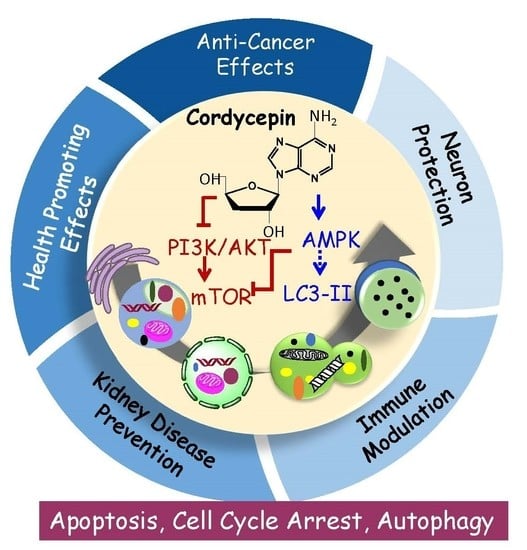
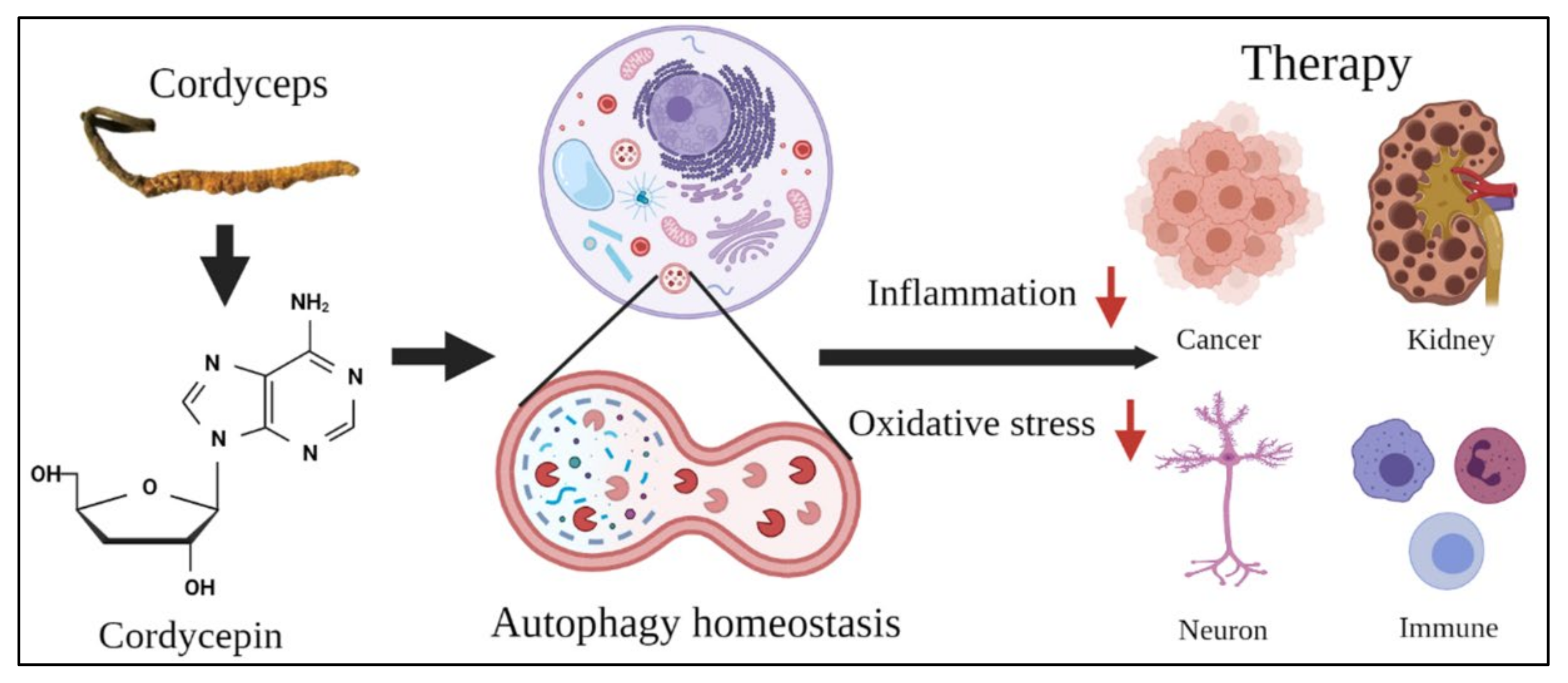
The Role of Autophagy in Anti-Cancer and Health Promoting Effects of Cordycepin
Abstract
1. Introduction
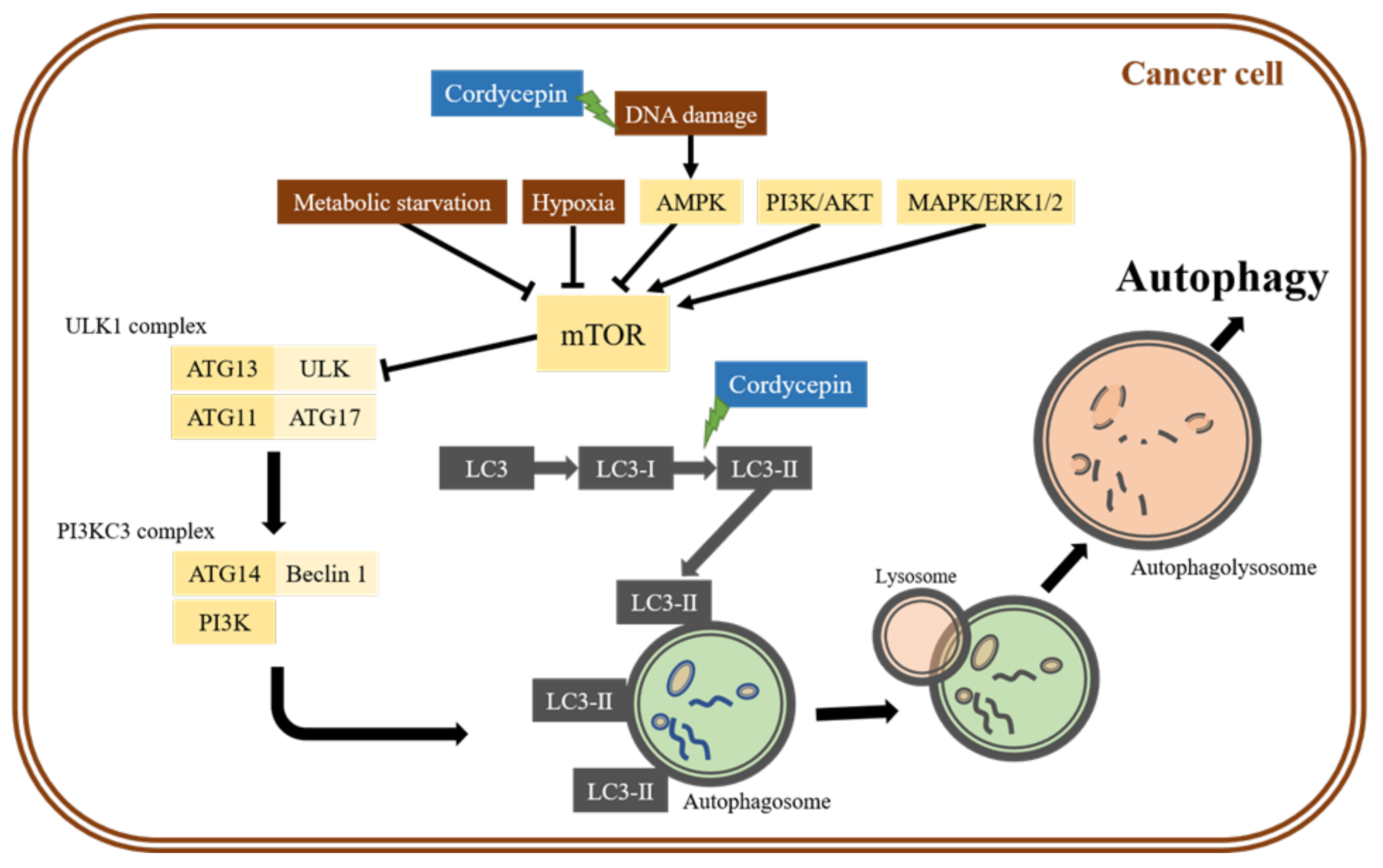
2. The Role of Autophagy in the Anti-Cancer Effects of Cordycepin
2.1. Cordycepin-Induced Cancer Cell Death through Autophagy Induction
2.2. The Effects of Combination Therapy of Cordycepin and Anti-Cancer Therapy
3. The Role of Autophagy in the Health-Promoting Effects of Cordycepin
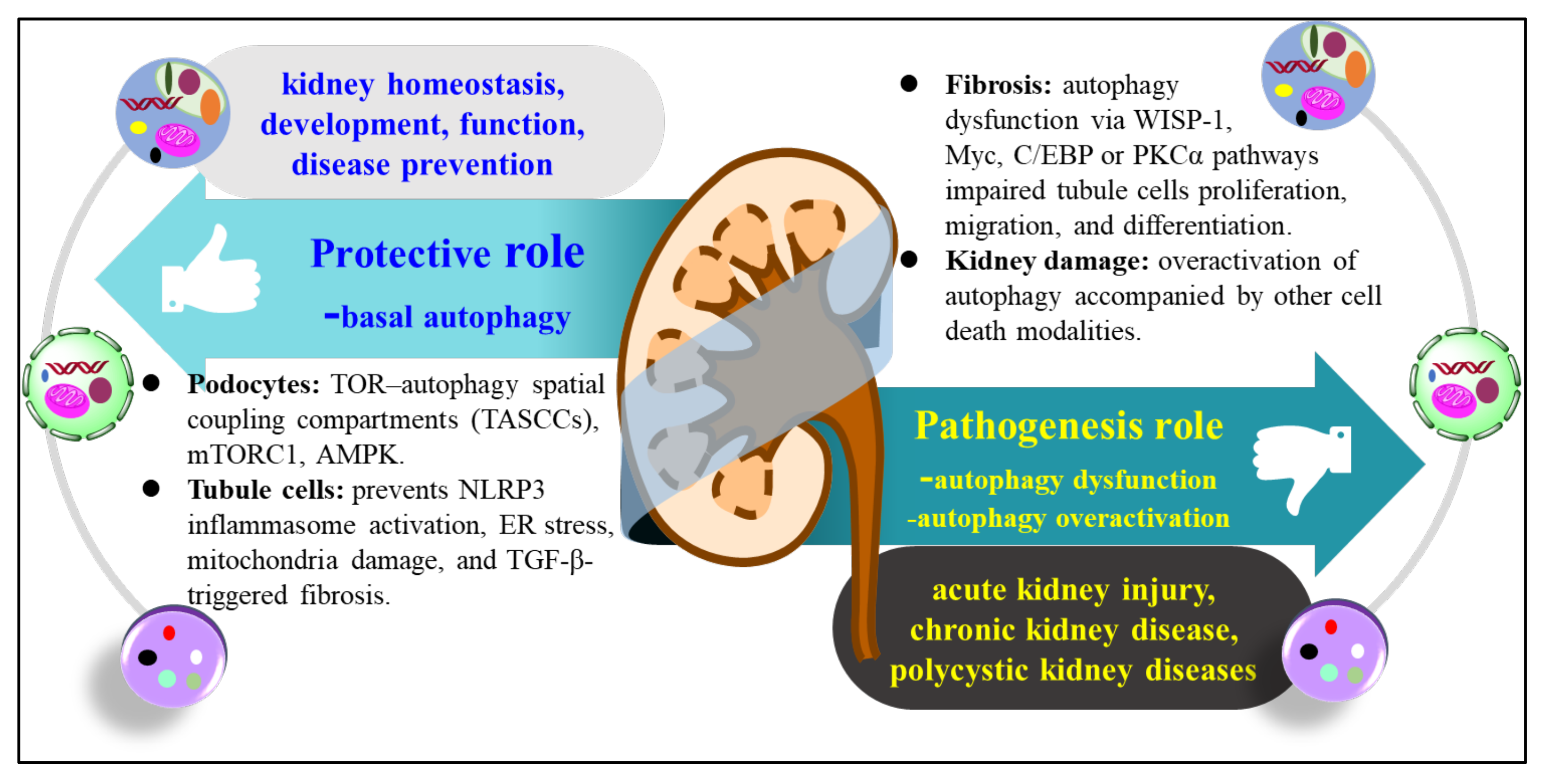
3.1. The Role of Autophagy in Kidney Disease
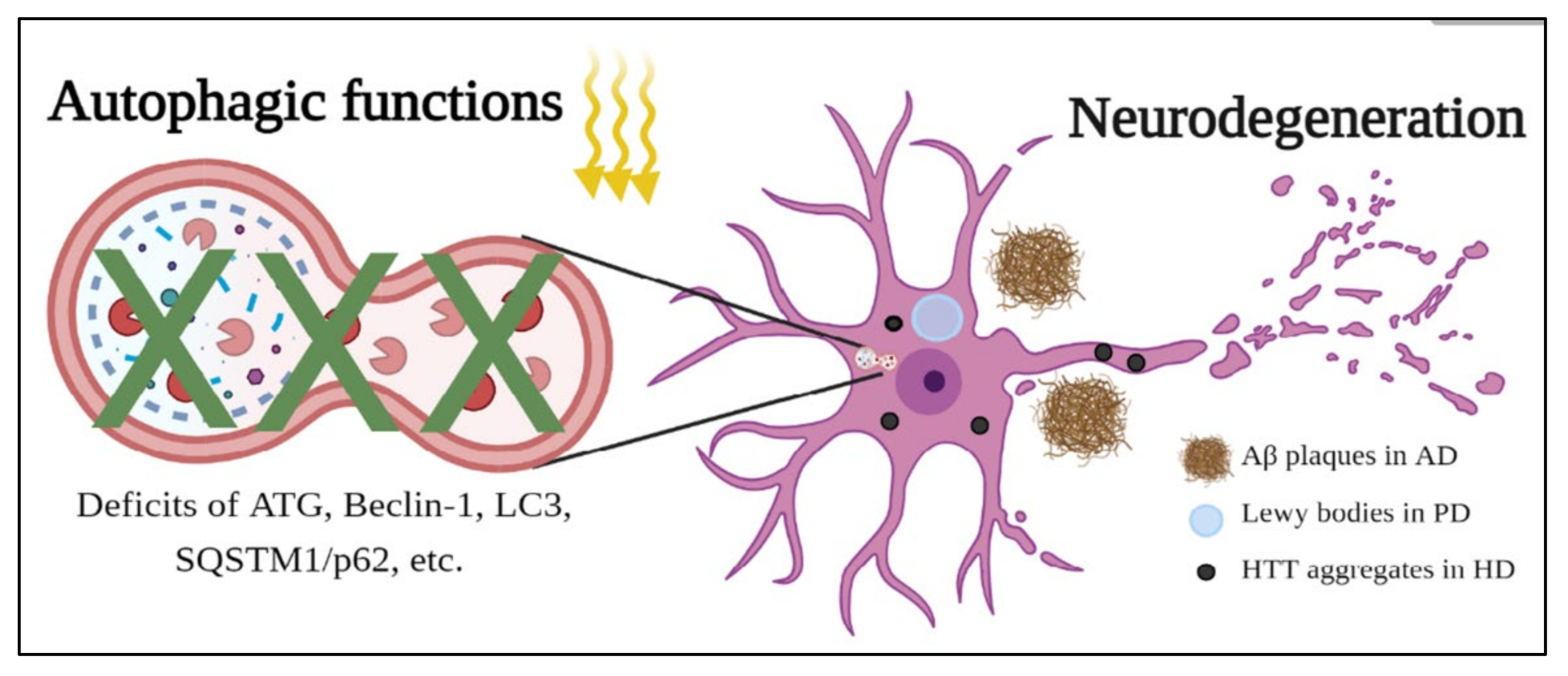
3.2. The Role of Autophagy in Neurodegenerative Diseases
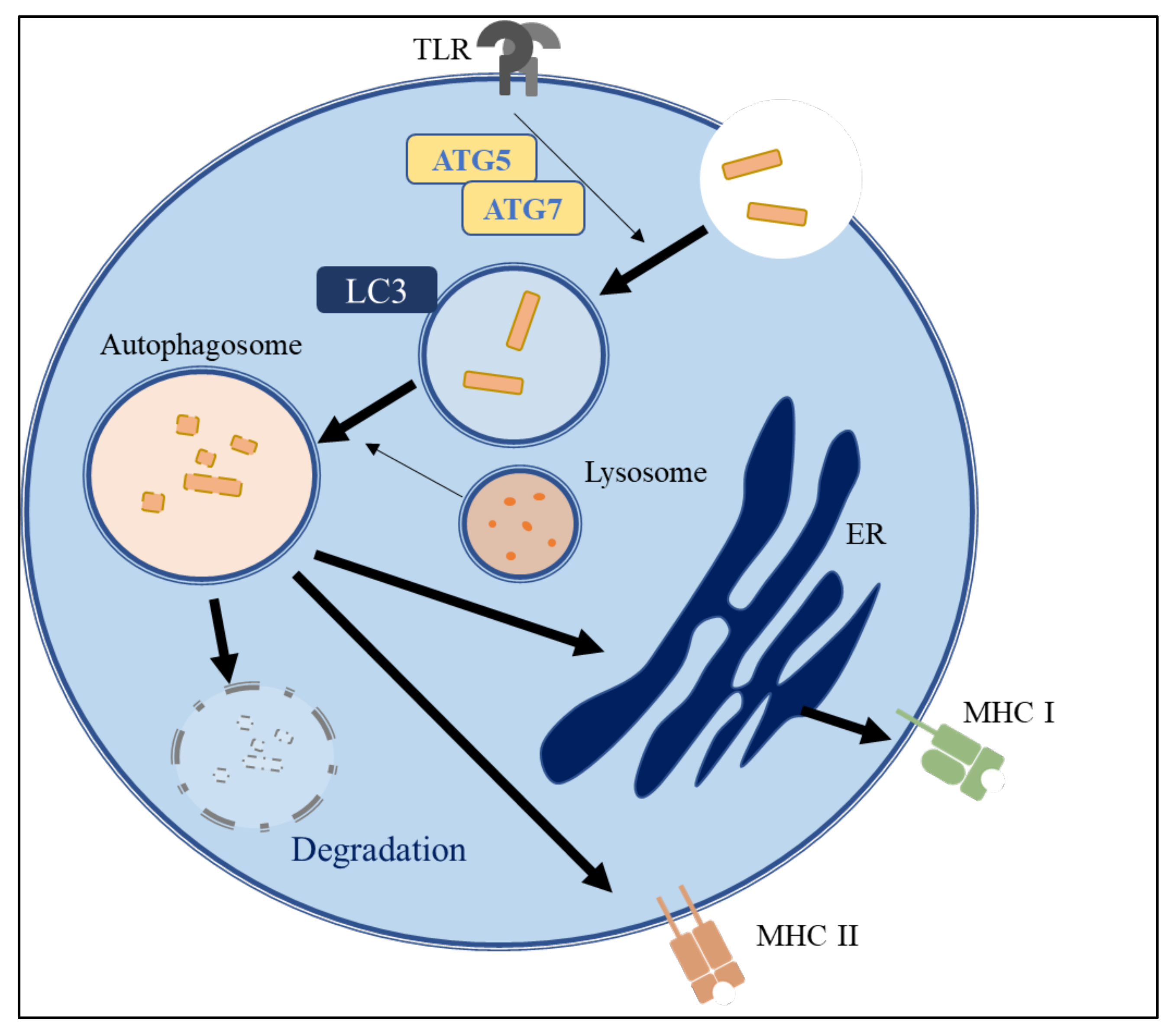
3.3. The Role of Autophagy in Immune Systems
3.4. Potential Health-Promoting Effects of Cordycepin via Autophagy Induction
4. Cordycepin-Loaded Nanoparticles as a Promising Anti-Cancer and Health-Promoting Agent
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Liu, Q.; Li, W.; Li, Q.; Qian, Z.; Liu, X.; Dong, C. A breakthrough in the artificial cultivation of Chinese cordyceps on a large-scale and its impact on science, the economy, and industry. Crit. Rev. Biotechnol. 2018, 39, 181–191. [Google Scholar] [CrossRef]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nat. Cell Biol. 1950, 166, 949. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-S.; Halpern, G.M.; Jones, K. The scientific rediscovery of a precious ancient chinese herbal regimen: Cordyceps sinensis part II. J. Altern. Complement. Med. 1998, 4, 429–457. [Google Scholar] [CrossRef]
- Xu, J.; Huang, Y.; Chen, X.-X.; Zheng, S.-C.; Chen, P.; Mo, M.-H. The mechanisms of pharmacological activities of ophiocordyceps sinensis fungi. Phytother. Res. 2016, 30, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-Y.; Chen, A.; Kuo, Y.-C.; Lin, C.-Y. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J. Lab. Clin. Med. 1999, 134, 492–500. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Tsai, W.-J.; Wang, J.C.-Y.; Chang, S.-C.; Lin, C.-Y.; Shiao, M.-S. Regulation of bronchoalveolar lavage fluids cell function by the immunomodulatory agents from Cordyceps sinensis. Life Sci. 2001, 68, 1067–1082. [Google Scholar] [CrossRef]
- Manabe, N.; Sugimoto, M.; Azuma, Y.; Taketomo, N.; Yamashita, A.; Tsuboi, H.; Tsunoo, A.; Kinjo, N.; Nian-Lai, H.; Miyamoto, H. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism in the mouse. Jpn. J. Pharmacol. 1996, 70, 85–88. [Google Scholar] [CrossRef][Green Version]
- Chiou, W.-F.; Chang, P.-C.; Chou, C.-J.; Chen, C.-F. Protein constituent contributes to the hypotensive and vasorelaxant acttvtties of cordyceps sinensis. Life Sci. 2000, 66, 1369–1376. [Google Scholar] [CrossRef]
- Manabe, N.; Azuma, Y.; Sugimoto, M.; Uchio, K.; Miyamoto, M.; Taketomo, N.; Tsuchita, H. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism and blood flow in dietary hypoferric anaemic mice. Br. J. Nutr. 2000, 83, 197–204. [Google Scholar] [CrossRef]
- Dai, G.; Bao, T.; Xu, C.; Cooper, R.; Zhu, J.-S. CordyMax™ Cs-4 improves steady-state bioenergy status in mouse liver. J. Altern. Complement. Med. 2001, 7, 231–240. [Google Scholar] [CrossRef]
- Wang, S.-M.; Lee, L.-J.; Lin, W.W.; Chang, C.-M. Effects of a water-soluble extract of Cordyceps sinensis on steroidogenesis and capsular morphology of lipid droplets in cultured rat adrenocortical cells. J. Cell. Biochem. 1998, 69, 483–489. [Google Scholar] [CrossRef]
- Leu, S.-F.; Poon, S.L.; Pao, H.-Y.; Huang, B.-M. The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci. Biotechnol. Biochem. 2011, 75, 723–731. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.-H.; Pan, B.-S.; Chang, M.-M.; Huang, B.-M. Functional study of Cordyceps sinensis and cordycepin in male reproduction: A review. J. Food Drug Anal. 2017, 25, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-Y.; Huang, W.J.; Hsieh, H.-G.; Lin, C.-Y. H1-A extracted from Cordyceps sinensis suppresses the proliferation of human mesangial cells and promotes apoptosis, probably by inhibiting the tyrosine phosphorylation of Bcl-2 and Bcl-XL. J. Lab. Clin. Med. 2003, 141, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Huang, Y.-L.; Huang, B.-M. Cordyceps sinensis mycelium activates PKA and PKC signal pathways to stimulate steroidogenesis in MA-10 mouse Leydig tumor cells. Int. J. Biochem. Cell Biol. 2005, 37, 214–223. [Google Scholar] [CrossRef]
- Nakamura, K.; Shinozuka, K.; Yoshikawa, N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J. Pharmacol. Sci. 2015, 127, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Takamura, S.; Yamaguchi, N.; Ren, L.J.; Chen, H.; Koshimura, S.; Suzuki, S. Antitumor activity of an extract of Cordyceps sinensis (Berk.) Sacc. against murine tumor cell lines. Jpn. J. Exp. Med. 1989, 59, 157–161. [Google Scholar]
- Shashidhar, M.; Giridhar, P.; Sankar, K.U.; Manohar, B. Bioactive principles from Cordyceps sinensis: A potent food supplement—A review. J. Funct. Foods 2013, 5, 1013–1030. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of Cordycepin: A review. Phytotherapy Res. 2021, 35, 1284–1297. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, S.O.; Kim, G.-Y.; Moon, S.-K.; Kim, W.-J.; Jeong, Y.K.; Yoo, Y.H.; Choi, Y.H. Involvement of autophagy in cordycepin-induced apoptosis in human prostate carcinoma LNCaP cells. Environ. Toxicol. Pharmacol. 2014, 38, 239–250. [Google Scholar] [CrossRef]
- Ashraf, S.A.; ElKhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; AwadElkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for health and wellbeing: A potent bioactive metabolite of an entomopathogenic cordyceps medicinal fungus and its nutraceutical and therapeutic potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.-S.; Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Cortes, H.; Singh, Y.D.; Panda, M.K.; Mishra, A.P.; Nigam, M.; Saklani, S. Cordyceps spp.: A review on its immune-stimulatory and other biological potentials. Front. Pharmacol. 2021, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Busceti, C.L.; Biagioni, F.; Lazzeri, G.; Forte, M.; Schiavon, S.; Sciarretta, S.; Frati, G.; Fornai, F. Cell clearing systems as targets of polyphenols in viral infections: Potential implications for COVID-19 pathogenesis. Antioxidants 2020, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Li, X.; Yang, H.; Wang, Z.Y.; Lu, D. Therapeutic potential and biological applications of Cordycepin and metabolic mechanisms in Cordycepin-producing fungi. Molecules 2019, 24, 2231. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sharma, A.; Sandhu, S.S.; Kashyap, D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013, 93, 863–869. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer properties of cordycepin and their underlying mechanisms. Int. J. Mol. Sci. 2018, 19, 3027. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M. Cordycepin in anticancer research: Molecular mechanism of therapeutic effects. Curr. Med. Chem. 2020, 27, 983–996. [Google Scholar] [CrossRef]
- Pan, B.-S.; Wang, Y.-K.; Lai, M.-S.; Mu, Y.-F.; Huang, B.-M. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis by regulating p38 MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 1–17. [Google Scholar] [CrossRef]
- Jin, Y.; Meng, X.; Qiu, Z.; Su, Y.; Yu, P.; Qu, P. Anti-tumor and anti-metastatic roles of cordycepin, one bioactive compound of Cordyceps militaris. Saudi J. Biol. Sci. 2018, 25, 991–995. [Google Scholar] [CrossRef]
- Tania, M.; Shawon, J.; Saif, K.; Kiefer, R.; Khorram, M.S.; Halim, M.A.; Khan, A. Cordycepin downregulates Cdk-2 to interfere with cell cycle and increases apoptosis by generating ROS in cervical cancer cells: In vitro and in silico study. Curr. Cancer Drug Targets 2019, 19, 152–159. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Y.; Liu, T.S.; Di Yan, W.; Chen, L.Y.; Li, Z.H.; Piao, Y.S.; An, R.B.; Lin, Z.H.; Ren, X.S. Cordycepin suppresses cell proliferation and migration by targeting CLEC2 in human gastric cancer cells via Akt signaling pathway. Life Sci. 2019, 223, 110–119. [Google Scholar] [CrossRef]
- Wu, W.-C.; Hsiao, J.-R.; Lian, Y.-Y.; Lin, C.-Y.; Huang, B.-M. The apoptotic effect of cordycepin on human OEC-M1 oral cancer cell line. Cancer Chemother. Pharmacol. 2006, 60, 103–111. [Google Scholar] [CrossRef]
- Chang, M.-M.; Hong, S.-Y.; Yang, S.-H.; Wu, C.-C.; Wang, C.-Y.; Huang, B.-M. Anti-Cancer Effect of Cordycepin on FGF9-Induced Testicular Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 8336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Sun, J.; Li, W.; Li, S.; Zhang, K. Cordycepin induces apoptosis in human tongue cancer cells in vitro and has antitumor effects in vivo. Arch. Oral Biol. 2020, 118, 104846. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qi, M.; Li, L.; Yuan, Y.; Wu, X.; Fu, J. Natural cordycepin induces apoptosis and suppresses metastasis in breast cancer cells by inhibiting the Hedgehog pathway. Food Funct. 2020, 11, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Pan, B.; Wang, C.; Huang, B. Cordycepin-induced unfolded protein response-dependent cell death, and AKT/MAPK-mediated drug resistance in mouse testicular tumor cells. Cancer Med. 2019, 8, 3949–3964. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Tsai, S.-W.; Chien, H.-H.; Chen, T.-Y.; Sheu, S.-Y.; So, E.C.; Huang, B.-M. Cordycepin inhibits human gestational choriocarcinoma cell growth by disrupting centrosome homeostasis. Drug Des. Dev. Ther. 2020, 14, 2987–3000. [Google Scholar] [CrossRef]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of autophagy in cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy in protein and organelle turnover. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 397–402. [Google Scholar] [CrossRef]
- Green, D.R.; Levine, B. To Be or Not to Be? How selective autophagy and cell death govern cell fate. Cell 2014, 157, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Karsli-Uzunbas, G.; Guo, J.Y.; Price, S.; Teng, X.; Laddha, S.V.; Khor, S.; Kalaany, N.Y.; Jacks, T.; Chan, C.S.; Rabinowitz, J.D.; et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 2014, 4, 914–927. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Bielawski, K.; Bielawska, A. Autophagy modulators in cancer therapy. Int. J. Mol. Sci. 2021, 22, 5804. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, L.; Fu, X.; Wang, W.; Ma, J.; Tian, T.; Nan, K.; Liang, X. Cytoplasmic liver kinase B1 promotes the growth of human lung adenocarcinoma by enhancing autophagy. Cancer Sci. 2018, 109, 3055–3067. [Google Scholar] [CrossRef]
- Boya, P.; Reggiori, F.; Codogno, P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013, 15, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Park, C.W.; Yoon, G.; Hong, S.M.; Choi, K.Y. NNMT depletion contributes to liver cancer cell survival by enhancing autophagy under nutrient starvation. Oncogenesis 2018, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Choi, S.; Lim, M.-H.; Kim, K.M.; Jeon, B.H.; Song, W.O.; Kim, T.W. Cordycepin-induced apoptosis and autophagy in breast cancer cells are independent of the estrogen receptor. Toxicol. Appl. Pharmacol. 2011, 257, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Zhu, S.; Zhou, R.; Wang, L.; Du, J.; Wang, Y.; Zhou, B.; Mai, L. Cordycepin induces apoptosis and autophagy in human neuroblastoma SK-N-SH and BE(2)-M17 cells. Oncol. Lett. 2015, 9, 2541–2547. [Google Scholar] [CrossRef]
- Yu, X.; Ling, J.; Liu, X.; Guo, S.; Lin, Y.; Liu, X.; Su, L. Cordycepin induces autophagy-mediated c-FLIPL degradation and leads to apoptosis in human non-small cell lung cancer cells. Oncotarget 2017, 8, 6691–6699. [Google Scholar] [CrossRef] [PubMed]
- Chaicharoenaudomrung, N.; Jaroonwitchawan, T.; Noisa, P. Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol. Vitr. 2018, 46, 113–121. [Google Scholar] [CrossRef]
- Jang, H.-J.; Yang, K.E.; Hwang, I.-H.; Huh, Y.H.; Kim, D.J.; Yoo, H.-S.; Park, S.J.; Jang, I.-S. Cordycepin inhibits human ovarian cancer by inducing autophagy and apoptosis through Dickkopf-related protein 1/β-catenin signaling. Am. J. Transl. Res. 2019, 11, 6890–6906. [Google Scholar] [PubMed]
- Ho, S.-Y.; Wu, W.-S.; Lin, L.-C.; Chiu, H.-W.; Yeh, Y.-L.; Huang, B.-M.; Wang, Y.-J. Cordycepin enhances radiosensitivity in oral squamous carcinoma cells by inducing autophagy and apoptosis through cell cycle arrest. Int. J. Mol. Sci. 2019, 20, 5366. [Google Scholar] [CrossRef]
- Hwang, J.H.; Park, S.J.; Ko, W.G.; Kang, S.-M.; Bin Lee, D.; Bang, J.; Park, B.-J.; Wee, C.-B.; Kim, D.; Jang, I.-S.; et al. Cordycepin induces human lung cancer cell apoptosis by inhibiting nitric oxide mediated ERK/Slug signaling pathway. Am. J. Cancer Res. 2017, 7, 417–432. [Google Scholar] [PubMed]
- Huang, B.-M.; Chen, Y.-H.; Wang, J.-Y.; Pan, B.-S.; Mu, Y.-F.; Lai, M.-S.; So, E.C.; Wong, T.-S. Cordycepin enhances cisplatin apoptotic effect through caspase/MAPK pathways in human head and neck tumor cells. OncoTargets Ther. 2013, 6, 983–998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.-H.; Hao, L.-J.; Hung, C.-P.; Chen, J.-W.; Leu, S.-F.; Huang, B.-M. Apoptotic effect of cisplatin and cordycepin on OC3 human oral cancer cells. Chin. J. Integr. Med. 2014, 20, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-M.; Kang, F.-C.; Chen, P.-J.; Pan, B.-S.; Lai, M.-S.; Chen, Y. Apoptotic effect of cordycepin combined with cisplatin and/or paclitaxel on MA-10 mouse Leydig tumor cells. OncoTargets Ther. 2015, 8, 2345–2360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, C.; Yao, X.; Jiang, Z.; Wang, Y.; Zhang, D.; Chen, X.; Fan, X.; Xie, C.; Cheng, J.; Fu, J.; et al. Cordycepin inhibits drug-resistance non-small cell lung cancer progression by activating AMPK signaling pathway. Pharmacol. Res. 2019, 144, 79–89. [Google Scholar] [CrossRef]
- Bi, Y.; Li, H.; Yi, D.; Sun, Y.; Bai, Y.; Zhong, S.; Song, Y.; Zhao, G.; Chen, Y. Cordycepin augments the chemosensitivity of human glioma cells to temozolomide by activating AMPK and inhibiting the AKT signaling pathway. Mol. Pharm. 2018, 15, 4912–4925. [Google Scholar] [CrossRef]
- Su, N.-W.; Wu, S.-H.; Chi, C.-W.; Tsai, T.-H.; Chen, Y.-J. Cordycepin, isolated from medicinal fungus Cordyceps sinensis, enhances radiosensitivity of oral cancer associated with modulation of DNA damage repair. Food Chem. Toxicol. 2019, 124, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chu, M.; Liu, H. Autophagy and metabolism. Kaohsiung J. Med Sci. 2021, 37, 12–19. [Google Scholar] [CrossRef]
- Liu, P.-W.; Li, C.-I.; Huang, K.-C.; Liu, C.-S.; Chen, H.-L.; Lee, C.-C.; Chiou, Y.-Y.; Chen, R.-J. 3-MCPD and glycidol coexposure induces systemic toxicity and synergistic nephrotoxicity via NLRP3 inflammasome activation, necroptosis, and autophagic cell death. J. Hazard. Mater. 2021, 405, 124241. [Google Scholar] [CrossRef]
- Livingston, M.J.; Ding, H.-F.; Huang, S.; Hill, J.A.; Yin, X.-M.; Dong, Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 2016, 12, 976–998. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, C.-C.; Yi, H.; Geng, Z.-J.; Wu, X.-Y.; Zhang, Y.; Liu, Y.; Fan, G. Traditional tibetan medicine in cancer therapy by targeting apoptosis pathways. Front. Pharmacol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Bork, T.; Liang, W.; Yamahara, K.; Lee, P.; Tian, Z.; Liu, S.; Schell, C.; Thedieck, K.; Hartleben, B.; Patel, K.; et al. Podocytes maintain high basal levels of autophagy independent of mtor signaling. Autophagy 2020, 16, 1932–1948. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Gomez, I.G.; Ren, S.; Hudkins, K.; Roach, A.; Alpers, C.E.; Shankland, S.J.; D’Agati, V.D.; Duffield, J.S. Deficient autophagy results in mitochondrial dysfunction and FSGS. J. Am. Soc. Nephrol. 2014, 26, 1040–1052. [Google Scholar] [CrossRef]
- Bechtel, W.; Helmstädter, M.; Balica, J.; Hartleben, B.; Kiefer, B.; Hrnjic, F.; Schell, C.; Kretz, O.; Liu, S.; Geist, F.; et al. Vps34 Deficiency reveals the importance of endocytosis for podocyte homeostasis. J. Am. Soc. Nephrol. 2013, 24, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef]
- Kang, Y.-L.; Saleem, M.A.; Chan, K.W.; Yung, B.Y.-M.; Law, H.K.-W. The cytoprotective role of autophagy in puromycin aminonucleoside treated human podocytes. Biochem. Biophys. Res. Commun. 2013, 443, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Chen, Y.-Y.; Hsiao, C.-M.; Pan, M.-H.; Wang, B.-J.; Chen, Y.-C.; Ho, C.-T.; Huang, K.-C.; Chen, R.-J. Induction of autophagy by pterostilbene contributes to the prevention of renal fibrosis via attenuating NLRP3 inflammasome activation and epithelial-mesenchymal transition. Front. Cell Dev. Biol. 2020, 8, 436. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, L.; Liu, Y.; Livingston, M.J.; Chen, J.-K.; Nahman, N.S.; Liu, F.; Dong, Z. Autophagy is activated to protect against podocyte injury in adriamycin-induced nephropathy. Am. J. Physiol. Physiol. 2017, 313, F74–F84. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, O.; Jasiek, M.; Hénique, C.; Guyonnet, L.; Hartleben, B.; Bork, T.; Chipont, A.; Flosseau, K.; Bensaada, I.; Schmitt, A.; et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 2015, 11, 1130–1145. [Google Scholar] [CrossRef]
- Tagawa, A.; Yasuda, M.; Kume, S.; Yamahara, K.; Nakazawa, J.; Chin-Kanasaki, M.; Araki, H.; Araki, S.-I.; Koya, D.; Asanuma, K.; et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 2015, 65, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, Y.C.; Park, J.Y.; Lee, J.; An, J.N.; Kim, C.T.; Oh, S.; Park, S.; Kim, D.K.; Oh, Y.K.; et al. The Long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care 2020, 43, 948–955. [Google Scholar] [CrossRef]
- Li, H.; Peng, X.; Wang, Y.; Cao, S.; Xiong, L.; Fan, J.; Wang, Y.; Zhuang, S.; Yu, X.; Mao, H. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy 2016, 12, 1472–1486. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kimura, T.; Takahashi, A.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.-Y.; Matsui, I.; Kitamura, H.; Matsusaka, T.; et al. Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 2016, 12, 801–813. [Google Scholar] [CrossRef]
- Nam, S.A.; Kim, W.-Y.; Kim, J.W.; Park, S.H.; Kim, H.L.; Lee, M.-S.; Komatsu, M.; Ha, H.; Lim, J.H.; Park, C.W.; et al. Autophagy attenuates tubulointerstital fibrosis through regulating transforming growth factor-β and NLRP3 inflammasome signaling pathway. Cell Death Dis. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Canaud, G.; Brooks, C.R.; Kishi, S.; Taguchi, K.; Nishimura, K.; Magassa, S.; Scott, A.; Hsiao, L.-L.; Ichimura, T.; Terzi, F.; et al. Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci. Transl. Med. 2019, 11, eaav4754. [Google Scholar] [CrossRef] [PubMed]
- Lemos, D.R.; McMurdo, M.; Karaca, G.; Wilflingseder, J.; Leaf, I.A.; Gupta, N.; Miyoshi, T.; Susa, K.; Johnson, B.G.; Soliman, K.; et al. Interleukin-1β activates a MYC-dependent metabolic switch in kidney stromal cells necessary for progressive tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2018, 29, 1690–1705. [Google Scholar] [CrossRef]
- Noh, M.; Woo, C.-H.; Park, M.-J.; Kim, J.I.; Park, K.M. Ablation of C/EBP homologous protein attenuates renal fibrosis after ureteral obstruction by reducing autophagy and microtubule disruption. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1634–1641. [Google Scholar] [CrossRef]
- Xue, X.; Ren, J.; Sun, X.; Gui, Y.; Feng, Y.; Shu, B.; Wei, W.; Lu, Q.; Liang, Y.; He, W.; et al. Protein kinase Cα drives fibroblast activation and kidney fibrosis by stimulating autophagic flux. J. Biol. Chem. 2018, 293, 11119–11130. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Tu, Y.; Li, Y.; Zou, Y.; Li, G.; Wang, L.; Zhong, X. WNT1-inducible signaling protein-1 mediates TGF-β1-induced renal fibrosis in tubular epithelial cells and unilateral ureteral obstruction mouse models via autophagy. J. Cell. Physiol. 2020, 235, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Chang-Panesso, M.; Kadyrov, F.F.; Lalli, M.; Wu, H.; Ikeda, S.; Kefalogianni, E.; Abdelmageed, M.M.; Herrlich, A.; Kobayashi, A.; Humphreys, B.D. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Investig. 2019, 129, 5501–5517. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, R.; Wu, J.; Li, X. Self-eating: Friend or foe? The emerging role of autophagy in fibrotic diseases. Theranostics 2020, 10, 7993–8017. [Google Scholar] [CrossRef]
- Ma, Z.; Li, L.; Livingston, M.J.; Zhang, D.; Mi, Q.; Zhang, M.; Ding, H.F.; Huo, Y.; Mei, C.; Dong, Z. p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney disease. J. Clin. Investig. 2020, 130, 5011–5026. [Google Scholar] [CrossRef]
- Fu, M.-H.; Li, C.-L.; Lin, H.-L.; Chen, P.-C.; Calkins, M.J.; Chang, Y.-F.; Cheng, P.-H.; Yang, S.-H. Stem cell transplantation therapy in Parkinson’s disease. Springerplus 2015, 4, 597. [Google Scholar] [CrossRef]
- John, A.; Kubosumi, A.; Reddy, P.H. Mitochondrial microRNAs in aging and neurodegenerative diseases. Cells 2020, 9, 1345. [Google Scholar] [CrossRef]
- Yusuf, I.O.; Chen, H.M.; Cheng, P.H.; Chang, C.Y.; Tsai, S.J.; Chuang, J.I.; Wu, C.C.; Huang, B.M.; Sun, H.S.; Yang, S.H. Fibroblast growth factor 9 activates anti-oxidative functions of Nrf2 through ERK signalling in striatal cell models of Huntington’s disease. Free Radic. Biol. Med. 2019, 130, 256–266. [Google Scholar] [CrossRef]
- Yusuf, I.; Cheng, P.-H.; Chen, H.-M.; Chang, Y.-F.; Chang, C.-Y.; Yang, H.-I.; Lin, C.-W.; Tsai, S.-J.; Chuang, J.-I.; Wu, C.-C.; et al. Fibroblast growth factor 9 suppresses striatal cell death dominantly through ERK signaling in Huntington’s disease. Cell. Physiol. Biochem. 2018, 48, 605–617. [Google Scholar] [CrossRef]
- Ciechanover, A.; Kwon, Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015, 47, e147. [Google Scholar] [CrossRef]
- Kabir, T.; Uddin, S.; Abdeen, A.; Ashraf, G.M.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Evidence linking protein misfolding to quality control in progressive neurodegenerative diseases. Curr. Top. Med. Chem. 2020, 20, 2025–2043. [Google Scholar] [CrossRef]
- Nijholt, D.A.; De Kimpe, L.; Elfrink, H.L.; Hoozemans, J.J.; Scheper, W. Removing protein aggregates: The role of proteolysis in neurodegeneration. Curr. Med. Chem. 2011, 18, 2459–2476. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bustos, V.; Flajolet, M.; Greengard, P. A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 2011, 25, 1934–1942. [Google Scholar] [CrossRef]
- Xu, W.; Ocak, U.; Gao, L.; Tu, S.; Lenahan, C.J.; Zhang, J.; Shao, A. Selective autophagy as a therapeutic target for neurological diseases. Cell. Mol. Life Sci. 2021, 78, 1369–1392. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, M.; Jeong, Y.Y.; Margolis, D.J.; Cai, Q. The role of mitophagy in the regulation of mitochondrial energetic status in neurons. Autophagy 2021, 1–20. [Google Scholar] [CrossRef]
- Nobili, A.; La Barbera, L.; D’Amelio, M. Targeting autophagy as a therapeutic strategy to prevent dopamine neuron loss in early stages of Alzheimer disease. Autophagy 2021, 17, 1278–1280. [Google Scholar] [CrossRef]
- Pedrioli, G.; Paganetti, P. Hijacking endocytosis and autophagy in extracellular vesicle communication: Where the inside meets the outside. Front. Cell Dev. Biol. 2021, 8, 595515. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Malampati, S.; Zeng, Y.; Durairajan, S.S.K.; Yang, C.B.; Tong, B.C.; Iyaswamy, A.; Shang, W.B.; Sreenivasmurthy, S.G.; Zhu, Z.; et al. A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and Tau pathology in Alzheimer’s disease models. Aging Cell 2020, 19, e13069. [Google Scholar] [CrossRef]
- Umeda, T.; Ono, K.; Sakai, A.; Yamashita, M.; Mizuguchi, M.; Klein, W.L.; Yamada, M.; Mori, H.; Tomiyama, T. Rifampicin is a candidate preventive medicine against amyloid-β and tau oligomers. Brain 2016, 139, 1568–1586. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhu, C.; Wang, X.; Feng, X.; Pang, S.; Huang, W.; Hawley, R.G.; Yan, B. A novel and functional variant within the ATG5 gene promoter in sporadic Parkinson’s disease. Neurosci. Lett. 2013, 538, 49–53. [Google Scholar] [CrossRef]
- El Manaa, W.; Duplan, E.; Goiran, T.; Lauritzen, I.; Beuchot, L.V.; Lacas-Gervais, S.; Morais, V.A.; You, H.; Qi, L.; Salazar, M.; et al. Transcription- and phosphorylation-dependent control of a functional interplay between XBP1s and PINK1 governs mitophagy and potentially impacts Parkinson disease pathophysiology. Autophagy. 2021, 1–23. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Pang, S.; Wang, H.; Zhang, A.; Hawley, R.G.; Yan, B. Novel and functional ATG12 gene variants in sporadic Parkinson’s disease. Neurosci. Lett 2017, 643, 22–26. [Google Scholar] [CrossRef]
- Svarcbahs, R.; Jäntti, M.; Kilpeläinen, T.; Julku, U.H.; Urvas, L.; Kivioja, S.; Norrbacka, S.; Myöhänen, T.T. Prolyl oligopeptidase inhibition activates autophagy via protein phosphatase 2A. Pharmacol. Res. 2020, 151, 104558. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Lu, Y.; Tian, H.; Duan, C.; Lu, L.; Gao, G.; Wu, X.; Wang, X.; Yang, H. Piperlongumine restores the balance of autophagy and apoptosis by increasing BCL2 phosphorylation in rotenone-induced Parkinson disease models. Autophagy 2018, 14, 845–861. [Google Scholar] [CrossRef]
- Kovács, T.; Billes, V.; Komlós, M.; Hotzi, B.; Manzéger, A.; Tarnóci, A.; Papp, D.; Szikszai, F.; Szinyákovics, J.; Rácz, Á.; et al. The small molecule AUTEN-99 (autophagy enhancer-99) prevents the progression of neurodegenerative symptoms. Sci. Rep. 2017, 7, 42014. [Google Scholar] [CrossRef] [PubMed]
- Her, L.S.; Mao, S.H.; Chang, C.Y.; Cheng, P.H.; Chang, Y.F.; Yang, H.I.; Chen, C.M.; Yang, S.H. miR-196a enhances neuronal morphology through suppressing RANBP10 to provide neuroprotection in Huntington’s disease. Theranostics 2017, 7, 2452–2462. [Google Scholar] [CrossRef]
- Yusuf, I.O.; Chen, H.M.; Cheng, P.H.; Chang, C.Y.; Tsai, S.J.; Chuang, J.I.; Wu, C.C.; Huang, B.M.; Sun, H.S.; Chen, C.M.; et al. FGF9 induces neurite outgrowth upon ERK signaling in knock-in striatal Huntington’s disease cells. Life Sci. 2021, 267, 118952. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, I.O.; Chen, H.M.; Cheng, P.H.; Chang, C.Y.; Tsai, S.J.; Chuang, J.I.; Wu, C.C.; Huang, B.M.; Sun, H.S.; Chen, C.M.; et al. Fibroblast growth factor 9 stimulates neuronal length through NF-kB signaling in striatal cell Huntington’s disease models. Mol. Neurobiol. 2021, 58, 2396–2406. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Bento, C.F.; Ricketts, T.; Vicinanza, M.; Siddiqi, F.; Pavel, M.; Squitieri, F.; Hardenberg, M.; Imarisio, S.; Menzies, F.M.; et al. Polyglutamine tracts regulate beclin 1-dependent autophagy. Nat. Cell Biol. 2017, 545, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Franco-Iborra, S.; Plaza-Zabala, A.; Montpeyo, M.; Sebastian, D.; Vila, M.; Martinez-Vicente, M. Mutant HTT (huntingtin) impairs mitophagy in a cellular model of Huntington disease. Autophagy 2021, 17, 672–689. [Google Scholar] [CrossRef]
- Shibata, M.; Lu, T.; Furuya, T.; Degterev, A.; Mizushima, N.; Yoshimori, T.; MacDonald, M.; Yankner, B.; Yuan, J. Regulation of intracellular accumulation of mutant huntingtin by Beclin 1. J. Biol. Chem. 2006, 281, 14474–14485. [Google Scholar] [CrossRef] [PubMed]
- Her, L.S.; Lin, J.Y.; Fu, M.H.; Chang, Y.F.; Li, C.L.; Tang, T.Y.; Jhang, Y.L.; Chang, C.Y.; Shih, M.C.; Cheng, P.H.; et al. The differential profiling of ubiquitin-proteasome and autophagy systems in different tissues before the onset of Huntington’s disease models. Brain Pathol. 2015, 25, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Erie, C.; Lu, M.L.; Wei, J. Aberrant subcellular localization of SQSTM1/p62 contributes to increased vulnerability to proteotoxic stress recovery in Huntington’s disease. Mol. Cell Neurosci. 2018, 88, 43–52. [Google Scholar] [CrossRef]
- Kataura, T.; Tashiro, E.; Nishikawa, S.; Shibahara, K.; Muraoka, Y.; Miura, M.; Sakai, S.; Katoh, N.; Totsuka, M.; Onodera, M.; et al. A chemical genomics-aggrephagy integrated method studying functional analysis of autophagy inducers. Autophagy 2020, 1–17. [Google Scholar] [CrossRef]
- Roscic, A.; Baldo, B.; Crochemore, C.; Marcellin, D.; Paganetti, P. Induction of autophagy with catalytic mTOR inhibitors reduces huntingtin aggregates in a neuronal cell model. J. Neurochem. 2011, 119, 398–407. [Google Scholar] [CrossRef]
- Tsvetkov, A.S.; Miller, J.; Arrasate, M.; Wong, J.S.; Pleiss, M.A.; Finkbeiner, S. A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc. Natl. Acad. Sci. USA 2010, 107, 16982–16987. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Sarkar, S.; Cuddon, P.; Ttofi, E.K.; Saiki, S.; Siddiqi, F.H.; Jahreiss, L.; Fleming, A.; Pask, D.; Goldsmith, P.; et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008, 4, 295–305. [Google Scholar] [CrossRef]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the Integrated Stress Response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nat. Cell Biol. 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Hampe, J.; Franke, A.; Rosenstiel, P.; Till, A.; Teuber, M.; Huse, K.; Albrecht, M.; Mayr, G.; De La Vega, F.M.; Briggs, J.; et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2006, 39, 207–211. [Google Scholar] [CrossRef]
- Rioux, J.D.; Xavier, R.J.; Taylor, K.D.; Silverberg, M.S.; Goyette, P.; Huett, A.; Green, T.; Kuballa, P.; Barmada, M.M.; Datta, L.W.; et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 2007, 39, 596–604. [Google Scholar] [CrossRef]
- Dengjel, J.; Schoor, O.; Fischer, R.; Reich, M.; Kraus, M.; Muller, M.; Kreymborg, K.; Altenberend, F.; Brandenburg, J.; Kalbacher, H.; et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 7922–7927. [Google Scholar] [CrossRef]
- Kasai, M.; Tanida, I.; Ueno, T.; Kominami, E.; Seki, S.; Ikeda, T.; Mizuochi, T. Autophagic compartments gain access to the MHC class II compartments in thymic epithelium. J. Immunol. 2009, 183, 7278–7285. [Google Scholar] [CrossRef]
- Munz, C. Antigen processing via autophagy—Not only for MHC class II presentation anymore? Curr. Opin. Immunol. 2010, 22, 89–93. [Google Scholar] [CrossRef]
- Huang, J.; Canadien, V.; Lam, G.Y.; Steinberg, B.E.; Dinauer, M.C.; Magalhaes, M.; Glogauer, M.; Grinstein, S.; Brumell, J.H. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA 2009, 106, 6226–6231. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nat. Cell Biol. 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Zhao, Z.; Fux, B.; Goodwin, M.; Dunay, I.R.; Strong, D.; Miller, B.; Cadwell, K.; Delgado, M.A.; Ponpuak, M.; Green, K.G.; et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 2008, 4, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.A.; Deretic, V. Toll-like receptors in control of immunological autophagy. Cell Death Differ. 2009, 16, 976–983. [Google Scholar] [CrossRef]
- Wen, X.; Klionsky, D.J. How bacteria can block xenophagy: An insight from Salmonella. Autophagy 2019, 16, 193–194. [Google Scholar] [CrossRef]
- Martinez, J.; Almendinger, J.; Oberst, A.; Ness, R.; Dillon, C.P.; Fitzgerald, P.; Hengartner, M.; Green, D.R. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17396–17401. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.A.; Grinstein, S. Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol. Rev. 2014, 262, 193–215. [Google Scholar] [CrossRef]
- Henault, J.; Martinez, J.; Riggs, J.M.; Tian, J.; Mehta, P.; Clarke, L.; Sasai, M.; Latz, E.; Brinkmann, M.M.; Iwasaki, A.; et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity 2012, 37, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-C.; Devenish, R.J. LC3-associated phagocytosis (LAP): Connections with host autophagy. Cells 2012, 1, 396–408. [Google Scholar] [CrossRef]
- Martinez, J.; Malireddi, R.; Lu, Q.; Cunha, L.D.; Pelletier, S.; Gingras, S.; Orchard, R.; Guan, J.-L.; Tan, H.; Peng, J.; et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 2015, 17, 893–906. [Google Scholar] [CrossRef]
- Martinez, J.; Cunha, L.D.; Park, S.; Yang, M.; Lu, Q.; Orchard, R.; Li, Q.Z.; Yan, M.; Janke, L.; Guy, C.; et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 2016, 533, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wu, H.; Chen, Y.; Zhang, J.; Zheng, M.; Chen, G.; Li, L.; Lu, Q. The Therapeutic and pathogenic role of autophagy in autoimmune diseases. Front. Immunol. 2018, 9, 1512. [Google Scholar] [CrossRef]
- Lee, H.K.; Mattei, L.; Steinberg, B.E.; Alberts, P.; Lee, Y.H.; Chervonsky, A.; Mizushima, N.; Grinstein, S.; Iwasaki, A. In vivo requirement for atg5 in antigen presentation by dendritic cells. Immunity 2010, 32, 227–239. [Google Scholar] [CrossRef]
- Chemali, M.; Radtke, K.; Desjardins, M.; English, L. Alternative pathways for MHC class I presentation: A new function for autophagy. Cell. Mol. Life Sci. 2011, 68, 1533–1541. [Google Scholar] [CrossRef]
- Nedjic, J.; Aichinger, M.; Emmerich, J.; Mizushima, N.; Klein, L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nat. Cell Biol. 2008, 455, 396–400. [Google Scholar] [CrossRef]
- Morris, S.; Swanson, M.; Lieberman, A.; Reed, M.; Yue, Z.; Lindell, D.M.; Lukacs, N.W. Autophagy-mediated dendritic cell activation is essential for innate cytokine production and apc function with respiratory syncytial virus responses. J. Immunol. 2011, 187, 3953–3961. [Google Scholar] [CrossRef]
- English, L.; Chemali, M.; Duron, J.; Rondeau, C.; Laplante, A.; Gingras, D.; Alexander, D.; Leib, D.; Norbury, C.; Lippe, R.; et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009, 10, 480–487. [Google Scholar] [CrossRef]
- Schmid, D.; Pypaert, M.; Münz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 2007, 26, 79–92. [Google Scholar] [CrossRef]
- Yang, Z.; Goronzy, J.J.; Weyand, C.M. Autophagy in autoimmune disease. J. Mol. Med. 2015, 93, 707–717. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, M.; Li, H.-H.; Long, Q.; Liang, W.; Wen, S.; Wang, M.; Guo, H.-P.; Cheng, X.; Liao, Y.-H. Autophagy contributes to IL-17-induced plasma cell differentiation in experimental autoimmune myocarditis. Int. Immunopharmacol. 2014, 18, 98–105. [Google Scholar] [CrossRef]
- Miller, B.; Zhao, Z.; Stephenson, L.M.; Cadwell, K.; Pua, H.H.; Lee, H.K.; Mizushima, N.; Iwasaki, A.; He, Y.-W.; Swat, W.; et al. The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy 2008, 4, 309–314. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Zhang, J.-J.; Gao, C.-C.; Zhao, M.; Liu, S.-Y.; Gao, G.-M.; Zheng, Z.-H. Expression of autophagy related genes mTOR, Becline-1, LC3 and p62 in the peripheral blood mononuclear cells of systemic lupus erythematosus. Am. J. Clin. Exp. Immunol. 2017, 6, 1–8. [Google Scholar]
- Cao, C.; Yang, S.; Zhou, Z. The potential application of Cordyceps in metabolic-related disorders. Phytotherapy Res. 2020, 34, 295–305. [Google Scholar] [CrossRef]
- Cao, T.; Xu, R.; Xu, Y.; Liu, Y.; Qi, D.; Wan, Q. The protective effect of cordycepin on diabetic nephropathy through autophagy induction in vivo and in vitro. Int. Urol. Nephrol. 2019, 51, 1883–1892. [Google Scholar] [CrossRef]
- Yong, T.; Chen, S.; Xie, Y.; Chen, D.; Su, J.; Shuai, O.; Jiao, C.; Zuo, D. Cordycepin, a characteristic bioactive constituent in cordyceps militaris, ameliorates hyperuricemia through URAT1 in hyperuricemic mice. Front. Microbiol. 2018, 9, 58. [Google Scholar] [CrossRef]
- Sun, T.; Dong, W.; Jiang, G.; Yang, J.; Liu, J.; Zhao, L.; Ma, P. Cordyceps militarisImproves chronic kidney disease by affecting TLR4/NF-κB redox signaling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Gu, L.; Johno, H.; Nakajima, S.; Kato, H.; Takahashi, S.; Katoh, R.; Kitamura, M. Blockade of Smad signaling by 3′-deoxyadenosine: A mechanism for its anti-fibrotic potential. Lab. Investig. 2013, 93, 450–461. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Z.; Shang, Y.; Yan, W.; Wu, B.; Li, C. Cordycepin improves behavioral-LTP and dendritic structure in hippocampal CA1 area of rats. J. Neurochem. 2019, 151, 79–90. [Google Scholar] [CrossRef]
- Liu, Z.-B.; Liu, C.; Zeng, B.; Huang, L.-P.; Yao, L.-H. Modulation effects of cordycepin on voltage-gated sodium channels in rat hippocampal CA1 pyramidal neurons in the presence/absence of oxygen. Neural Plast. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Yao, L.; Huang, J.; Li, C.; Li, H.-H.; Yan, W.; Cai, Z.; Liu, W.; Xiao, P. Cordycepin suppresses excitatory synaptic transmission in rat hippocampal slices via a presynaptic mechanism. CNS Neurosci. Ther. 2013, 19, 216–221. [Google Scholar] [CrossRef]
- Yao, L.-H.; Li, C.-H.; Yan, W.-W.; Huang, J.-N.; Liu, W.-X.; Xiao, P. Cordycepin decreases activity of hippocampal CA1 pyramidal neuron through membrane hyperpolarization. Neurosci. Lett. 2011, 503, 256–260. [Google Scholar] [CrossRef]
- Cheng, Z.; He, W.; Zhou, X.; Lv, Q.; Xu, X.; Yang, S.; Zhao, C.; Guo, L. Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur. J. Pharmacol. 2011, 664, 20–28. [Google Scholar] [CrossRef]
- Marcelo, A.; Brito, F.; Silva, S.C.; Matos, C.; Alves-Cruzeiro, J.; Vasconcelos-Ferreira, A.; Koppenol, R.; Mendonça, L.; de Almeida, L.P.; Nóbrega, C. Cordycepin activates autophagy through AMPK phosphorylation to reduce abnormalities in Machado–Joseph disease models. Hum. Mol. Genet. 2018, 28, 51–63. [Google Scholar] [CrossRef]
- Yao, L.-H.; Wang, J.; Liu, C.; Wei, S.; Li, G.; Wang, S.; Meng, W.; Liu, Z.-B.; Huang, L.-P. Cordycepin protects against β-amyloid and ibotenic acid-induced hippocampal CA1 pyramidal neuronal hyperactivity. Korean J. Physiol. Pharmacol. 2019, 23, 483–491. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Huang, W.-M.; Tang, P.-C.; Sun, Y.; Zhang, X.; Qiu, L.; Yu, B.-C.; Hong, Y.-X.; He, Y.; Ge, X.-Q. Anti-inflammatory and neuroprotective effects of natural cordycepin in rotenone-induced PD models through inhibiting Drp1-mediated mitochondrial fission. NeuroToxicology 2021, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huang, L.-P.; Li, Y.; Liu, C.; Wang, S.; Meng, W.; Wei, S.; Liu, X.-P.; Gong, Y.; Yao, L.-H. Neuroprotective effects of cordycepin inhibit Aβ-induced apoptosis in hippocampal neurons. NeuroToxicology 2018, 68, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wei, Y.; Song, P.; Li, Y.; Zhang, T.; Feng, Q.; Xu, G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2018, 818, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.-Z.; Hylemon, P.B.; Zhang, L.-Y.; Zhou, H.-P. Cordycepin inhibits LPS-induced inflammatory responses by modulating NOD-Like Receptor Protein 3 inflammasome activation. Biomed. Pharmacother. 2017, 95, 1777–1788. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, Z. Using Cordyceps militaris extracellular polysaccharides to prevent Pb2+-induced liver and kidney toxicity by activating Nrf2 signals and modulating gut microbiota. Food Funct. 2020, 11, 9226–9239. [Google Scholar] [CrossRef]
- An, Y.; Li, Y.; Wang, X.; Chen, Z.; Xu, H.; Wu, L.; Li, S.; Wang, C.; Luan, W.; Wang, X.; et al. Cordycepin reduces weight through regulating gut microbiota in high-fat diet-induced obese rats. Lipids Heal. Dis. 2018, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mao, Y.; Shergis, J.L.; Coyle, M.E.; Wu, L.; Chen, Y.; Zhang, A.L.; Lin, L.; Xue, C.C.; Xu, Y. Effectiveness and safety of oral cordyceps sinensis on stable COPD of GOLD stages 2-3: Systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2019, 2019, 4903671. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, H.; Bao, H.; Zhang, D.; Feng, L.; Xiao, Y.; Zhu, K.; Hou, Y.; Luo, S.; Zhang, Y.; et al. Cordycepin (3′-deoxyadenosine) promotes remyelination via suppression of neuroinflammation in a cuprizone-induced mouse model of demyelination. Int. Immunopharmacol. 2019, 75, 105777. [Google Scholar] [CrossRef]
- Wu, P.-K.; Tao, Z.; Ouyang, Z.; Cao, J.-Y.; Geng, D.; Liu, J.; Wang, C.-M. The anti-tumor effects of cordycepin-loaded liposomes on the growth of hepatoma 22 tumors in mice and human hepatoma BEL-7402 cells in culture. Drug Dev. Ind. Pharm. 2016, 42, 1424–1433. [Google Scholar] [CrossRef]
- Marslin, G.; Khandelwal, V.; Franklin, G. Cordycepin nanoencapsulated in poly(lactic-co-glycolic acid) exhibits better cytotoxicity and lower hemotoxicity than free drug. Nanotechnol. Sci. Appl. 2020, 13, 37–45. [Google Scholar] [CrossRef]
- Aramwit, P.; Porasuphatana, S.; Srichana, T.; Nakpheng, T. Toxicity evaluation of cordycepin and its delivery system for sustained in vitro anti-lung cancer activity. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Chen, C.-H.; Lin, W.-C.; Tung, C.-W.; Chen, Y.-C.; Yang, S.-H.; Huang, B.-M.; Chen, R.-J. The Role of Autophagy in Anti-Cancer and Health Promoting Effects of Cordycepin. Molecules 2021, 26, 4954. https://doi.org/10.3390/molecules26164954
Chen Y-Y, Chen C-H, Lin W-C, Tung C-W, Chen Y-C, Yang S-H, Huang B-M, Chen R-J. The Role of Autophagy in Anti-Cancer and Health Promoting Effects of Cordycepin. Molecules. 2021; 26(16):4954. https://doi.org/10.3390/molecules26164954
Chicago/Turabian StyleChen, Yu-Ying, Chun-Hsien Chen, Wei-Chen Lin, Chih-Wei Tung, Yung-Chia Chen, Shang-Hsun Yang, Bu-Miin Huang, and Rong-Jane Chen. 2021. "The Role of Autophagy in Anti-Cancer and Health Promoting Effects of Cordycepin" Molecules 26, no. 16: 4954. https://doi.org/10.3390/molecules26164954
APA StyleChen, Y.-Y., Chen, C.-H., Lin, W.-C., Tung, C.-W., Chen, Y.-C., Yang, S.-H., Huang, B.-M., & Chen, R.-J. (2021). The Role of Autophagy in Anti-Cancer and Health Promoting Effects of Cordycepin. Molecules, 26(16), 4954. https://doi.org/10.3390/molecules26164954