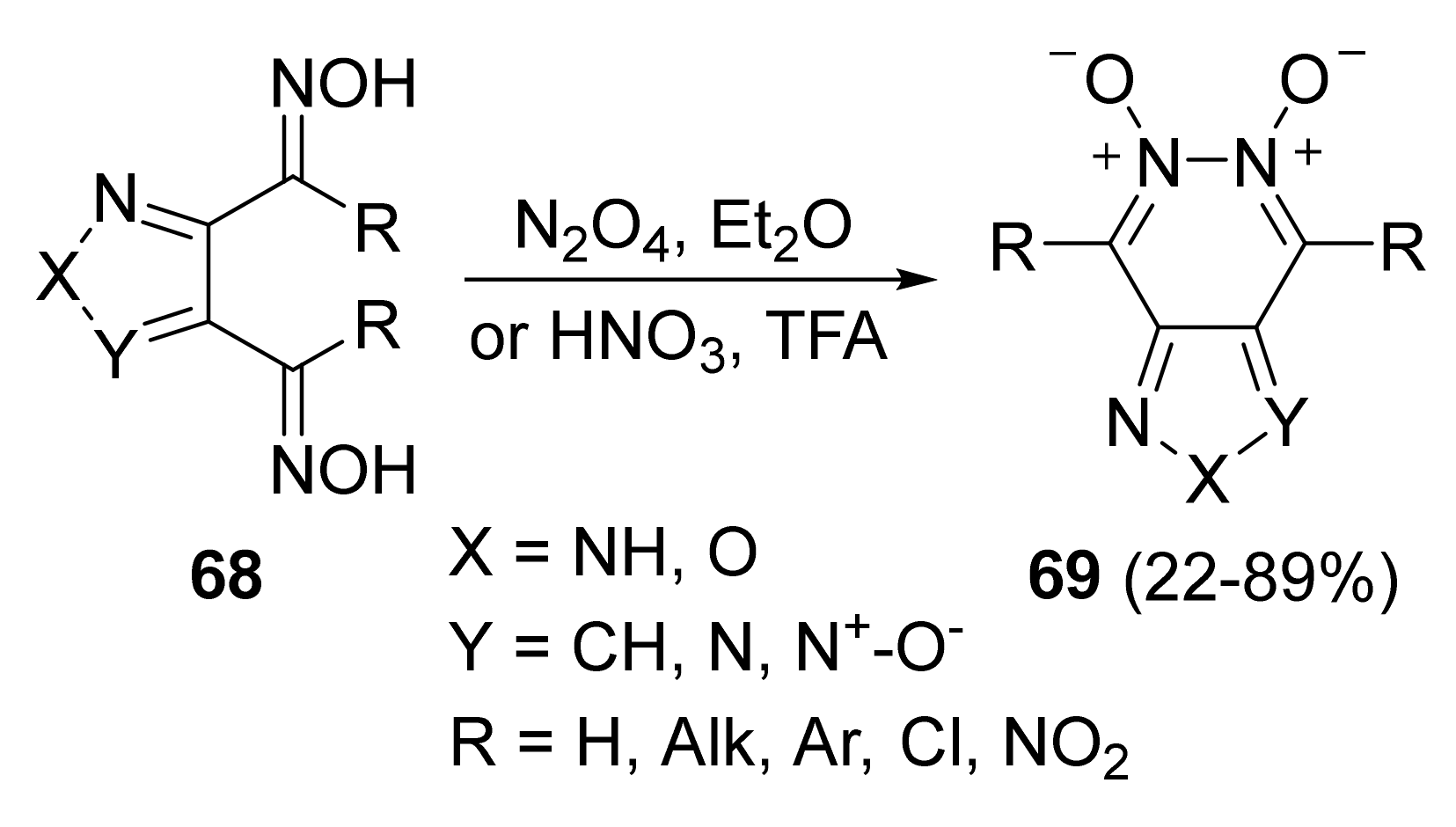
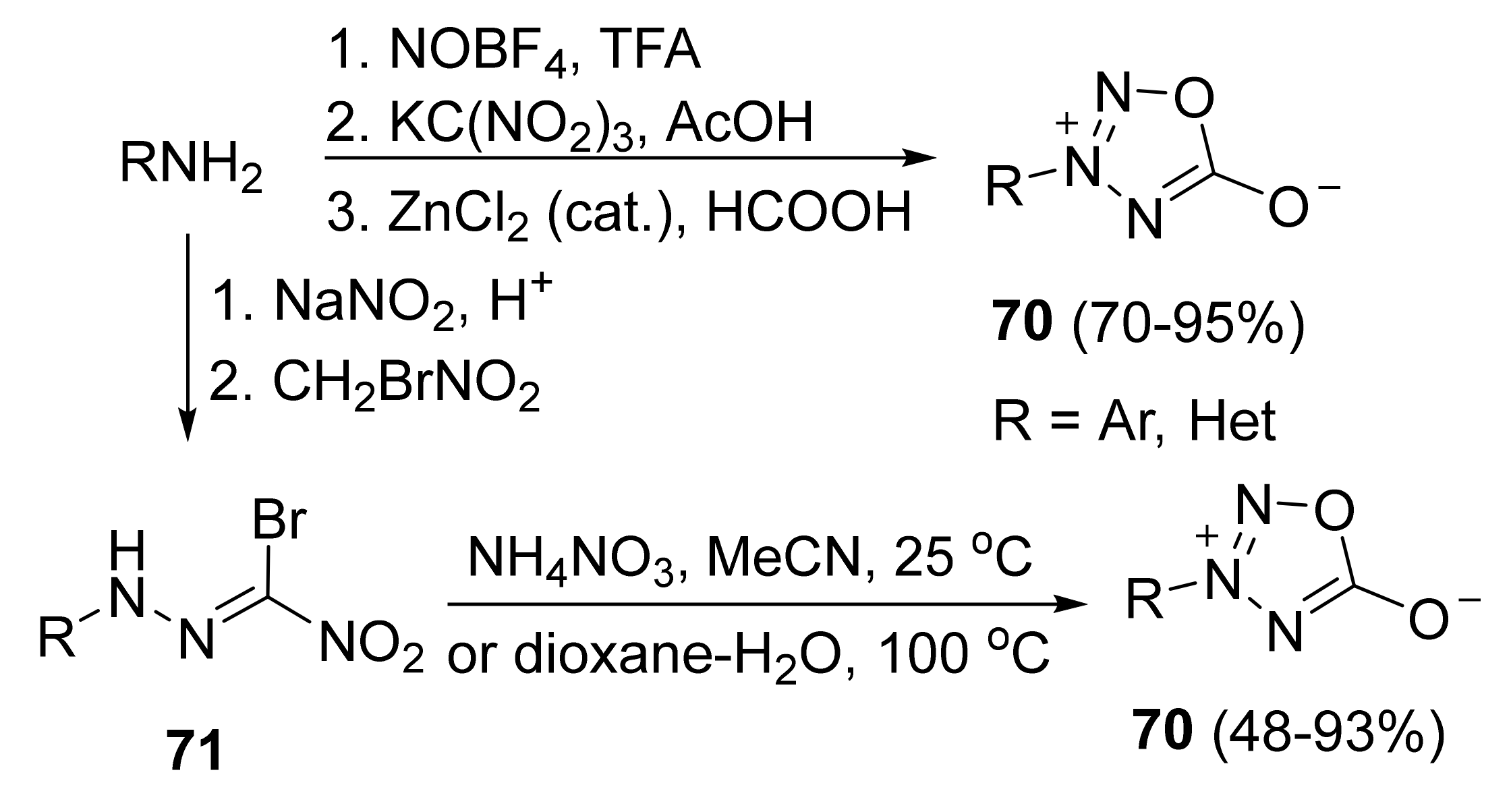
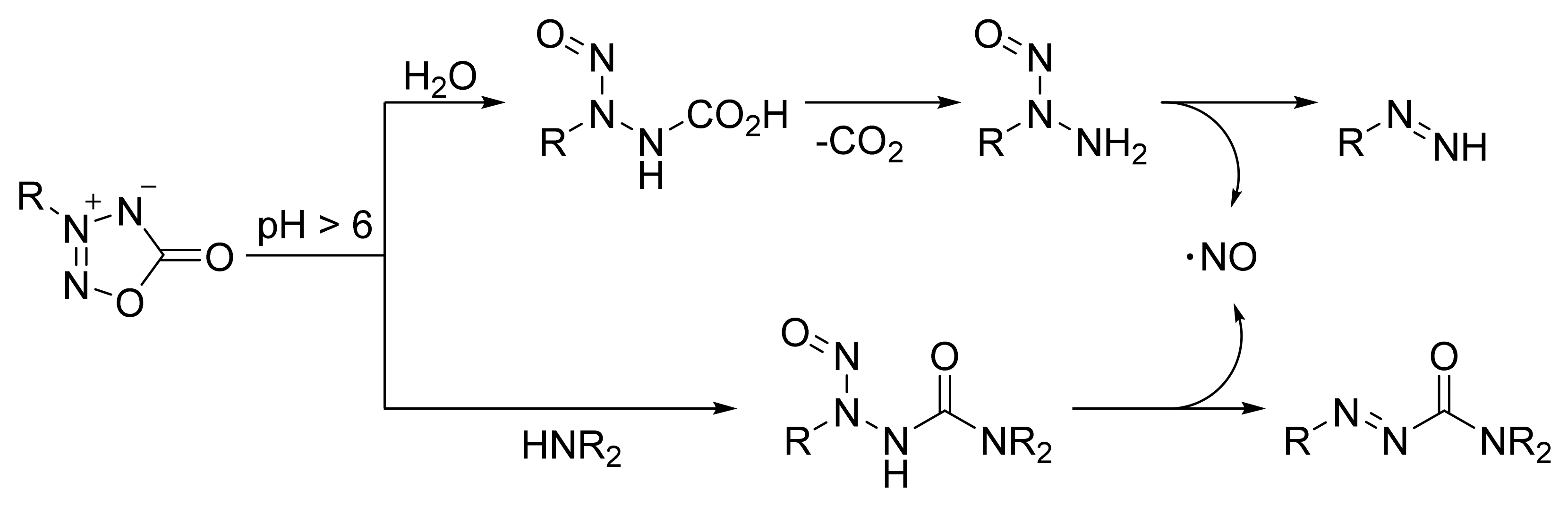
4. Pyridazine Dioxides and Azasydnones
5. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W.S. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Z.; Li, L.-L. Advanced nitric oxide donors: Chemical structure of NO drugs, NO nanomedicines and biomedical applications. Nanoscale 2021, 13, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.; Costa, D.E.F.R.; Bonaventura, D.; Lunardi, C.N.; Bendhack, L.M. Nitric Oxide Donors as Potential Drugs for the Treatment of Vascular Diseases Due to Endothelium Dysfunction. Curr. Pharm. Des. 2020, 26, 3748–3759. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Ritter, J.; Ferro, A. Platelet-Derived Nitric Oxide Signaling and Regulation. Circ. Res. 2007, 101, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Krol, M.; Kepinska, M. Human Nitric Oxide Synthase—Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Furchgott, R.F. Endothelium-Derived Relaxing Factor: Discovery, Early Studies, and Identifcation as Nitric Oxide (Nobel Lecture). Angew. Chem. Int. Ed. 1999, 38, 1870–1880. [Google Scholar] [CrossRef]
- Ignarro, L.J. Nitric Oxide: A Unique Endogenous Signaling Molecule in Vascular Biology (Nobel Lecture). Angew. Chem. Int. Ed. 1999, 38, 1882–1892. [Google Scholar] [CrossRef]
- Murad, F. Discovery of Some of the Biological Effects of Nitric Oxide and Its Role in Cell Signaling (Nobel Lecture). Angew. Chem. Int. Ed. 1999, 38, 1856–1868. [Google Scholar] [CrossRef]
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The Role of Nitric Oxide in Cancer: Master Regulator or NOt? Int. J. Mol. Sci. 2020, 21, 9393. [Google Scholar] [CrossRef]
- Heinrich, T.A.; da Silva, R.S.; Miranda, K.M.; Switzer, C.H.; Wink, D.A.; Fukuto, J.M. Biological nitric oxide signalling: Chemistry and terminology. Br. J. Pharmacol. 2013, 169, 1417–1429. [Google Scholar] [CrossRef]
- Basudhar, D.; Ridnour, L.A.; Cheng, R.; Kesarwala, A.H.; Heinecke, J.; Wink, D.A. Biological signaling by small inorganic molecules. Coord. Chem. Rev. 2016, 306, 708–723. [Google Scholar] [CrossRef]
- Majumder, S.; Sinha, S.; Siamwala, J.H.; Muley, A.; Seerapu, H.R.; Kolluru, G.K.; Veeriah, V.; Nagarajan, S.; Sridhara, S.R.C.; Priya, M.K.; et al. A comparative study of NONOate based NO donors: Spermine NONOate is the best suited NO donor for angiogenesis. Nitric Oxide 2014, 36, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Nagarajan, S.; Saran, U.; Kumar, R.; Keshri, G.K.; Suryakumar, G.; Chatterjee, S. Nitric oxide restores peripheral blood mononuclear cell adhesion against hypoxia via NO-cGMP signaling. Cell Biochem. Funct. 2020, 38, 319–329. [Google Scholar] [CrossRef]
- Swaminathan, A.; Kasiviswanathan, D.; Balaguru, U.M.; Kolluru, G.K.; Suryakumar, G.; Chatterjee, S. Hypoxia perturbs endothelium by re-organizing cellular actin architecture: Nitric oxide offers limited protection. Tissue Cell 2018, 50, 114–124. [Google Scholar] [CrossRef]
- Siamwala, J.H.; Kumar, P.; Veeriah, V.; Muley, A.; Rajendran, S.; Konikkat, S.; Majumder, S.; Mani, K.P.; Chatterjee, S. Nitric Oxide Reverses the Position of the Heart during Embryonic Development. Int. J. Mol. Sci. 2019, 20, 1157. [Google Scholar] [CrossRef]
- Kumar, P.; Sundaresan, L.; Chatterjee, S. Nitrosative Stress and Cardiogenesis: Cardiac Remodelling Perturbs Embryonic Metabolome. In Modulation of Oxidative Stress in Heart Disease; Chakraborti, S., Dhalla, N.S., Dikshit, M., Ganguly, N.K., Eds.; Springer: Singapore, 2019; pp. 377–392. [Google Scholar]
- Giri, S.; Thakar, S.; Majumder, S.; Chatterjee, S. Regulation of Oxidative Stress by Nitric Oxide Defines Lung Development and Diseases. In Oxidative Stress in Lung Diseases; Chakraborti, S., Parinandi, N.L., Ghosh, R., Ganguly, N.K., Chakraborti, T., Eds.; Springer: Singapore, 2019; pp. 445–464. [Google Scholar]
- Serafim, R.A.M.; Pernichelle, F.G.; Ferreira, E.I. The latest advances in the discovery of nitric oxide hybrid drug compounds. Expert Opin. Drug. Discov. 2017, 12, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Natural Product Chemistry for Nitric Oxide Based Therapeutics. Isr. J. Chem. 2019, 59, 414–419. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef] [PubMed]
- Gasco, A.; Schoenafinger, K. Nitric Oxide Donors: For Pharmaceutical and Biological Applications; Wang, P.G., Cai, T.B., Taniguchi, N., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 131–175. [Google Scholar]
- Bath, P.M.W.; Krishnan, K.; Appleton, J.P. Nitric oxide donors (nitrates), L-arginine, or nitric oxide synthase inhibitors for acute stroke (Review). Cochrane Database Syst. Rev. 2017, 4, CD000398. [Google Scholar]
- Steven, S.; Oelze, M.; Hausding, M.; Roohani, S.; Kashani, F.; Kröller-Schön, S.; Helmstädter, J.; Jansen, T.; Baum, C.; Iglarz, M.; et al. Oxidative Stress and Cardiovascular Dysfunction: From Basic Science to Applied Investigations. Oxid. Med. Cell. Longevity 2018, 2018, 7845629. [Google Scholar]
- Kuchurov, I.V.; Arabadzhi, S.S.; Zharkov, M.N.; Fershtat, L.L.; Zlotin, S.G. Sustainable Synthesis of Polynitroesters in the Freon Medium and their in Vitro Evaluation as Potential Nitric Oxide Donors. ACS Sustain. Chem. Eng. 2018, 6, 2535–2540. [Google Scholar] [CrossRef]
- Schönafinger, K. Heterocyclic NO prodrugs. Il Pharmaco 1999, 54, 316–320. [Google Scholar] [CrossRef]
- Feelisch, M.; Ostrowski, J.; Noack, E. On the mechanism of NO release from sydnonimines. J. Cardiovasc. Pharmacol. 1989, 14, 13–22. [Google Scholar] [CrossRef]
- Ullrich, T.; Oberle, S.; Abate, A.; Schröder, H. Photoactivation of the nitric oxide donor SIN-1. FEBS Lett. 1997, 406, 66–68. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Moiseev, S.K. Recent developments in the chemistry of sydnones and sydnone imines. Adv. Heterocycl. Chem. 2020, 131, 49–164. [Google Scholar]
- Beal, E.N.; Tumbull, K. An efficient, one-pot synthesis of 3-alkyl or aryl sydnoneimines. Synth. Commun. 1992, 22, 673–676. [Google Scholar] [CrossRef]
- Gotz, M.; Grozinger, K. 3-Hydroxysydnone imines. Tetrahedron 1971, 27, 4449–4456. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Samarskaya, A.S.; Godovikov, I.A.; Lyssenko, K.A.; Pankratova, A.A.; Kalinin, V.N. N6-tert-Butoxycarbonyl derivatives of sydnone imines: Preparation and synthetic use. Tetrahedron Lett. 2018, 59, 727–729. [Google Scholar] [CrossRef]
- Acharya, S.; Rogers, P.; Krishnamoorthy, R.R.; Stankowska, D.L.; Dias, H.V.R.; Yorio, T. Design and synthesis of novel hybrid sydnonimine and prodrug useful for glaucomatous optic neuropathy. Bioorg. Med. Chem. Lett. 2016, 26, 1490–1494. [Google Scholar] [CrossRef]
- Cai, T.B.; Lu, D.; Tang, X.; Zhang, Y.; Landerholm, M.; Wang, P.G. New glycosidase activated nitric oxide donors: Glycose and 3-morphorlinosydnonimine conjugates. J. Org. Chem. 2005, 70, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Audisio, D.; Riomet, M.; Bregant, S.; Sallustrau, A.; Plougastel, A.; Decuypere, E.; Gabillet, S.; Kumar, R.A.; Elyian, J.; et al. Bioorthogonal Click and Release Reaction of Iminosydnones with Cycloalkynes. Angew. Chem., Int. Ed. 2017, 56, 15612–15616. [Google Scholar] [CrossRef]
- Samarskaya, A.S.; Cherepanov, I.A.; Godovikov, I.A.; Kalinin, V.A. N6-α-haloacyl sydnone imine derivatives. Dokl. Chem. 2015, 463, 199–203. [Google Scholar] [CrossRef]
- Shao, Z.; Liu, W.; Tao, H.; Liu, F.; Zeng, R.; Champagne, P.A.; Cao, Y.; Houk, K.N.; Liang, Y.; Cao, Y.; et al. Bioorthogonal release of sulfonamides and mutually orthogonal liberation of two drugs. Chem. Commun. 2018, 54, 14089–14092. [Google Scholar] [CrossRef] [PubMed]
- Riomet, M.; Decuypere, E.; Porte, K.; Bernard, S.; Plougastel, L.; Kolodych, S.; Audisio, D.; Taran, F. Design and Synthesis of Iminosydnones for Fast Click and Release Reactions with Cycloalkynes. Chem. Eur. J. 2018, 24, 8535–8541. [Google Scholar] [CrossRef]
- Riomet, M.; Porte, K.; Madegard, L.; Thuéry, P.; Audisio, D.; Taran, F. Access to N-Carbonyl Derivatives of Iminosydnones by Carbonylimidazolium Activation. Org. Lett. 2020, 22, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Samarskaya, A.S.; Cherepanov, I.A.; Godovikov, I.A.; Dmitrienko, A.O.; Moiseev, S.K.; Kalinin, V.N.; Hey-Hawkins, E. Synthesis of N6-phosphorylated sydnone imines and their functionalization via 4-Li derivatives. Novel bicyclic sydnone imines. Tetrahedron 2018, 74, 2693–2702. [Google Scholar] [CrossRef]
- Beal, E.N.; Turnbull, K. Bromination/Debromination of 6-Benzoyl-3-alkyl or 3-Aryl Sydnoneimines. Synth. Commun. 1992, 22, 1515–1522. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Kusaeva, L.H.; Godovikov, I.A.; Kalinin, V.N. 4-Formylsydnonimine derivatives. Russ. Chem. Bull. Int. Ed. 2009, 58, 2474–2477. [Google Scholar] [CrossRef]
- Freese, T.; Lücke, A.-L.; Schmidt, C.A.S.; Polamo, M.; Nieger, M.; Namyslo, J.C.; Schmidt, A. Anionic N-heterocyclic carbenes derived from sydnone imines such as molsidomine. Trapping reactions with selenium, palladium, and gold. Tetrahedron 2017, 73, 5350–5357. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Kalinin, V.N. Synthesis and reactivity of 4-lithium and 4-copper derivatives of sydnone imines. Mendeleev Commun. 2000, 5, 181–182. [Google Scholar] [CrossRef]
- Kalinin, V.N.; Lebedev, S.N.; Cherepanov, I.A.; Godovikov, I.A.; Lyssenko, K.A.; Hey-Hawkins, E. 4-Diphenylphosphinosydnone imines as bidentate ligands. Polyhedron 2009, 28, 2411–2417. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Lebedev, S.N.; Samarskaya, A.S.; Godovikov, I.A.; Nelyubina, Y.V.; Kalinin, V.N. 4-Thio derivatives of sydnone imines. Mendeleev Commun. 2009, 19, 322–323. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Shevaldina, E.V.; Lapshin, D.A.; Spiridonov, Y.Y.; Abubikerov, V.C.; Moiseev, S.K. 4-Lithiosydnone imines: Generation and stability. Plant growth regulating activity of 4-hydroxymethyl derivatives of sydnone imines. J. Organomet. Chem. 2021, 943, 121841. [Google Scholar] [CrossRef]
- Freese, T.; Lücke, A.-L.; Namyslo, J.C.; Nieger, M.; Schmidt, A. Heterocycle Syntheses with Anionic N-Heterocyclic Carbenes: Ring Transformations of Sydnone Imine Anions. Eur. J. Org. Chem. 2018, 2018, 1646–1654. [Google Scholar] [CrossRef]
- Freese, T.; Nieger, M.; Namyslo, J.C.; Schmidt, A. Cycloadditions of anionic N-heterocyclic carbenes of sydnone imines. Tetrahedron Lett. 2019, 60, 1272–1276. [Google Scholar] [CrossRef]
- Freese, T.; Namyslo, J.C.; Nieger, M.; Schmidt, A. Sulfur, mercury, and boron adducts of sydnone imine derived anionic N-heterocyclic carbenes. RSC Adv. 2019, 9, 4781–4788. [Google Scholar] [CrossRef]
- Riomet, M.; Porte, K.; Wijkhuisen, A.; Audisio, D.; Taran, F. Fluorogenic iminosydnones: Bioorthogonal tools for double turn-on click-and-release reactions. Chem. Commun. 2020, 56, 7183–7186. [Google Scholar] [CrossRef]
- Porte, K.; Renoux, B.; Péraudeau, E.; Clarhaut, J.; Eddhif, B.; Poinot, P.; Gravel, E.; Doris, E.; Wijkhuisen, A.; Audisio, D.; et al. Controlled Release of Micelle Payload via Sequential Enzymatic and Bioorthogonal Reactions in Living Systems. Angew. Chem., Int. Ed. 2019, 58, 6366–6370. [Google Scholar] [CrossRef]
- Porte, K.; Riomet, M.; Figliola, C.; Audisio, D.; Taran, F. Click and Bio-Orthogonal Reactions with Mesoionic Compounds. Chem. Rev. 2021, 121, 6718–6743. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, E.; Bernard, S.; Feng, M.; Porte, K.; Riomet, M.; Thuery, P.; Audisio, D.; Taran, F. Copper-Catalyzed Aza-Iminosydnone-Alkyne Cycloaddition Reaction Discovered by Screening. ACS Catal. 2018, 8, 11882–11888. [Google Scholar] [CrossRef]
- Khmel’;nitskaya, E.Y.; Levina, V.I.; Trukhacheva, L.A.; Grigoriev, N.B.; Kalinin, V.N.; Cherepanov, I.A.; Lebedev, S.N.; Granik, V.G. Sydnonimines as exogenous NO donors. Russ. Chem. Bull. Int. Ed. 2004, 53, 2840–2844. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hirata, M.; Nagaoka, A. Hypotensive action of N-ethoxycarbonyl-3-morpholinosydnonimine, SIN-10. Jap. J. Pharmacol. 1970, 20, 102–115. [Google Scholar] [CrossRef]
- Drummer, C.; Valta-Seufzer, U.; Karrenbrock, B.; Heim, J.M.; Gerzer, R. Comparison of anti-platelet properties of molsidomine, isosorbide-5-mononitrate and placebo in healthy volunteers. Eur. Heart J. 1991, 12, 541–549. [Google Scholar] [CrossRef]
- Nortcliffe, A.; Botting, N.P.; O’Hagan, D. Novel amino acids: Synthesis of furoxan and sydnonimine containing amino acids and peptides as potential nitric oxide releasing motifs. Org. Biomol. Chem. 2013, 11, 4657–5671. [Google Scholar] [CrossRef]
- Nortcliffe, A.; Fleming, I.N.; Botting, N.P.; O’Hagan, D. Synthesis and anticancer properties of RGD peptides conjugated to nitric oxide releasing functional groups and abiraterone. Tetrahedron 2014, 70, 8343–8347. [Google Scholar] [CrossRef]
- Nortcliffe, A.; Ekstrom, A.G.; Black, J.R.; Ross, J.A.; Habib, F.K.; Botting, N.P.; O’Hagan, D. Synthesis and biological evaluation of nitric oxide-donating analogues of sulindac for prostate cancer treatment. Bioorg. Med. Chem. 2014, 22, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Savtchenko, N.; Mashkovsky, M.; Beekman, M.; Munzar, P.; Gasior, M.; Goldberg, S.R.; Ungard, J.T.; Kim, J.; Shippenberg, T.; et al. Behavioral, Toxic, and Neurochemical Effects of Sydnocarb, a Novel Psychomotor Stimulant: Comparisons with Methamphetamine. J. Pharmacol. Exp. Ther. 1999, 288, 1298–1310. [Google Scholar]
- Marvasi, M.; Chen, C.; Carrazana, M.; Durie, I.A.; Teplitski, M. Systematic analysis of the ability of Nitric Oxide donors to dislodge biofilms formed by Salmonella enterica and Escherichia coli O157:H7. AMB Express 2014, 4, 42. [Google Scholar] [CrossRef]
- Soulère, L.; Hoffmanna, P.; Bringaud, F. Synthesis of sydnonimine derivatives as potential trypanocidal agents. J. Heterocycl. Chem. 2003, 40, 943–947. [Google Scholar] [CrossRef]
- Du, S.; Hu, X.; Li, M.; Jiang, X.; Xu, X.; Cheng, J.; Qian, X. Discovery of novel iminosydnone compounds with insecticidal activities based on the binding mode of triflumezopyrim. Bioorg. Med. Chem. Lett. 2021, 46, 128120. [Google Scholar] [CrossRef]
- Pruschinski, L.; Lücke, A.-L.; Freese, T.; Kahnert, S.-R.; Mummel, S.; Schmidt, A. Suzuki–Miyaura Cross-Couplings under Acidic Conditions. Synthesis 2020, 52, 882–892. [Google Scholar] [CrossRef]
- Lücke, A.-L.; Pruschinski, L.; Freese, T.; Schmidt, A. Sonogashira-Hagihara and Buchwald-Hartwig cross-coupling reactions with sydnone and sydnone imine derived catalysts. Arkivoc 2020, vii, 94–104. [Google Scholar] [CrossRef]
- Gettings, M.L.; Byrd, E.F.C.; Zeller, M.; Piercey, D. Methyl sydnone imine and its energetic salts. New J. Chem. 2021, 45, 2228–2236. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Advances in the synthesis of non-annelated polynuclear heterocyclic systems comprising the 1,2,5-oxadiazole ring. Russ. Chem. Rev. 2016, 85, 1097–1145. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Molecular Hybridization Tools in the Development of Furoxan-Based NO-Donor Prodrugs. ChemMedChem 2017, 12, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Makhova, N.N.; Fershtat, L.L. Recent advances in the synthesis and functionalization of 1,2,5-oxadiazole 2-oxides. Tetrahedron Lett. 2018, 59, 2317–2326. [Google Scholar] [CrossRef]
- Makhova, N.N.; Belen’kii, L.I.; Gazieva, G.A.; Dalinger, I.L.; Konstantinova, L.S.; Kuznetsov, V.V.; Kravchenko, A.N.; Krayushkin, M.M.; Rakitin, O.A.; Starosotnikov, A.M.; et al. Progress in the chemistry of nitrogen-, oxygen- and sulfur-containing heterocyclic systems. Russ. Chem. Rev. 2020, 89, 55–124. [Google Scholar] [CrossRef]
- Ferioli, R.; Folco, G.C.; Ferretti, C.; Gasco, A.M.; Medana, C.; Fruttero, R.; Civelli, M.; Gasco, A. A new class of furoxan derivatives as NO donors: Mechanism of action and biological activity. Br. J. Pharmacol. 1995, 114, 816–820. [Google Scholar] [CrossRef]
- Gasco, A.; Fruttero, R.; Sorba, G.; Di Stilo, A.; Calvino, R. NO donors: Focus on furoxan derivatives. Pure Appl. Chem. 2004, 76, 973–981. [Google Scholar] [CrossRef]
- Bohn, H.; Brendel, J.; Martorana, P.A.; Schönafinger, K. Cardiovascular actions of the furoxan CAS 1609, a novel nitric oxide donor. Br. J. Pharmacol. 1995, 114, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant activity of water-soluble furoxans. Mendeleev Commun. 2018, 28, 49–51. [Google Scholar] [CrossRef]
- Civelli, M.; Giossi, M.; Caruso, P.; Razzetti, R.; Bergamaschi, M.; Bongrani, S.; Gasco, A. The involvement of the release of nitric oxide in the pharmacological activity of the new furoxan derivative CHF 2363. Br. J. Pharmacol. 1996, 118, 923–928. [Google Scholar] [CrossRef]
- Balbo, S.; Lazzarato, L.; di Stilo, A.; Fruttero, R.; Lombaert, N.; Kirsch-Volders, M. Studies of the potential genotoxic effects of furoxans: The case of CAS 1609 and of the water-soluble analogue of CHF 2363. Toxic. Lett. 2008, 178, 44–51. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Bystrov, D.M.; Zhilin, E.S.; Makhova, N.N. N-Oxide-Controlled Chemoselective Reduction of Nitrofuroxans. Synthesis 2019, 51, 747–756. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Fershtat, L.L.; Bystrov, D.M.; Kulikov, A.S.; Dmitrienko, A.O.; Ananyev, I.V.; Makhova, N.N. Renaissance of 1,2,5-Oxadiazolyl Diazonium Salts: Synthesis and Reactivity. Eur. J. Org. Chem. 2019, 2019, 4248–4259. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Polkovnichenko, M.S.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Novel Arylazo-1,2,5-oxadiazole Photoswitches: Synthesis, Photoisomerization and Nitric Oxide Releasing Properties. ChemPhotoChem 2020, 4, 5346–5354. [Google Scholar] [CrossRef]
- Bystrov, D.M.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Direct Synthesis of N-(1,2,5-Oxadiazolyl)hydrazones through a Diazotization/Reduction/Condensation Cascade. J. Org. Chem. 2020, 85, 15466–15475. [Google Scholar] [CrossRef]
- Dos Santos Fernandes, G.F.; Pavan, A.R.; dos Santos, J.L. Heterocyclic N-oxides—A Promising Class of Agents against Tuberculosis, Malaria and Neglected Tropical Diseases. Curr. Pharm. Des. 2018, 24, 1325–1340. [Google Scholar] [CrossRef]
- Serafim, R.A.M.; Gonçalves, J.E.; de Souza, F.P.; de Melo Loureiro, A.P.; Storpirtis, S.; Krogh, R.; Andricopulo, A.D.; Dias, L.C.; Ferreira, E.I. Design, synthesis and biological evaluation of hybrid bioisoster derivatives of N-acylhydrazone and furoxan groups with potential and selective anti-Trypanosoma cruzi activity. Eur. J. Med. Chem. 2014, 82, 418–425. [Google Scholar] [CrossRef]
- Hernández, P.; Rojas, R.; Gilman, R.H.; Sauvain, M.; Lima, L.M.; Barreiro, E.J.; González, M.; Cerecetto, H. Hybrid furoxanyl N-acylhydrazone derivatives as hits for the development of neglected diseases drug candidates. Eur. J. Med. Chem. 2013, 59, 64–74. [Google Scholar] [CrossRef]
- Guglielmo, S.; Cortese, D.; Vottero, F.; Rolando, B.; Kommer, V.P.; Williams, D.L.; Fruttero, R.; Gasco, A. New praziquantel derivatives containing NO-donor furoxans and related furazans as active agents against Schistosoma mansoni. Eur. J. Med. Chem. 2014, 84, 135–145. [Google Scholar] [CrossRef]
- Epishina, M.A.; Kulikov, A.S.; Fershtat, L.L.; Ananyev, I.V.; Makhova, N.N. Synthesis of new pharmacologically oriented heterocyclic ensembles, [2-(1H-pyrazol-1-yl)thiazol-4-yl]furoxans. Mendeleev Commun. 2019, 29, 288–291. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Epishina, M.A.; Fershtat, L.L.; Makhova, N.N. Effective synthesis of 7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Chem. Heterocycl. Compd. 2018, 54, 669–672. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Epishina, M.A.; Fershtat, L.L.; Romanova, A.A.; Makhova, N.N. Effective synthesis of 6-substituted 7H-tetrazolo[5,1-b][1,3,4]thiadiazines via a one-pot condensation/nitrosation/azide-tetrazole tautomerism reaction sequence. Tetrahedron Lett. 2017, 58, 3998–4002. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Epishina, M.A.; Churakov, A.I.; Anikina, L.V.; Fershtat, L.L.; Makhova, N.N. Regioselective synthesis, structural diversification and cytotoxic activity of (thiazol-4-yl)furoxans. Mendeleev Commun. 2018, 28, 623–625. [Google Scholar] [CrossRef]
- Matsubara, R.; Kim, H.; Sakaguchi, T.; Xie, W.; Zhao, X.; Nagoshi, Y.; Wang, C.; Tateiwa, M.; Ando, A.; Hayashi, M.; et al. Modular Synthesis of Carbon-Substituted Furoxans via Radical Addition Pathway. Useful Tool for Transformation of Aliphatic Carboxylic Acids Based on “Build-and-Scrap” Strategy. Org. Lett. 2020, 22, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, R.; Ando, A.; Hasebe, H.; Kim, H.; Tsuneda, T.; Hayashi, M. Synthesis and Synthetic Application of Chloro- and Bromofuroxans. J. Org. Chem. 2020, 85, 5959–5972. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Hayashi, M.; Matsubara, R. Borylfuroxans: Synthesis and Applications. Org. Lett. 2021, 23, 4317–4321. [Google Scholar] [CrossRef]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Sorokina, I.V.; Zhukova, N.A.; Frolova, T.S.; Tolstikova, T.G.; Shults, E.E.; Turks, M. Lupane-type conjugates with aminoacids, 1,3,4- oxadiazole and 1,2,5-oxadiazole-2-oxide derivatives: Synthesis, anti-inflammatory activity and in silico evaluation of target affinity. Steroids 2019, 150, 108443. [Google Scholar] [CrossRef]
- Prabhuling, S.; Tamboli, Y.; Choudhari, P.B.; Bhatia, M.S.; Mohanta, T.K.; Al-Harrasi, A.; Pudukulathan, Z.K. Synthesis and Modeling Studies of Furoxan Coupled Spiro-Isoquinolino Piperidine Derivatives as NO Releasing PDE 5 Inhibitors. Biomedicines 2020, 8, 121. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Fan, J.; Cao, W.; Zeng, G.; Wang, Y.; Li, Y.; Zhou, Y.; Deng, X. Dual-targeting Rutaecarpine-NO donor hybrids as novel antihypertensive agents by promoting release of CGRP. Eur. J. Med. Chem. 2019, 168, 146–153. [Google Scholar] [CrossRef]
- Dos Santos Fernandes, G.F.; de Souza, P.C.; Marino, L.B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chung, M.C.; Pavan, F.R.; dos Santos, J.L. Synthesis and biological activity of furoxan derivatives against Mycobacterium tuberculosis. Eur. J. Med. Chem. 2016, 123, 523–531. [Google Scholar] [CrossRef]
- De Souza, P.C.; Fernandes, G.F.S.; Marino, L.B.; Ribeiro, C.M.; da Silva, P.B.; Chorilli, M.; Silva, C.S.P.; Resende, F.A.; Solcia, M.C.; de Grandis, R.A.; et al. Furoxan derivatives demonstrated in vivo efficacy by reducing Mycobacterium tuberculosis to undetectable levels in a mouse model of infection. Biomed. Pharmacother. 2020, 130, 110592. [Google Scholar] [CrossRef]
- Niu, X.; Cao, J.; Zhang, Y.; Gao, X.; Cheng, M.; Liu, Y.; Wang, W.; Yuan, Z. A glutathione responsive nitric oxide release system based on charge-reversal chitosan nanoparticles for enhancing synergistic effect against multidrug resistance tumor. Nanomed. Nanotechnol. 2019, 20, 102015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Meng, T.; Qin, Y.; Li, T.; Fu, J.; Yin, J. Nitric oxide-donating and reactive oxygen species-responsive prochelators based on 8-hydroxyquinoline as anticancer agents. Eur. J. Med. Chem. 2021, 212, 113153. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Deng, Y.; Huang, Y.; Yu, Z.; Wang, C.; Wang, K.; Dong, J.; Chen, Y. Synthesis and Antitumor Evaluation of Novel Hybrids of Phenylsulfonylfuroxan and Estradiol Derivatives. ChemistryOpen 2020, 9, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sodano, F.; Gazzano, E.; Rolando, B.; Marini, E.; Lazzarato, L.; Fruttero, R.; Riganti, C.; Gasco, A. Tuning NO release of organelle-targeted furoxan derivatives and their cytotoxicity against lung cancer cells. Bioorg. Chem. 2021, 111, 104911. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lai, F.; Fu, J.; Li, C.; Ma, J.; Chen, C.; Liu, K.; Zhang, T.; Chen, X.; Zhang, D. Novel nitric oxide-releasing derivatives of triptolide as antitumor and anti-inflammatory agents: Design, synthesis, biological evaluation, and nitric oxide release studies. Eur. J. Med. Chem. 2020, 190, 112079. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, Y.; Cheng, J.; Shen, J.; Huang, H.; Liu, M.; Chen, Z.; Liu, Y. Nitric oxide-releasing platinum(IV) prodrug efficiently inhibits proliferation and metastasis of cancer cells. Chem. Commun. 2020, 56, 14051. [Google Scholar] [CrossRef]
- Zhao, J.; Gou, S.; Sun, Y.; Fang, L.; Wang, Z. Antitumor Platinum(II) Complexes Containing Platinum-Based Moieties of Present Platinum Drugs and Furoxan Groups as Nitric Oxide Donors: Synthesis, DNA Interaction, and Cytotoxicity. Inorg. Chem. 2012, 51, 10317–10324. [Google Scholar] [CrossRef] [PubMed]
- Larin, A.A.; Fershtat, L.L.; Ustyuzhanina, N.E.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New hybrid furoxan structures with antiaggregant activity. Mendeleev Commun. 2018, 28, 595–597. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Campos, D.L.; da Silva, I.C.; Prates, J.L.B.; Pavan, A.R.; Pavan, F.R.; dos Santos, J.L. Benzofuroxan Derivatives as Potent Agents against Multidrug-Resistant Mycobacterium tuberculosis. ChemMedChem 2021, 16, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Fedik, N.S.; Kletskii, M.E.; Burov, O.N.; Lisovin, A.V.; Kurbatov, S.V.; Chistyakov, V.A.; Morozov, P.G. Comprehensive study of nitrofuroxanoquinolines. New perspective donors of NO molecules. Nitric Oxide 2019, 93, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Frenna, V.; Consiglio, G.; Micheletti, G.; Boga, C.; Akylbekov, N.; Burilov, A.; Spinelli, D. On the Nucleophilic Reactivity of 4,6-Dichloro-5-nitrobenzofuroxan with Some Aliphatic and Aromatic Amines: Selective Nucleophilic Substitution. J. Org. Chem. 2020, 85, 13472–13480. [Google Scholar] [CrossRef]
- Chugunova, E.; Micheletti, G.; Telese, D.; Boga, C.; Islamov, D.; Usachev, K.; Burilov, A.; Tulesinova, A.; Voloshina, A.; Lyubina, A.; et al. Novel Hybrid Compounds Containing Benzofuroxan and Aminothiazole Scaffolds: Synthesis and Evaluation of Their Anticancer Activity. Int. J. Mol. Sci. 2021, 22, 7497. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New insight into the antiaggregant activity of furoxans. Mendeleev Commun. 2016, 26, 513–515. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Bolognese, F.; Rolando, B.; Guglielmo, S.; Lazzarato, L.; Fruttero, R. Anti-Pseudomonas activity of 3-nitro-4-phenylfuroxan. Microbiology 2018, 164, 1557–1566. [Google Scholar] [CrossRef]
- Fei, Y.; Wu, J.; An, H.-W.; Zhu, K.; Peng, B.; Cai, J.; Zhang, Y.; Li, L.-L.; Wang, H.; Huang, Z. Identification of New Nitric Oxide-Donating Peptides with Dual Biofilm Eradication and Antibacterial Activities for Intervention of Device-Related Infections. J. Med. Chem. 2020, 63, 9127–9135. [Google Scholar] [CrossRef]
- Huang, L.Y.; Tsui, D.Y.; Williams, C.M.; Wyse, B.D.; Smith, M.T. The furoxan nitric oxide donor, PRG150, evokes dose-dependent analgesia in a rat model of painful diabetic neuropathy. Clin. Exp. Pharmacol. Physiol. 2015, 42, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Pippin, A.B.; Arshad, Z.H.M.; Voll, R.J.; Nye, J.A.; Ghassabian, S.; Williams, C.M.; Mancini, A.; Liotta, D.C.; Smith, M.T.; Goodman, M.M. In Vitro Metabolic Stability and in Vivo Biodistribution of 3-Methyl-4-furoxancarbaldehyde Using PET Imaging in Rats. ACS Med. Chem. Lett. 2016, 7, 563–567. [Google Scholar]
- Huang, L.; Wyse, B.D.; Williams, C.M.; Smith, M.T. Nitric oxide modulates μ-opioid receptor function in vitro. Clin. Exp. Pharmacol. Physiol. 2019, 46, 676–685. [Google Scholar] [CrossRef]
- Eaton, J.K.; Ruberto, R.A.; Kramm, A.; Viswanathan, V.S.; Schreiber, S.L. Diacylfuroxans Are Masked Nitrile Oxides That Inhibit GPX4 Covalently. J. Am. Chem. Soc. 2019, 141, 20407–20415. [Google Scholar] [CrossRef] [PubMed]
- Larin, A.A.; Bystrov, D.M.; Fershtat, L.L.; Konnov, A.A.; Makhova, N.N.; Monogarov, K.A.; Meerov, D.B.; Melnikov, I.N.; Pivkina, A.N.; Kiselev, V.G.; et al. Nitro-, Cyano-, and Methylfuroxans, and Their Bis-Derivatives: From Green Primary to Melt-Cast Explosives. Molecules 2020, 25, 5836. [Google Scholar] [CrossRef]
- Larin, A.A.; Shaferov, A.V.; Epishina, M.A.; Melnikov, I.N.; Muravyev, N.V.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Pushing the Energy-Sensitivity Balance with High-Performance Bifuroxans. ACS Appl. Energy Mater. 2020, 3, 7764–7771. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. 1,2,5-Oxadiazole-Based High-Energy-Density Materials: Synthesis and Performance. ChemPlusChem 2020, 85, 13–42. [Google Scholar] [CrossRef]
- Larin, A.A.; Muravyev, N.V.; Pivkina, A.N.; Suponitsky, K.Y.; Ananyev, I.V.; Khakimov, D.V.; Fershtat, L.L.; Makhova, N.N. Assembly of Tetrazolylfuroxan Organic Salts: Multipurpose Green Energetic Materials with High Enthalpies of Formation and Excellent Detonation Performance. Chem. Eur. J. 2019, 25, 4225–4233. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Ovchinnikov, I.V.; Epishina, M.A.; Romanova, A.A.; Lempert, D.B.; Muravyev, N.V.; Makhova, N.N. Assembly of Nitrofurazan and Nitrofuroxan Frameworks for High-Performance Energetic Materials. ChemPlusChem 2017, 82, 1315–1319. [Google Scholar] [CrossRef]
- Nakadate, M.; Sueyoshi, S.; Suzuki, I. Studies on Pyridazine 1,2-Dioxides. I. Syntheses of Pyridazine 1,2-Dioxides. Chem. Pharm. Bull. 1970, 18, 1211–1218. [Google Scholar] [CrossRef][Green Version]
- Rozen, S.; Shaffer, A. Synthesis of N,N-Dioxopyridazines. Org. Lett. 2017, 19, 4707–4709. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.S.; Epishina, M.A.; Zhilin, E.S.; Shuvaev, A.D.; Fershtat, L.L.; Makhova, N.N. Design and synthesis of pyrazolo[3,4-d]pyridazine 5,6-dioxides as novel NO-donors. Mendeleev Commun. 2021, 31, 42–45. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Averina, E.B.; Kuznetsova, T.S.; Zefirov, N.S. Synthesis of new 3,4-disubstituted furazans. Chem. Heterocycl. Compd. 2000, 36, 1091–1096. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Fakhrutdinov, A.N.; Zlotin, S.G.; Rakitin, O.A. [1,2,5]Oxadiazolo[3,4-d]pyridazine 1,5,6-trioxides: Efficient synthesis via the reaction of 3,4-bis(hydroxyimino)methyl)-1,2,5-oxadiazole 2-oxides with a mixture of concentrated nitric and trifluoroacetic acids and structural characterization. Tetrahedron Lett. 2018, 59, 3143–3146. [Google Scholar] [CrossRef]
- Obruchnikova, N.V.; Novikov, R.A.; Zlotin, S.G.; Dorovatovskii, P.V.; Khrustalev, V.N.; Rakitin, O.A. Synthesis and structural investigation of 4,4’;-dimethyl-[3,3’;-bi(1,2,5-oxadiazole)] 5,5’;-dioxide. Russ. Chem. Bull. Int. Ed. 2018, 67, 2044–2048. [Google Scholar] [CrossRef]
- Spyroudis, S.; Varvoglis, A. A New Synthesis of Pyridazine 1,2-Dioxides. Synthesis 1976, 1976, 837–838. [Google Scholar] [CrossRef]
- Ohsawa, A.; Arai, H.; Igeta, H. Oxidative Cyclization of 2-Unsaturated 1,4-Dioximes. Heterocycles 1978, 9, 1367–1373. [Google Scholar]
- Ohsawa, A.; Arai, H.; Igeta, H.; Akimoto, T.; Tsuji, A.; Iitaka, Y. Oxidative cyclization of dioximes and bis(hydrazones) of 2-unsaturated 1,4-diketones. J. Org. Chem. 1979, 44, 3524–3529. [Google Scholar] [CrossRef]
- Kots, A.Y.; Grafov, M.A.; Khropov, Y.V.; Betin, V.L.; Belushkina, N.N.; Busygina, O.G.; Yazykova, M.Y.; Ovchinnikov, I.V.; Kulikov, A.S.; Makhova, N.N.; et al. Vasorelaxant and antiplatelet activity of 4,7-dimethyl-1,2,5-oxadiazolo[3,4-d]pyridazine 1,5,6-trioxide: Role of soluble guanylate cyclase, nitric oxide and thiols. Br. J. Pharmacol. 2000, 129, 1163–1177. [Google Scholar] [CrossRef]
- Shevelev, S.A.; Dalinger, I.L.; Gulevskaya, V.I.; Cherkasova, T.I.; Vinogradov, V.M.; Ugrak, B.I.; Starosotnikov, A.M. Synthesis of mesoionic 3-aryl(hetaryl)-1,2,3,4-oxatriazol-5-ones based on N-aryl- and N-hetarylhydrazones of bromonitroformaldehyde. Chem. Heterocycl. Compd. 1999, 35, 363–373. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Bystrov, D.M.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Straightforward Access to the Nitric Oxide Donor Azasydnone Scaffold by Cascade Reactions of Amines. Chem. Eur. J. 2019, 25, 14284–14289. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.Q.; Kier, L.B.; Glennon, R.A.; Egle, J.L., Jr. Preliminary studies of mesoionic 3-(substituted-aryl)-.psi.-oxatriazoles as potential antihypertensive agents. J. Med. Chem. 1982, 25, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.L.; Fedorchuk, M.; Shetty, B.V.; Anderson, F.E. Synthesis and activity of some 3-substituted 1,2,3,4-pseudooxatriazol-5-ones and their precursors and related compounds. J. Med. Chem. 1970, 13, 196–203. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Ustyuzhanina, N.E.; Fershtat, L.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant effects of (1,2,5-oxadiazolyl)azasydnone ring assemblies as novel antiplatelet agents. Chem. Biol. Drug Des. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Gettings, M.; Piercey, D. Azasydnones and their use in Energetic Materials. Energ. Mater. Front. 2020, 1, 136–140. [Google Scholar] [CrossRef]
- Gettings, M.L.; Thoenen, M.T.; Byrd, E.F.C.; Sabatini, J.J.; Zeller, M.; Piercey, D.G. Tetrazole Azasydnone (C2N7O2H) And Its Salts: High-Performing Zwitterionic Energetic Materials Containing A Unique Explosophore. Chem. Eur. J. 2020, 26, 14530–14535. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Serushkina, O.V.; Muravyev, N.V.; Meerov, D.B.; Miroshnichenko, E.A.; Kon’kova, T.S.; Suponitsky, K.Y.; Vener, M.V.; Sheremetev, A.B. Azasydnone—Novel “green” building block for designing high energetic compounds. J. Mater. Chem. A 2018, 6, 18669–18676. [Google Scholar] [CrossRef]
- Serushkin, V.V.; Sinditskii, V.P.; Filatov, S.A.; Kulagina, P.D.; Nguyen, V.T.; Vatsadze, I.A.; Dalinger, I.L.; Sheremetev, A.B. Thermal stability and combustion behaviors of energetic materials based on a new heterocycle azasydnone. Int. J. Energ. Mater. Chem. Propul. 2018, 17, 147–170. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fershtat, L.L.; Zhilin, E.S. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules 2021, 26, 5705. https://doi.org/10.3390/molecules26185705
Fershtat LL, Zhilin ES. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules. 2021; 26(18):5705. https://doi.org/10.3390/molecules26185705
Chicago/Turabian StyleFershtat, Leonid L., and Egor S. Zhilin. 2021. "Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors" Molecules 26, no. 18: 5705. https://doi.org/10.3390/molecules26185705
APA StyleFershtat, L. L., & Zhilin, E. S. (2021). Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules, 26(18), 5705. https://doi.org/10.3390/molecules26185705




