Phytochemicals from Polyalthia Species: Potential and Implication on Anti-Oxidant, Anti-Inflammatory, Anti-Cancer, and Chemoprevention Activities
Abstract
:1. Chemopreventive Concepts on Cancer Progression by Using Natural Products against Chronic Inflammation or Oxidative Stress
2. Polyalthia Genus Plants
Methods for Extraction of Phytochemical Compounds from Polyalthia
3. Phytochemical Constituents in Species of Polyalthia
4. Anti-Oxidant Phytochemicals in Polyalthia
5. Anti-Inflammatory Phytochemicals in Polyalthia
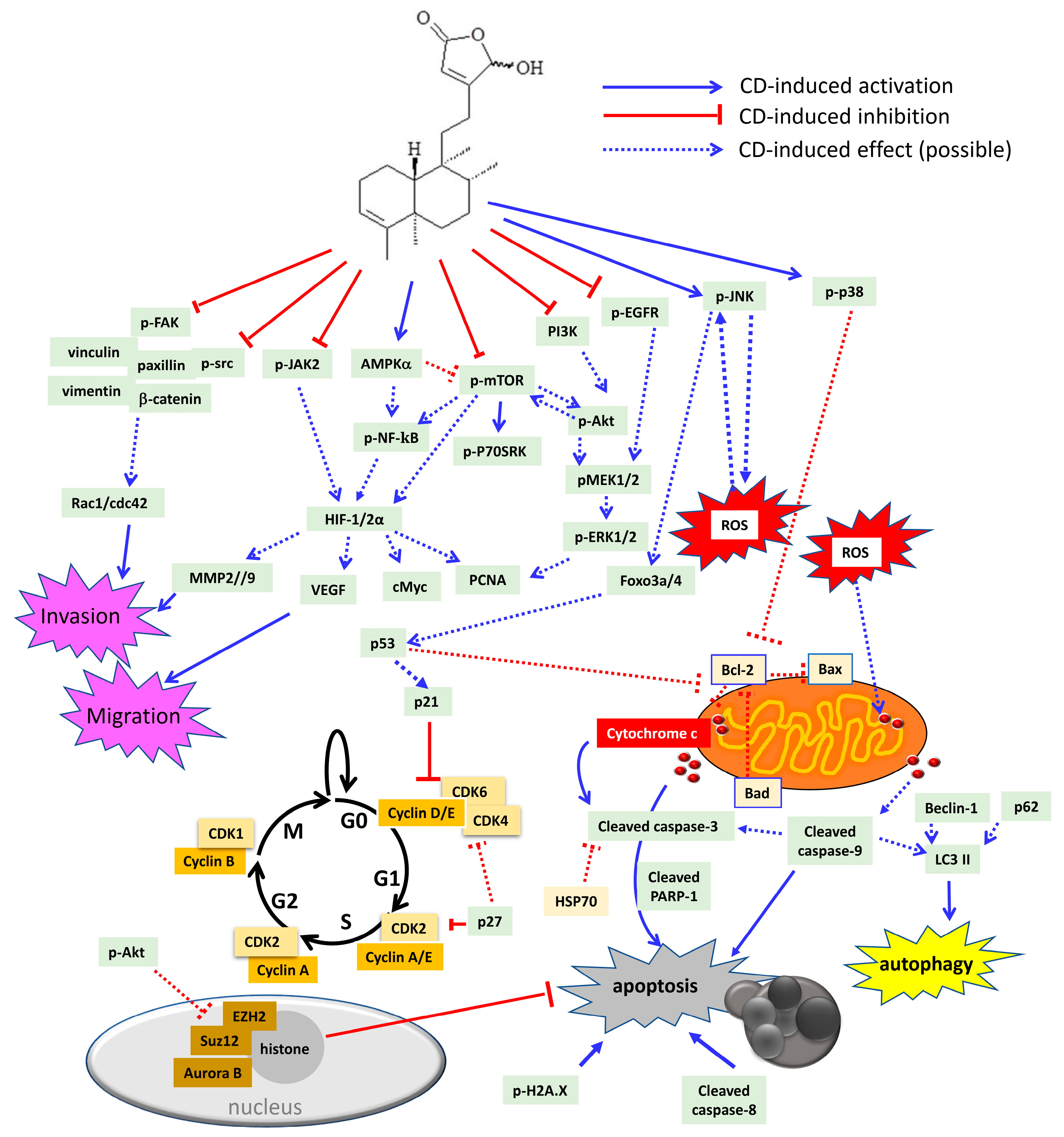
6. Cytotoxic/Anti-Tumor Phytochemicals in Polyalthia and the Molecular Mechanism of CD-Induced Tumor Cell Death
7. Anti-Cancer Potential of Polyalthia Genus
8. Chemoprevention Potential of Phytochemical Compounds from Polyalthia
8.1. Phytochemical Compounds with Anti-Bacterial and Anti-Fungal Activities
8.2. Phytochemical Compounds with Anti-Viral Activity
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of cancer. CA Cancer J. Clin. 2004, 54, 150–180. [Google Scholar] [CrossRef] [Green Version]
- Gu, K.J.; Li, G. An overview of cancer prevention: Chemoprevention and immunoprevention. J. Cancer Prev. 2020, 25, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- De Melo, F.H.M.; Oliveira, J.S.; Sartorelli, V.O.B.; Montor, W.R. Cancer chemoprevention: Classic and epigenetic mechanisms inhibiting tumorigenesis. What have we learned so far? Front. Oncol. 2018, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Afaq, F.; Mukhtar, H. Cancer chemoprevention through dietary antioxidants: Progress and promise. Antioxid. Redox. Signal. 2008, 10, 475–510. [Google Scholar] [CrossRef]
- Chatrou, L.; Erkens, R.J.; Richardson, J.E.; Saunders, R.K.; Fay, M. The natural history of Annonaceae. Bot J. Linn. Soc. 2012, 169, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Wang, M.J.; Chen, P.Y.; Wu, T.Y.; Wen, W.C.; Tsai, F.Y.; Lee, C.K. Constituents of Polyalthia longifolia var. pendula. J. Nat. Prod. 2009, 72, 1960–1963. [Google Scholar] [CrossRef] [PubMed]
- Jothy, S.L.; Yeng, C.; Sasidharan, S. Chromatographic and spectral fingerprinting of Polyalthia longifolia, a source of phytochemicals. Bioresources 2013, 8, 5102–5119. [Google Scholar] [CrossRef] [Green Version]
- Jothy, S.L.; Saito, T.; Kanwar, J.R.; Chen, Y.; Aziz, A.; Yin-Hui, L.; Sasidharan, S. Radioprotective activity of Polyalthia longifolia standardized extract against X-ray radiation injury in mice. Phys. Med. 2016, 32, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Vijayarathna, S.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Standardized Polyalthia longifolia leaf extract (PLME) inhibits cell proliferation and promotes apoptosis: The anti-cancer study with various microscopy methods. Biomed. Pharmacother. 2017, 91, 366–377. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Shih, Y.C.; Hsieh, T.J.; Chia, Y.C.; Tseng, H.Y.; Chen, H.C.; Chen, S.J.; Hsu, M.C.; Wu, Y.C. Cytotoxic constituents of Polyalthia longifolia var. pendula. J. Nat. Prod. 2000, 63, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Dave, R.; Kaneria, M.; Shukla, V. Acute oral toxicity of Polyalthia longifolia var. pendula leaf extract in Wistar albino rats. Pharm. Biol. 2012, 50, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Jothy, S.L.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Evaluation of the genotoxic potential against H2O2-radical-mediated DNA damage and acute oral toxicity of standardized extract of Polyalthia longifolia leaf. Evid Based Complement. Alternat. Med. 2013, 2013, 925380. [Google Scholar] [CrossRef] [Green Version]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Faizi, S.; Khan, R.A.; Azher, S.; Khan, S.A.; Tauseef, S.; Ahmad, A. New antimicrobial alkaloids from the roots of Polyalthia longifolia var. pendula. Planta Med. 2003, 69, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Lekphrom, R. Bioactive constituents of the roots of Polyalthia cerasoides. J. Nat. Prod. 2007, 70, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Marthanda Murthy, M.; Subramanyam, M.; Hima Bindu, M.; Annapurna, J. Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 2005, 76, 336–339. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Chand, H.R.; John, J.; Deshpande, M.V. Clerodane type diterpene as a novel antifungal agent from Polyalthia longifolia var. pendula. Eur. J. Med. Chem. 2015, 94, 1–7. [Google Scholar] [CrossRef]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Kantikeaw, I.; Phonkerd, N. 2-substituted furans from the roots of Polyalthia evecta. J. Nat. Prod. 2006, 69, 68–72. [Google Scholar] [CrossRef]
- Li, H.Y.; Sun, N.J.; Kashiwada, Y.; Sun, L.; Snider, J.V.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents, 9. Suberosol, a new C31 lanostane-type triterpene and anti-HIV principle from Polyalthia suberosa. J. Nat. Prod. 1993, 56, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.; Sashidhara, K.V.; Singh, S.P.; Kumar, A.; Gupta, R.; Chaudhaery, S.S.; Gupta, S.S.; Majumder, H.K.; Saxena, A.K.; Dube, A. 16alpha-Hydroxycleroda-3,13 (14)Z-dien-15,16-olide from Polyalthia longifolia: A safe and orally active antileishmanial agent. Br. J. Pharmacol. 2010, 159, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Gbedema, S.Y.; Bayor, M.T.; Annan, K.; Wright, C.W. Clerodane diterpenes from Polyalthia longifolia (Sonn) Thw. var. pendula: Potential antimalarial agents for drug resistant Plasmodium falciparum infection. J. Ethnopharmacol. 2015, 169, 176–182. [Google Scholar] [CrossRef]
- Ngantchou, I.; Nyasse, B.; Denier, C.; Blonski, C.; Hannaert, V.; Schneider, B. Antitrypanosomal alkaloids from Polyalthia suaveolens (Annonaceae): Their effects on three selected glycolytic enzymes of Trypanosoma brucei. Bioorganic Med. Chem. Lett. 2010, 20, 3495–3498. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.R.; Hwang, T.L.; Yang, Y.L.; Li, C.E.; Wu, C.C.; Issa, H.H.; Hsieh, W.B.; Wu, Y.C. Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. pendula. Planta Med. 2006, 72, 1344–1347. [Google Scholar] [CrossRef]
- Bermejo, A.; Collado, A.; Barrachina, I.; Marques, P.; El Aouad, N.; Franck, X.; Garibotto, F.; Dacquet, C.; Caignard, D.H.; Suvire, F.D.; et al. Polycerasoidol, a natural prenylated benzopyran with a dual PPARalpha/PPARgamma agonist activity and anti-inflammatory effect. J. Nat. Prod. 2019, 82, 1802–1812. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Vu, T.Y.; Chandi, V.; Polimati, H.; Tatipamula, V.B. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci. Rep. 2020, 10, 15965. [Google Scholar] [CrossRef]
- Olate, V.R.; Pertino, M.W.; Theoduloz, C.; Yesilada, E.; Monsalve, F.; Gonzalez, P.; Droguett, D.; Richomme, P.; Hadi, A.H.; Schmeda-Hirschmann, G. New gastroprotective labdeneamides from (4S,9R,10R) methyl 18-carboxy-labda-8,13(E)-diene-15-oate. Planta Med. 2012, 78, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Duh, C.Y.; Wang, S.K.; Chen, K.S.; Yang, T.H. Two new natural azafluorene alkaloids and a cytotoxic aporphine alkaloid from Polyalthia longifolia. J. Nat. Prod. 1990, 53, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.Y.; Guo, K.Y.; Lv, D.J.; Mei, R.Q.; Zhang, M.D. Terpenes isolated from Polyalthia simiarum and their cytotoxic activities. Fitoterapia 2020, 147, 104734. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lee, I.S.; Chai, H.B.; Zaw, K.; Farnsworth, N.R.; Soejarto, D.D.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic clerodane diterpenes from Polyalthia barnesii. Phytochem 1994, 37, 1659–1662. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Sivalingam, K.S.; Viswanadha, V.P.; Weng, C.F. 16-hydroxy-cleroda-3,13-dien-16,15-olide induced glioma cell autophagy via ROS generation and activation of p38 MAPK and ERK-1/2. Environ. Toxicol. Pharmacol. 2016, 45, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, S.O.; Olorundare, O.E.; Babatunde, A.; Albrecht, R.M.; Koketsu, M.; Syed, D.N.; Mukhtar, H. Polyalthia longifolia extract triggers ER stress in prostate cancer cells concomitant with induction of apoptosis: Insights from in vitro and in vivo studies. Oxid. Med. Cell Longev. 2019, 2019, 6726312. [Google Scholar] [CrossRef]
- Cheng, M.F.; Lin, S.R.; Tseng, F.J.; Huang, Y.C.; Tsai, M.J.; Fu, Y.S.; Weng, C.F. The autophagic inhibition oral squamous cell carcinoma cancer growth of 16-hydroxy-cleroda-3,14-dine-15,16-olide. Oncotarget 2017, 8, 78379–78396. [Google Scholar] [CrossRef] [Green Version]
- Paarakh, P.M.; Khosa, R. Phytoconstituents from the genus Polyalthia—A review. J. Pharm. Res. 2009, 2, 594–605. [Google Scholar]
- Yao, L.J.; Jalil, J.; Attiq, A.; Hui, C.C.; Zakaria, N.A. The medicinal uses, toxicities and anti-inflammatory activity of Polyalthia species (Annonaceae). J. Ethnopharmacol. 2019, 229, 303–325. [Google Scholar] [CrossRef]
- Wang, T.S.; Luo, Y.P.; Wang, J.; He, M.X.; Zhong, M.G.; Li, Y.; Song, X.P. (+)-rumphiin and polyalthurea, new compounds from the stems of Polyalthia rumphii. Nat. Prod. Commun. 2013, 8, 1427–1429. [Google Scholar] [CrossRef] [Green Version]
- Faizi, S.; Mughal, N.R.; Khan, R.A.; Khan, S.A.; Ahmad, A.; Bibi, N.; Ahmed, S.A. Evaluation of the antimicrobial property of Polyalthia longifolia var. pendula: Isolation of a lactone as the active antibacterial agent from the ethanol extract of the stem. Phytother. Res. 2003, 17, 1177–1181. [Google Scholar] [CrossRef]
- Rai, A.K.; Singh, S.P.; Pandey, A.R.; Ansari, A.; Ahmad, S.; Sashidhara, K.V.; Tamrakar, A.K. Flavonoids from Polyalthia longifolia prevents advanced glycation end products formation and protein oxidation aligned with fructose-induced protein glycation. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Doshi, G.M.; Nalawade, V.V.; Mukadam, A.S.; Chaskar, P.K.; Zine, S.P.; Somani, R.R.; Une, H.D. Elucidation of flavonoids from Carissa congesta, Polyalthia longifolia, and Benincasa hispida plant extracts by hyphenated technique of liquid chromatography-mass spectroscopy. Pharmacogn. Res. 2016, 8, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Thiyagarajan, V.; Lin, S.X.; Lee, C.H.; Weng, C.F. A focal adhesion kinase inhibitor 16-hydroxy-cleroda-3,13-dien-16,15-olide incorporated into enteric-coated nanoparticles for controlled anti-glioma drug delivery. Colloids Surf. B 2016, 141, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lee, W.C.; Huang, B.M.; Chia, Y.C.; Chen, Y.C.; Chen, Y.C. 16-Hydroxycleroda-3, 13-dien-15, 16-olide inhibits the proliferation and induces mitochondrial-dependent apoptosis through Akt, mTOR, and MEK-ERK pathways in human renal carcinoma cells. Phytomedicine 2017, 36, 95–107. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lee, C.C.; Chan, W.L.; Chang, W.H.; Wu, Y.C.; Chang, J.G. 16-Hydroxycleroda-3,13-dien-15,16-olide deregulates PI3K and Aurora B activities that involve in cancer cell apoptosis. Toxicology 2011, 285, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Srivastava, A.; Puri, A.; Chhonker, Y.S.; Bhatta, R.S.; Shah, P.; Siddiqi, M.I. Discovery of a new class of HMG-CoA reductase inhibitor from Polyalthia longifolia as potential lipid lowering agent. Eur. J. Med. Chem. 2011, 46, 5206–5211. [Google Scholar] [CrossRef]
- Beg, M.; Shankar, K.; Varshney, S.; Rajan, S.; Singh, S.P.; Jagdale, P.; Puri, A.; Chaudhari, B.P.; Sashidhara, K.V.; Gaikwad, A.N. A clerodane diterpene inhibit adipogenesis by cell cycle arrest and ameliorate obesity in C57BL/6 mice. Mol. Cell Endocrinol. 2015, 399, 373–385. [Google Scholar] [CrossRef]
- Gupta, V.K.; Tiwari, N.; Gupta, P.; Verma, S.; Pal, A.; Srivastava, S.K.; Darokar, M.P. A clerodane diterpene from Polyalthia longifolia as a modifying agent of the resistance of methicillin resistant Staphylococcus aureus. Phytomedicine 2016, 23, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Verma, S.; Pal, A.; Srivastava, S.K.; Srivastava, P.K.; Darokar, M.P. In vivo efficacy and synergistic interaction of 16alpha-hydroxycleroda-3, 13 (14) Z-dien-15, 16-olide, a clerodane diterpene from Polyalthia longifolia against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2013, 97, 9121–9131. [Google Scholar] [CrossRef]
- Chanda, S.; Baravalia, Y.; Kaneria, M. Protective effect of Polyalthia longifolia var. pendula leaves on ethanol and ethanol/HCl induced ulcer in rats and its antimicrobial potency. Asian Pac. J. Trop Med. 2011, 4, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.K.; Lin, S.R.; Riyaphan, J.; Fu, Y.S.; Weng, C.F. Polyalthia clerodane diterpene potentiates hypoglycemia via inhibition of dipeptidyl peptidase 4. Int. J. Mol. Sci. 2019, 20, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Wang, P.Y.; Huang, B.M.; Chen, Y.J.; Lee, W.C.; Chen, Y.C. 16-Hydroxycleroda-3,13-dien-15,16-olide induces apoptosis in human bladder cancer cells through cell cycle arrest, mitochondria ROS overproduction, and inactivation of EGFR-related signalling pathways. Molecules 2020, 25, 3958. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Lin, S.H.; Chia, Y.C.; Weng, C.F. A novel inhibitor, 16-hydroxy-cleroda-3,13-dien-16,15-olide, blocks the autophosphorylation site of focal adhesion kinase (Y397) by molecular docking. Biochim. Biophys. Acta 2013, 1830, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Srivastava, A.; Puri, A. Identification of the antioxidant principles of Polyalthia longifolia var. pendula using TEAC assay. Nat. Prod. Res. 2011, 25, 918–926. [Google Scholar] [CrossRef]
- Jothy, S.L.; Aziz, A.; Chen, Y.; Sasidharan, S. Antioxidant activity and hepatoprotective potential of Polyalthia longifolia and Cassia spectabilis leaves against paracetamol-induced liver Injury. Evid. Based Complement. Alternat. Med. 2012, 2012, 561284. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.X.; Liang, G.; Chai, W.M.; Feng, H.L.; Zhou, H.T.; Shi, Y.; Chen, Q.X. Antioxidant and antityrosinase proanthocyanidins from Polyalthia longifolia leaves. J. Biosci. Bioeng. 2014, 118, 583–587. [Google Scholar] [CrossRef]
- Kabir, S.; Rahman, M.S.; Chowdhury, A.M.; Hasan, C.M.; Rashid, M.A. An unusual bisnor-clerodane diterpenoid from Polyalthia simiarum. Nat. Prod. Commun. 2010, 5, 1543–1546. [Google Scholar] [CrossRef] [Green Version]
- Manjula, S.N.; Kenganora, M.; Parihar, V.K.; Kumar, S.; Nayak, P.G.; Kumar, N.; Ranganath Pai, K.S.; Rao, C.M. Antitumor and antioxidant activity of Polyalthia longifolia stem bark ethanol extract. Pharm. Biol. 2010, 48, 690–696. [Google Scholar] [CrossRef] [Green Version]
- Oyeyemi, A.O.; Oseni, O.A.; Babatunde, A.O.; Molehin, O.R. Modulatory effect of Polyalthia longifolia leaves against cadmium-induced oxidative stress and hepatotoxicity in rats. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Shih, Y.T.; Hsu, Y.Y.; Chang, F.R.; Wu, Y.C.; Lo, Y.C. 6-Hydroxycleroda-3,13-dien-15,16-olide protects neuronal cells from lipopolysaccharide-induced neurotoxicity through the inhibition of microglia-mediated inflammation. Planta Med. 2010, 76, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Cooke, J.P. Inflammation and its role in regeneration and repair. Circ. Res. 2019, 124, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Cheng, Y.Y.; Chen, C.J.; Ng, L.T.; Chou, L.C.; Huang, L.J.; Chen, Y.H.; Kuo, S.C.; El-Shazly, M.; Wu, Y.C.; et al. Three new clerodane diterpenes from Polyalthia longifolia var. pendula. Molecules 2014, 19, 2049–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, J.R.; Wu, T.Y.; Thang, T.D.; Hwang, T.L.; Wu, C.C.; Cheng, Y.B.; Chiang, M.Y.; Lan, Y.H.; El-Shazly, M.; Wu, S.L.; et al. Bioactive 6S-styryllactone constituents of Polyalthia parviflora. J. Nat. Prod. 2014, 77, 2626–2632. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobinski, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res. 2019, 68, 443–458. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and development of PPAR modulators in health and disease: An update of clinical evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, R.R.; Lincoff, A.M.; Mudaliar, S.; Rabbia, M.; Chognot, C.; Herz, M. Effect of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): A phase II, randomised, dose-ranging study. Lancet 2009, 374, 126–135. [Google Scholar] [CrossRef]
- Kalliora, C.; Kyriazis, I.D.; Oka, S.I.; Lieu, M.J.; Yue, Y.; Area-Gomez, E.; Pol, C.J.; Tian, Y.; Mizushima, W.; Chin, A.; et al. Dual peroxisome-proliferator-activated-receptor-alpha/gamma activation inhibits SIRT1-PGC1alpha axis and causes cardiac dysfunction. JCI Insight 2019, 4, e129556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadavid, A.P. Aspirin: The mechanism of action revisited in the context of pregnancy Complications. Front. Immunol. 2017, 8, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annan, K.; Dickson, R.; Sarpong, K.; Asare, C.; Amponsah, K.; Woode, E. Antipyretic activity of Polyalthia longifolia Benth. & Hook. F. var. pendula (Annonaceae), on lipopolysaccharide-induced fever in rats. J. Med. Biomed. Sci. 2013, 2, 8–12. [Google Scholar]
- Zheng, J.H.; Lin, S.R.; Tseng, F.J.; Tsai, M.J.; Lue, S.I.; Chia, Y.C.; Woon, M.; Fu, Y.S.; Weng, C.F. Clerodane diterpene ameliorates inflammatory bowel disease and potentiates cell apoptosis of colorectal cancer. Biomolecules 2019, 9, 762. [Google Scholar] [CrossRef] [Green Version]
- Shono, T.; Ishikawa, N.; Toume, K.; Arai, M.A.; Masu, H.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Cerasoidine, a bis-aporphine alkaloid isolated from Polyalthia cerasoides during screening for Wnt signal inhibitors. J. Nat. Prod. 2016, 79, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Q.; Chen, R.; Yu, D. Aporphine alkaloids from branches and leaves of Polyalthia nemoralis. Zhongguo Zhong Yao Za Zhi 2009, 34, 2343–2345. [Google Scholar]
- Pumsalid, K.; Thaisuchat, H.; Loetchutinat, C.; Nuntasaen, N.; Meepowpan, P.; Pompimon, W. A new azafluorenone from the roots of Polyalthia cerasoides and its biological activity. Nat. Prod. Commun. 2010, 5, 1931–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banjerdpongchai, R.; Khaw-On, P.; Ristee, C.; Pompimon, W. 6,8-dihydroxy-7-methoxy-1-methyl-azafluorenone induces caspase-8- and -9-mediated apoptosis in human cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 2637–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Wang, L.; Chen, G.; Han, C.; Wang, J. Isolation and crystal structure of marcanine A from Polyalthia plagioneura. Molecules 2010, 15, 6349–6356. [Google Scholar] [CrossRef] [Green Version]
- Panthama, N.; Kanokmedhakul, S.; Kanokmedhakul, K. Polyacetylenes from the roots of Polyalthia debilis. J. Nat. Prod. 2010, 73, 1366–1369. [Google Scholar] [CrossRef]
- Boonpangrak, S.; Cherdtrakulkiat, R.; Pingaew, R.; Manam, P.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and cytotoxic acetogenin from Polyalthia debilis. J. Appl. Pharm. Sci. 2015, 5, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Tuchinda, P.; Pohmakotr, M.; Reutrakul, V.; Thanyachareon, W.; Sophasan, S.; Yoosook, C.; Santisuk, T.; Pezzuto, J.M. 2-substituted furans from Polyalthia suberosa. Planta Med. 2001, 67, 572–575. [Google Scholar] [CrossRef]
- Wu, L.J.; Zheng, C.J.; Wang, L.K.; Han, C.R.; Song, X.P.; Chen, G.Y.; Zhou, X.M.; Wu, S.Y.; Li, X.B.; Bai, M.; et al. One new berberine from the branches and leaves of Polyalthia obliqua Hook.f. & Thomson. Nat. Prod. Res. 2016, 30, 2285–2290. [Google Scholar]
- Yu, Z.X.; Zhuo, M.Y.; Li, X.B.; Fu, Y.H.; Chen, G.Y.; Song, X.P.; Han, C.R.; Song, X.M.; Fan, Q.J. A new norsesquiterpene from the roots of Polyalthia laui. Nat. Prod. Res. 2017, 31, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Kant, R.; Maulik, P.R.; Sarkar, J.; Kanojiya, S.; Ravi Kumar, K. Cytotoxic cycloartane triterpene and rare isomeric bisclerodane diterpenes from the leaves of Polyalthia longifolia var. pendula. Bioorganic Med. Chem. Lett. 2010, 20, 5767–6571. [Google Scholar] [CrossRef]
- Afolabi, S.; Olorundare, O.; Ninomiya, M.; Babatunde, A.; Mukhtar, H.; Koketsu, M. Comparative antileukemic activity of a tetranorditerpene isolated from Polyalthia longifolia leaves and the derivative against human leukemia HL-60 cells. J. Oleo Sci. 2017, 66, 1169–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faizi, S.; Khan, R.A.; Mughal, N.R.; Malik, M.S.; Sajjadi, K.E.; Ahmad, A. Antimicrobial activity of various parts of Polyalthia longifolia var. pendula: Isolation of active principles from the leaves and the berries. Phytother. Res. 2008, 22, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.P.; Ninomiya, M.; Efdi, M.; Santoni, A.; Ibrahim, S.; Tanaka, K.; Koketsu, M. Clerodane diterpenes isolated from Polyalthia longifolia induce apoptosis in human leukemia HL-60 cells. J. Oleo Sci. 2013, 62, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.X.; Jung, J.H.; Smith, D.L.; Wood, K.V.; McLaughlin, J.L. Cytotoxic clerodane diterpenes from Polyalthia longifolia. Planta Med. 1991, 57, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Peng, W.; Yang, B.; Zhang, J.; Chen, Y. A new sesquiterpenoid from Polyalthia petelotii. Nat. Prod. Res. 2016, 30, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Shukla, P.K. Antimicrobial evaluation of clerodane diterpenes from Polyalthia longifolia var. pendula. Nat. Prod. Commun. 2009, 4, 327–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sashidhara, K.V.; Singh, S.P.; Sarkar, J.; Sinha, S. Cytotoxic clerodane diterpenoids from the leaves of Polyalthia longifolia. Nat. Prod. Res. 2010, 24, 1687–1694. [Google Scholar] [CrossRef]
- Yu, Z.X.; Fu, Y.H.; Chen, G.Y.; Song, X.P.; Han, C.R.; Li, X.B.; Song, X.M.; Wu, A.Z.; Chen, S.C. New clerodane diterpenoids from the roots of Polyalthia laui. Fitoterapia 2016, 111, 36–41. [Google Scholar] [CrossRef]
- Saepou, S.; Pohmakotr, M.; Reutrakul, V.; Yoosook, C.; Kasisit, J.; Napaswad, C.; Tuchinda, P. Anti-HIV-1 diterpenoids from leaves and twigs of Polyalthia sclerophylla. Planta Med. 2010, 76, 721–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.H.; Ji, M.H.; Shu, H.M.; Chen, G.Y.; Song, X.P.; Wang, J. Chemical constituents from the roots of Polyalthia obliqua. Chin. J. Nat. Med. 2012, 10, 303–306. [Google Scholar] [CrossRef]
- Suedee, A.; Mondranondra, I.O.; Kijjoa, A.; Pinto, M.; Nazareth, N.; Nascimento, M.S.; Silva, A.M.S.; Herz, W. Constituents of Polyalthia jucunda and their cytotoxic effect on human cancer cell lines. Pharm. Biol. 2007, 45, 575–579. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.K.; Zheng, C.J.; Li, X.B.; Chen, G.Y.; Han, C.R.; Chen, W.H.; Song, X.P. Two new lanostane triterpenoids from the branches and leaves of Polyalthia oblique. Molecules 2014, 19, 7621–7768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuchinda, P.; Munyoo, B.; Pohmakotr, M.; Thinapong, P.; Sophasan, S.; Santisuk, T.; Reutrakul, V. Cytotoxic styryl-lactones from the leaves and twigs of Polyalthia crassa. J. Nat. Prod. 2006, 69, 1728–1733. [Google Scholar] [CrossRef]
- Ravikumar, Y.S.; Mahadevan, K.M.; Manjunatha, H.; Satyanarayana, N.D. Antiproliferative, apoptotic and antimutagenic activity of isolated compounds from Polyalthia cerasoides seeds. Phytomedicine 2010, 17, 513–518. [Google Scholar] [CrossRef]
- Rupachandra, S.; Sarada, D.V. Anti-proliferative and apoptotic properties of a peptide from the seeds of Polyalthia longifolia against human cancer cell lines. Indian J. Biochem. Biophys. 2014, 51, 127–134. [Google Scholar] [PubMed]
- Lin, Y.H.; Lee, C.C.; Chang, F.R.; Chang, W.H.; Wu, Y.C.; Chang, J.G. 16-hydroxycleroda-3,13-dien-15,16-olide regulates the expression of histone-modifying enzymes PRC2 complex and induces apoptosis in CML K562 cells. Life Sci. 2011, 89, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Huang, B.M.; Lee, W.C.; Chen, Y.C. 16-Hydroxycleroda-3,13-dien-15,16-olide induces anoikis in human renal cell carcinoma cells: Involvement of focal adhesion disassembly and signaling. Oncol. Targets Ther. 2018, 11, 7679–7690. [Google Scholar] [CrossRef] [Green Version]
- Jasek-Gajda, E.; Jurkowska, H.; Jasinska, M.; Lis, G.J. Targeting the MAPK/ERK and PI3K/AKT signaling pathways affects NRF2, Trx and GSH antioxidant systems in leukemia cells. Antioxidants (Basel) 2020, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Lopez, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Nikulenkov, F.; Zawacka-Pankau, J.; Li, H.; Gabdoulline, R.; Xu, J.; Eriksson, S.; Hedstrom, E.; Issaeva, N.; Kel, A.; et al. ROS-dependent activation of JNK converts p53 into an efficient inhibitor of oncogenes leading to robust apoptosis. Cell Death Differ. 2014, 21, 612–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Wang, X.; Gu, C.; Zhang, H.; Zhang, R.; Dong, X.; Liu, C.; Hu, X.; Ji, X.; Huang, S.; et al. Celastrol ameliorates Cd-induced neuronal apoptosis by targeting NOX2-derived ROS-dependent PP5-JNK signaling pathway. J. Neurochem. 2017, 141, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Wu, T.; Li, B.; Liu, T.; Wang, R.; Liu, Q.; Liu, Z.; Gong, Y.; Shao, C. Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci. Rep. 2015, 5, 12579. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Shanmugapriya; Vijayarathna, S.; Sasidharan, S. Functional Validation of DownRegulated MicroRNAs in HeLa Cells Treated with Polyalthia longifolia Leaf Extract Using Different Microscopic Approaches: A Morphological Alteration-Based Validation. Microsc. Microanal. 2019, 25, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya; Sasidharan, S. Functional analysis of down-regulated miRNA-221-5p in HeLa cell treated with polyphenol-rich Polyalthia longifolia as regulators of apoptotic HeLa cell death. 3 Biotech 2020, 10, 206. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Longo, J.; van Leeuwen, J.E.; Elbaz, M.; Branchard, E.; Penn, L.Z. Statins as anticancer agents in the era of precision medicine. Clin. Cancer Res. 2020, 26, 5791–5800. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.K.; Wang, P.C.; Weng, C.F. 16-Hydroxycleroda-3,13-dien-15,16-olide and N-methyl-actinodaphine potentiate tamoxifen-induced cell death in breast cancer. Molecules 2018, 23, 1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.S.; Rafi, K.; Faizi, S.; Razzak, Z.A.; Simjee, S.U. A novel, semi-synthetic diterpenoid 16(R and S)-phenylamino-cleroda-3,13(14), Z-dien-15,16 olide (PGEA-AN) inhibits the growth and cell survival of human neuroblastoma cell line SH-SY5Y by modulating P53 pathway. Mol. Cell Biochem. 2018, 449, 105–115. [Google Scholar] [CrossRef]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Moss, S.F. The clinical evidence linking Helicobacter pylori to gastric cancer. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmond, M.P.; Mostafa, N.M.; El-Shazly, M.; Singab, A.N.B. Two clerodane diterpenes isolated from Polyalthia longifolia leaves: Comparative structural features, anti-histaminic and anti-Helicobacter pylori activities. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]

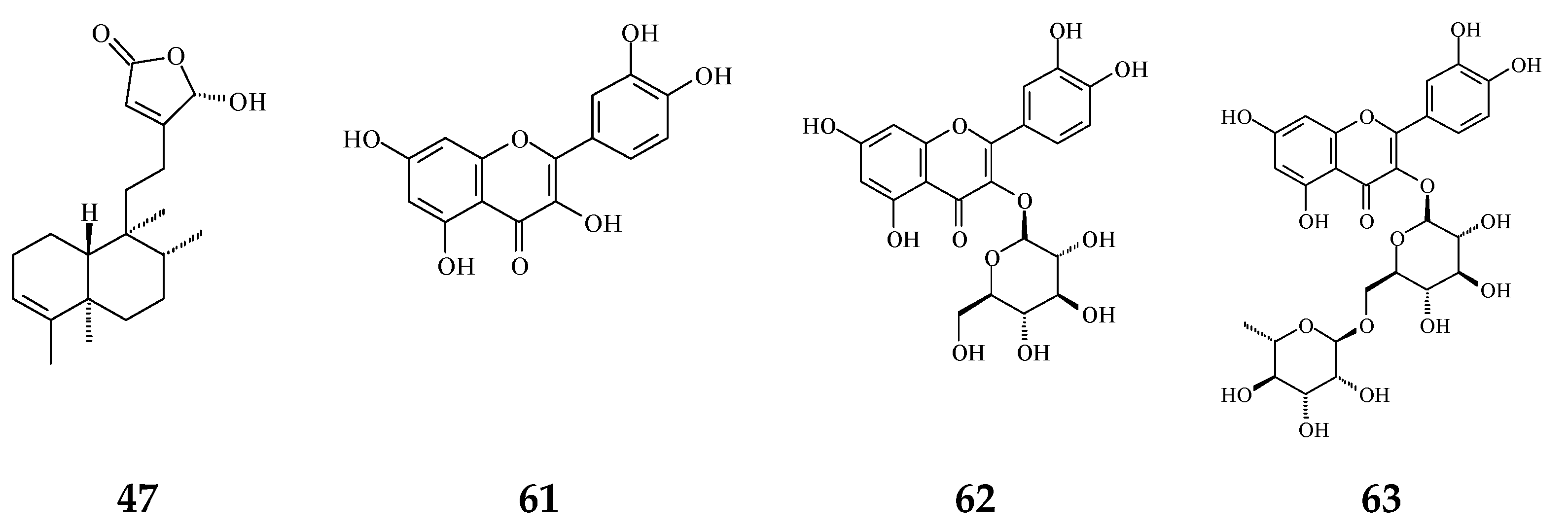
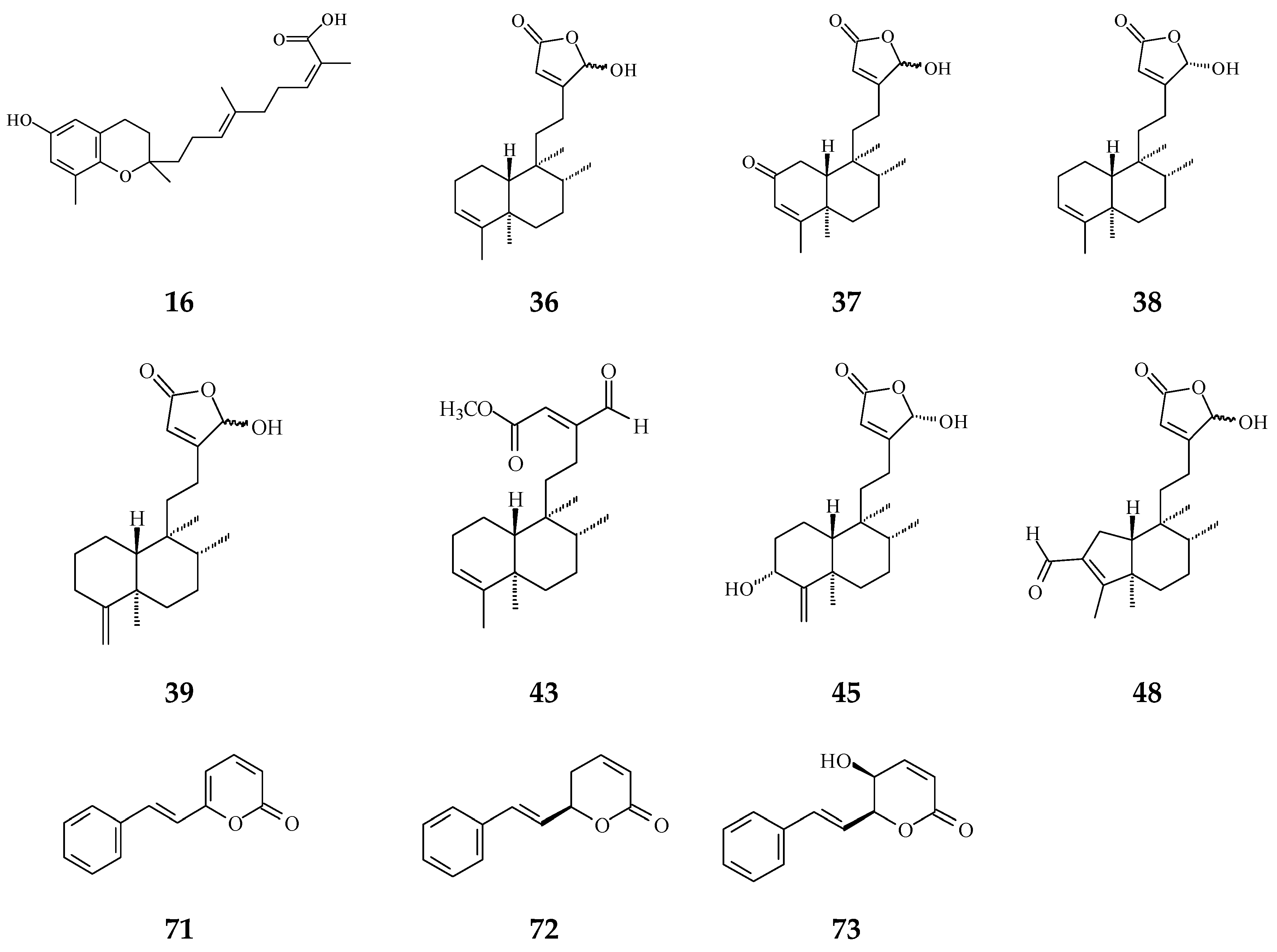
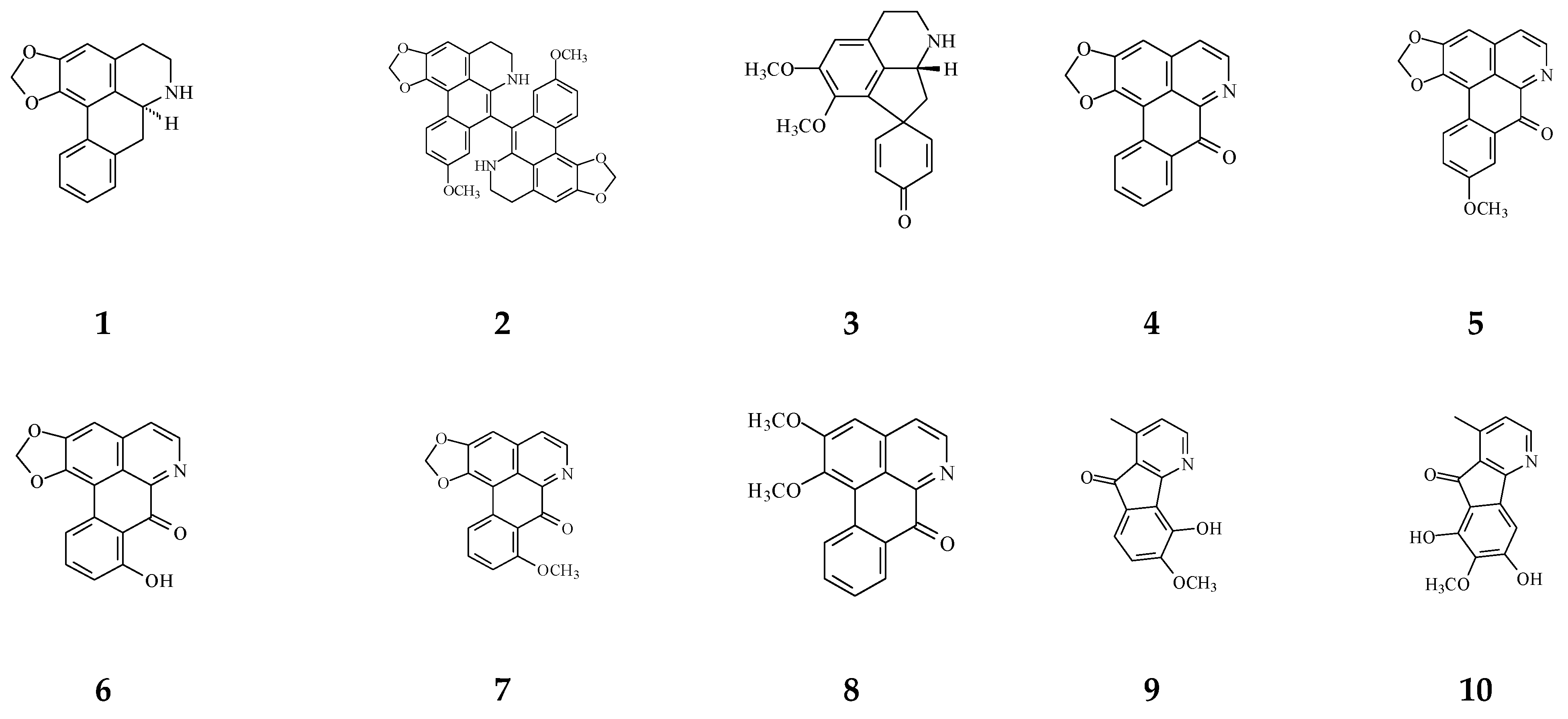
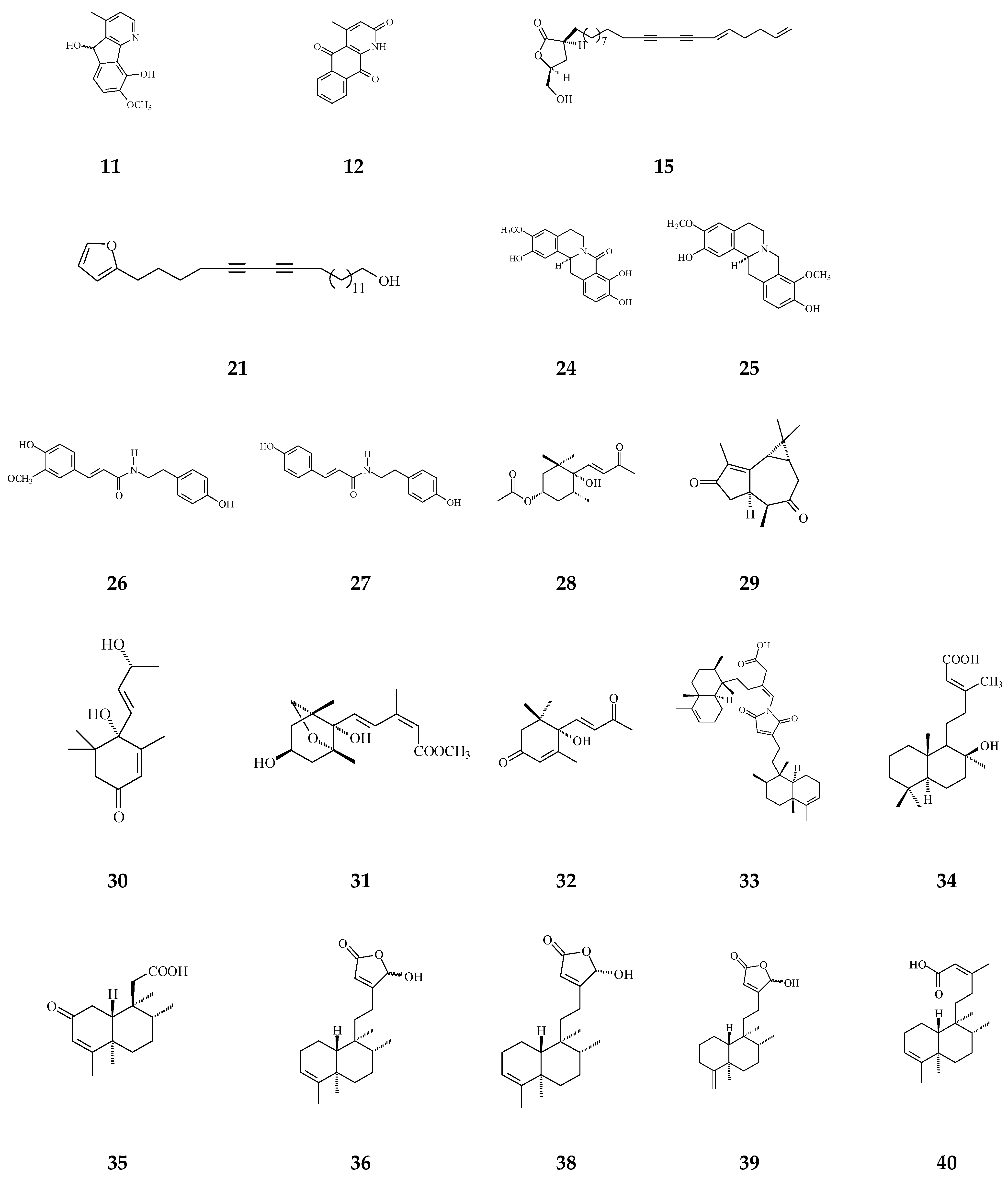
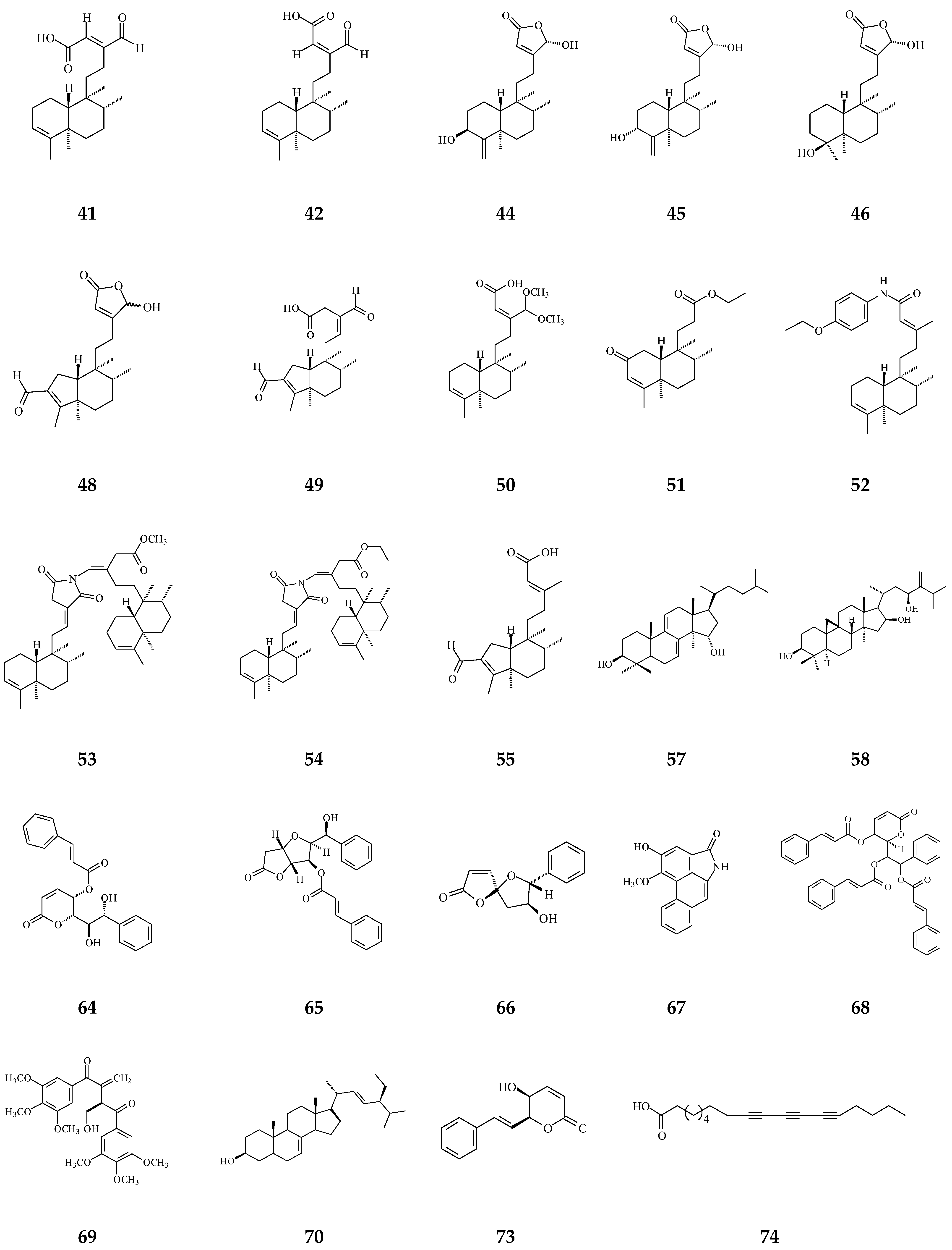
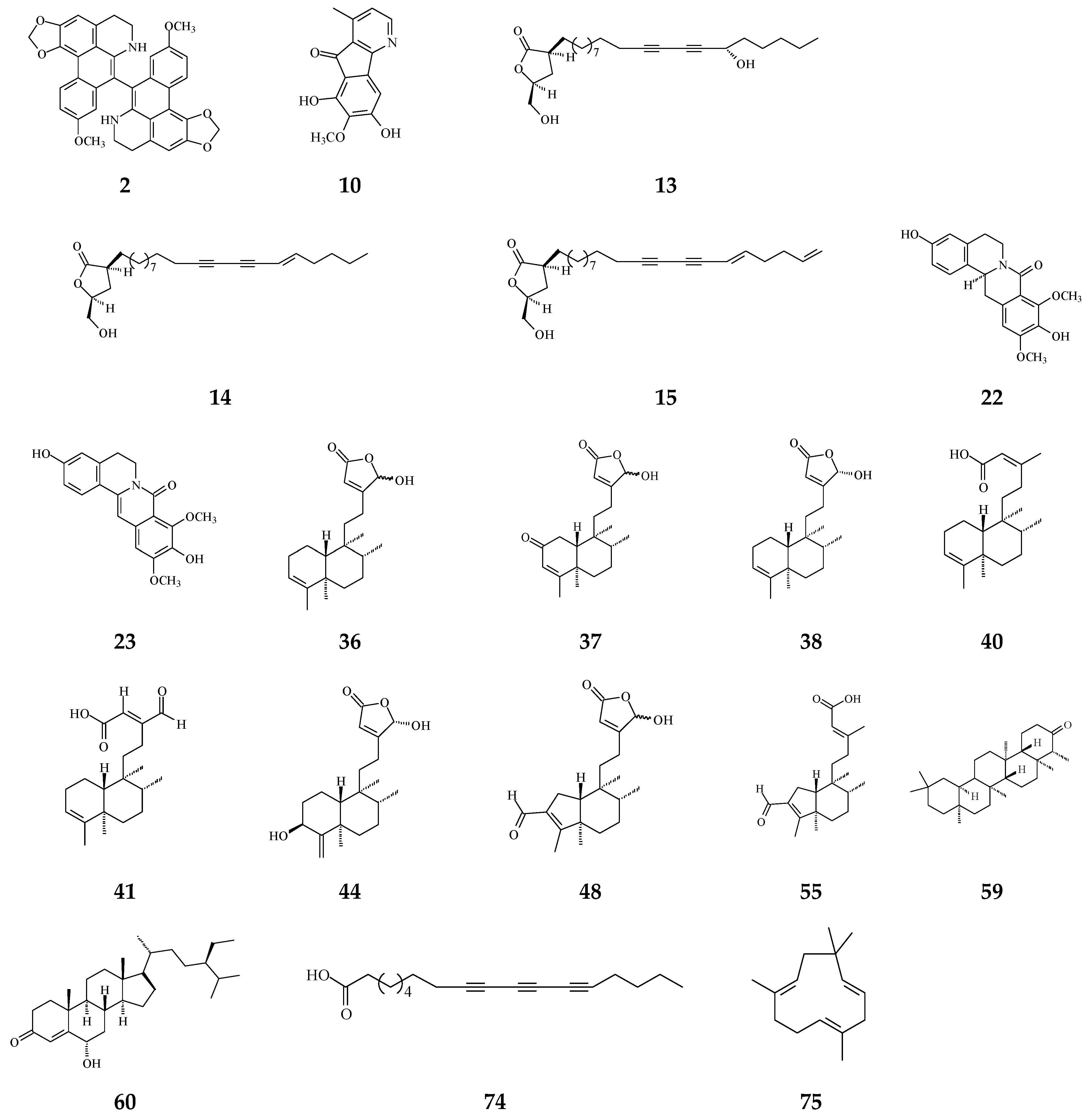
| Category | Name of Compound | Pharmacological Activities Described in References | Concentration | In Vitro/ In Vivo Model |
|---|---|---|---|---|
| IC50/EC50/ | ||||
| Minimal Inhibitory Concentration (MIC) | ||||
| Aporphine | (−)-Anonaine (1) | Cytotoxicity [14] | 8.6–28.9 μM | AGS, DLD1, HA59T, and HepG2 |
| Bidebiline E (2) | Anti-bacterial [19] | 6.25 μg/mL | Mycobacterium tuberculosis | |
| Inhibition of wnt protein [73] | 20.2 μM | SW480 | ||
| Proaporphine | (+)-Stepharine (3) | Cytotoxicity [27] | 9.4–9.9 μg/mL | MCF-7, MDA-MB-231 |
| Oxoaporphine | Liriodenine (4) | Cytotoxicity [27,31,74] | 4.46–10.28 μg/mL | MCF-7, MDA-MB-231 |
| 0.57–2.33 μg/mL | KB, A549, HCT-8, P-388, and L-1210, | |||
| Lanuginosine (oxoxylopine) (5) | Cytotoxicity [74] | 1 μg/mL | Unavalible | |
| Oxostephanosine (6) | Cytotoxicity [74] | 1 μg/mL | Unavalible | |
| Oxostephanine (7) | Cytotoxicity [39] | 1.47–1.73 μg/mL [39] | SPC-A-1 and BEL-7402 | |
| Lysicamine (8) | Cytotoxicity [27] | 8.94–16.75 μg/mL | MCF-7, MDA-MB-231 | |
| Azafluorene | 5-Hydroxy-6-methoxyonychine (isoursuline) (9) | Cytotoxicity [14] | 21.7 μg/mL | HA59T |
| 6,8-Dihydroxy-7-methoxy-1-methyl-azafluorenone (10) | Cytotoxicity [75] | 2.64–3.58 μg/mL | A549, GLC4, and adrinamycin-resistance GLC4 | |
| Apoptosis [76] | 20–55 μM | HL-60, U937, MOLT-4, MDA-MB-231, and HepG2 | ||
| anti-bacterial [75] | 0.78 μg/mL | M. tuberculosis | ||
| Polylongine (11) | Cytotoxicity [27] | 9.94–10.41 μg/mL | MCF-7 and MDA-MB-231 | |
| Anthraquinones | Marcanine A (12) | Cytotoxicity [77] | 1.53–11.78 μM | BEL-7402, K562, SPCA-1, and SGC-7409 |
| Acetogenin | Debilisone B (13) | Anti-bacterial [78] | 25 μg/mL | M. tuberculosis |
| Debilisone C (14) | Anti-bacterial [78] | 12.5 μg/mL | Same as above | |
| Debilisone E (15) | Anti-bacterial [78,79] | 25 μg/mL | M. tuberculosis | |
| 64 μg/mL | Morexella catarrhalis | |||
| Cytotoxicity [79] | 18.4–40.3 μg/mL | HepG2, A549, HCC-S102, HL-60, and P-388 | ||
| Prenylated Benzopyran | Polycerasoidol (16) | Anti-inflammatory [28] | 4.9 μΜ | Inhibition of mononuclear leukocyte adhesion to endothelium |
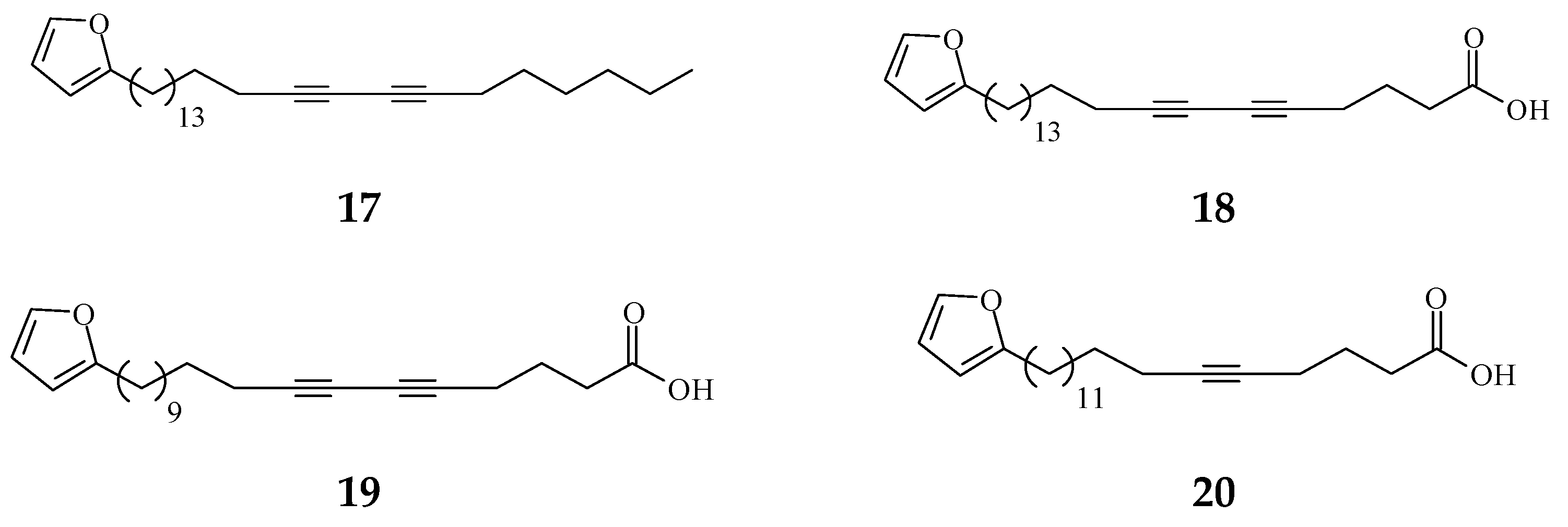
| 1-(2-furyl)pentacosa-16,18-diyne (17) | Anti-viral [80] | 43.3 μg/mL | ΔTat/RevMC99 syncytium assay for human immunodeficiency virus (HIV) | |
| 23-(2-furyl)tricosa-5,7-diynoic acid (18) | Anti-viral [80] | 8.9 μg/mL | Same as above | |
| 2-substituted furans | 19-(2-furyl)nonadeca-5,7-diynoic acid (19) | Anti-viral [22] | 47 μg/mL | Anti-viral assay against herpes simplex type 1 (HSV-1) |
| 19-(2-furyl)nonadeca-5-ynoic acid (20) | Anti-viral [22] | 19.4 μg/mL | Same as above | |
| 21-(2-furyl)heneicosa-14,16-diyne-1-ol (21) | Cytotoxicity [22] | 12.4 μg/mL | NCI-H187 | |
| 8-Oxoprotoberberine | pendulamine A (22) | Anti-bacterial [18] | 0.02 μg/mL | Gram-positive bacteria |
| Corynebacterium hoffmanii and Micrococcus lysodicklycus | ||||
| 0.2 μg/mL | Staphylococcus aureus | |||
| 2 μg/mL | Bacillus subtillis | |||
| 12.5 μg/mL | Streptococcus viridans | |||
| 20 μg/mL | S. pyogenes | |||
| 0.02 μg/mL | Gram-negative bacteria | |||
| Salmonella typhi | ||||
| 0.2 μg/mL | S. paratyphi A | |||
| 2 μg/mL | Klebsiella pneumoniae and Pseudomonas aeruginosa | |||
| pendulamine B (23) | Anti-bacterial [18] | 0.02 μg/mL | Gram-positive bacteria | |
| C. hoffmanii, S. viridans, and M. lysodicklycus | ||||
| 0.2 μg/mL | S. aureus, | |||
| 2 μg/mL | S.s faecalis | |||
| 20 μg/mL | S. pyogenes | |||
| 0.2 μg/mL | Gram-negative bacteria | |||
| S. paratyphi A and S. typhi | ||||
| 2 μg/mL | K. pneumoniae | |||
| (−)-8-oxo-2,9,10-Trihydroxy-3-methoxyberberine | Cytotoxicity [81] | 24.1 μM | MCF-7 | |
| (consanguine B) (24) | 33.5 μM | HeLa | ||
| Tetrahydroprotoberberine | (−)-stepholidine (25) | Cytotoxicity [27] | 16.56 μg/mL | MCF-7 |
| Amides | N-trans-Feruloyltyramine (26) | Cytotoxicity [27] | 21.17-25.54 μg/mL | MCF-7, MDA-MB-231, HepG2, Hep3B |
| N-trans-p-Coumaroyltyramine (27) | Cytotoxicity [27] | 17.35 μg/mL | MCF-7 | |
| Sesquiterpenes | Polyalone A (28) | Cytotoxicity [82] | 18.9–24.8 μΜ | HeLa, A549, MCF-7, and HL-60 |
| 9-Ketocyclocolorenone (29) | Cytotoxicity [82] | 20.5–26.2 μΜ | Same as above | |
| Blumenol A(30) | Cytotoxicity [82] | 24.5–28.2 μΜ | Same as above | |
| (−)-Methyl dihydrophaseate (31) | Cytotoxicity [82] | 22.6–27.1 μΜ | Same as above | |
| Bis-enone (32) | Cytotoxicity [82] | 25.6–30.1 μΜ | Same as above | |
| Diterpenoids | Longimide A (33) | Cytotoxicity [83] | 4.12–10.13 μg/mL | KB, MCF-7, A549, and C33A |
| 44.7 μg/mL | NIH3T3 | |||
| labd-13E-en-8-ol-15-oic acid (34) | Cytotoxicity [27] | 15.4–18.33 μg/mL | HepG2 and Hep3B | |
| 1-naphthaleneacetic-7-oxo-1,2,3,4,4a,7,8,8a-octahydro1,2,4a,5-tetramethyl acid (35) | Cytotoxicity [84] | 50 μM | HL-60 | |
| Clerodane diterpenoids | 16-Hydroxycleroda-3,13Z-dien-15,16-olide (36) | Cytotoxicity, apoptosis, anti-cancer [36,43] | Details are in Table 2 | 786-O, A498, HL-60, T24, C6, N18, Caco-2, K562, MCF-7, MDA-MB-231, GBM8401, |
| SW620, MOLT-4, HepG2, Hep3B, and A549 | ||||
| Anti-inflammatory [27,60,72] | 3.05 ± 1.13 μg/mL | Inhibition of fMLP/CB-induced superoxide anion production by human neutrophils. | ||
| 1–10 μM | LPS-treated RAW264.7 cells | |||
| 1.6–6.4 mg/kg | irritable bowel disease in mouse model (ADM/DSS-induced colitis) | |||
| Anti-bacterial [85] | Gram-negative bacteria | |||
| 125 μg/mL | S. typhi | |||
| 250 μg/mL | P. aeruginosa, K. ozaenae, and Proteus mirabilis | |||
| Gram-positive bacteria | ||||
| 7.8 μg/mL | S. aureus | |||
| 125 μg/mL | S. pyogenes | |||
| 500 μg/mL | C. hoffmanii | |||
| Anti-fungal [85] | 62.5 μg/mL | Aspergillus niger and Trichophyton metagrophyte | ||
| 250 μg/mL | Candida albicans | |||
| 16-Hydroxy-cleroda-3,13(14)Z-dien-15,16-olide-2-one (37) | Anti-bacterial [85] | Gram-positive bacteria | ||
| 15.6 μg/mL | B. subtilis | |||
| Anti-inflammatory [27] | 62.5 μg/mL | C. diphtheriae, C. xerosis, and S. aureus | ||
| 500 μg/mL | C. hoffmanii, and S. pyogenes | |||
| 7.96 ± 1.78 μg/mL | Inhibition of fMLP/CB-induced superoxide anion production by human neutrophils | |||
| 16α-Hydroxycleroda-3,13Z-dien-15,16-olide (38) | Cytotoxicity [14,33,84,86,87,88], | 0.5 μg/mL | P-388 | |
| 1.2 μg/mL | Mel2 | |||
| 3.4–8.7 μg/mL | A431, BC1, Col2, HT, KB, drug-resistance KB, LNCaP, Lu1, and ZR75-1 | |||
| 23.6–26.9 μM | AGS, HA59T | |||
| 21.28–34.89 μM | HT-29 | |||
| A549, MCF-7, HL-60, SMMC-7721, and SW-480 | ||||
| Anti-bacterial [20,48,49,89] | Gram-positive bacteria | |||
| 6.25 μg/mL | S. aureus, Sporothrix schenckii, and Arthrobacter citreus | |||
| 1.56 μg/mL | B. subtillis, B. polymyxa, and B. pumilus | |||
| 3.12 μg/mL | B. cereus, B. licheniformis, and Clostridium sp. | |||
| Gram-negative bacteria | ||||
| 0.78 μg/mL | E. coli, P. aeryginosa, and S. typhimurium | |||
| 1.56 μg/mL | K. aerogenes and Sarcina lutea | |||
| 3.12 μg/mL | P. putida and Nocardia sp. | |||
| Anti-inflammatory [29] | 9.46 ± 0.33 nM | COX1 inhibitory assay | ||
| 10.34 ± 0.26 nM | COX2 inhibitory assay | |||
| 14.38 ± 0.32 nM | 5-LOX inhibitory assay | |||
| 16-Hydroxycleroda-4(18),13 -dien-15,16-olide (39) | Cytotoxicity [27] | 1.97–10.43 μg/mL | MCF-7, MDA-MB-231, HepG2, and Hep3B | |
| Anti-inflammatory [29] | 11.85 ± 0.19 nM | COX1 inhibitory assay | ||
| 8.49 ± 0.55 nM | COX2 inhibitory assay | |||
| 14.38 ± 0.32 nM | 5-LOX inhibitory assay | |||
| Kolavenic acid (40) | cytotoxicity [87] | 1.39–3.34 μg/mL | A549, MCF-7, and HT-29 | |
| Anti-bacterial [85] | 31.25 μg/mL | B. subtilis and C. diphtheriae | ||
| 125 μg/mL | C. hoffmanii and C. xerosis | |||
| 16-Oxocleroda-3,13E-dien-15-oic acid (41) | Cytotoxicity [10] | 3.1–3.7 μM | MCF-7 and A549 | |
| anti-bacterial [85] | Gram-negative bacteria | |||
| 500 μg/mL | P. aeruginosa, S. typhi, K. ozaenae, K. aerogenes, E. coli, sarcina lutea, Nocardia sp., and P. mirabilis | |||
| Gram-positive bacteria | ||||
| 500 μg/mL | A. citreus, B. cereus, B. licheniformis, B. polymyxa, B. pumilus, B. subtilis, Clostridium sp. S. pyogenes, C. hoffmanii, and S. aureus | |||
| Anti-fungal [85] | 62.5 μg/mL | Trichonphyton mentagrophyte | ||
| 125 μg/mL | A. niger | |||
| 250 μg/mL | C. albicans | |||
| 16-Oxocleroda-3,13Z-dien-15-oic acid (polyalthialdoic acid) (42) | Cytotoxicity [87] | 0.552–0.753 μg/mL | A549, MCF-7, and HT-29 | |
| 16-Oxocelroda-3,13(14)E-dien-15-oic acid methyl ester (43) | Anti-inflammatory [27] | 0.6 ± 0.09 μg/mL | Inhibition of fMLP/CB-induced superoxide anion production by human neutrophils | |
| 3β,16α-dihydroxycleroda-4(18),13(14)Z-dien-15,16-olide (44) | Cytotoxicity [33] | 2.2–16μg/mL | P-388, BC1, Col2, LNCaP, Lu1, ZR75-1 | |
| 12–16 μg/mL | Mel2, A431, HT, and KB | |||
| Cytotoxicity [90] | 10.474–24.096 μg/mL | KB, C33A, PA1, MCF-7 | ||
| 18.564 μg/mL | Vero | |||
| anti-bacterial [85] | Gram-positive bacteria | |||
| 62.5 μg/mL | C. diphtheriae, C. xerosis, and S. pyogenes | |||
| 125 μg/mL | S. faecalis and C. hoffmanii | |||
| 250 μg/mL | S. saprophyticus | |||
| 500 μg/mL | B. subtilis | |||
| (−)-3α,16α-dihydroxycleroda-4(18),13(14)Z-dien-15,16-olide (45) | Cytotoxicity [90] | 13.415–29.778 μg/mL | KB, C33A, PA1, and MCF-7 | |
| 20.345 μg/mL | Vero | |||
| Anti-inflammatory [29] | 10.85 ± 0.17 nM | COX1 inhibitory assay | ||
| 12.82 ± 0.21 nM | COX2 inhibitory assay | |||
| 16.94 ± 0.56 nM | 5-LOX inhibitory assay | |||
| 4β,16α-dihydroxycleroda-13(14)Z-en-15,16-olide (46) | Cytotoxicity [33] | 5.1–16 μM | A431. BC1, Col2, HT, LNCaP, Lu1, Mel2, P-388, ZR75-1 | |
| 16β-Hydroxycleroda-3,13(14)Z-dien-15,16-olide (47) | Anti-oxidant [57] | 23.5 μg/mL | DPPH assay | |
| (4→2)-abeo-16(R&S)-2,13Z-clerodadien-15,16-olide-3-al (48) | Cytotoxicity [27] | 2.36–11.89 μg/mL | MCF-7, MDA-MB-231, HepG2, and Hep3B | |
| Anti-bacterial [85] | Gram-positive bacteria | |||
| 31.25 μg/mL | B. subtilis | |||
| 125 μg/mL | C. hoffmanii, C. xerosis, S. saprophyticus, S. faecalis, and S. pyogenes | |||
| Anti-inflammatory [27] | 4.32 ± 0.59 μg/mL | Inhibition of fMLP/CB-induced superoxide anion production by human neutrophils | ||
| (4→2)-abeo-2,13-diformyl-cleroda-2,12E-dien-14-oic acid (49) | Cytotoxicity [91] | 37.35–39.31 μM | HeLa, MCF-7, and A549 | |
| 16,16-dimethoxy-cleroda-3,13Z-dien-15-oic acid (50) | Cytotoxicity [32] | 22.43 μM | SMMC-7721 | |
| Polylauiester A (51) | Cytotoxicity [91] | 33.21–35.65 μM | HeLa, MCF-7, and A549 | |
| Polylauiamide B (52) | Cytotoxicity [91] | 28.09–29.25 μM | Same as above | |
| Polylauiamide C (53) | Cytotoxicity [91] | 25.01–30.30 μM | Same as above | |
| Polylauiamide D (54) | Cytotoxicity [91] | 26.73–28.88 μM | Same as above | |
| solidagonal acid (55) | Cytotoxicity [27] | 14.67–18.12 μg/mL | MCF-7 and MDA-MB-231 | |
| Anti-bacterial [85] | Gram-positive bacteria | |||
| 31.25 μg/mL | B. subtilis, C. hoffmanii, and S. saprophyticus | |||
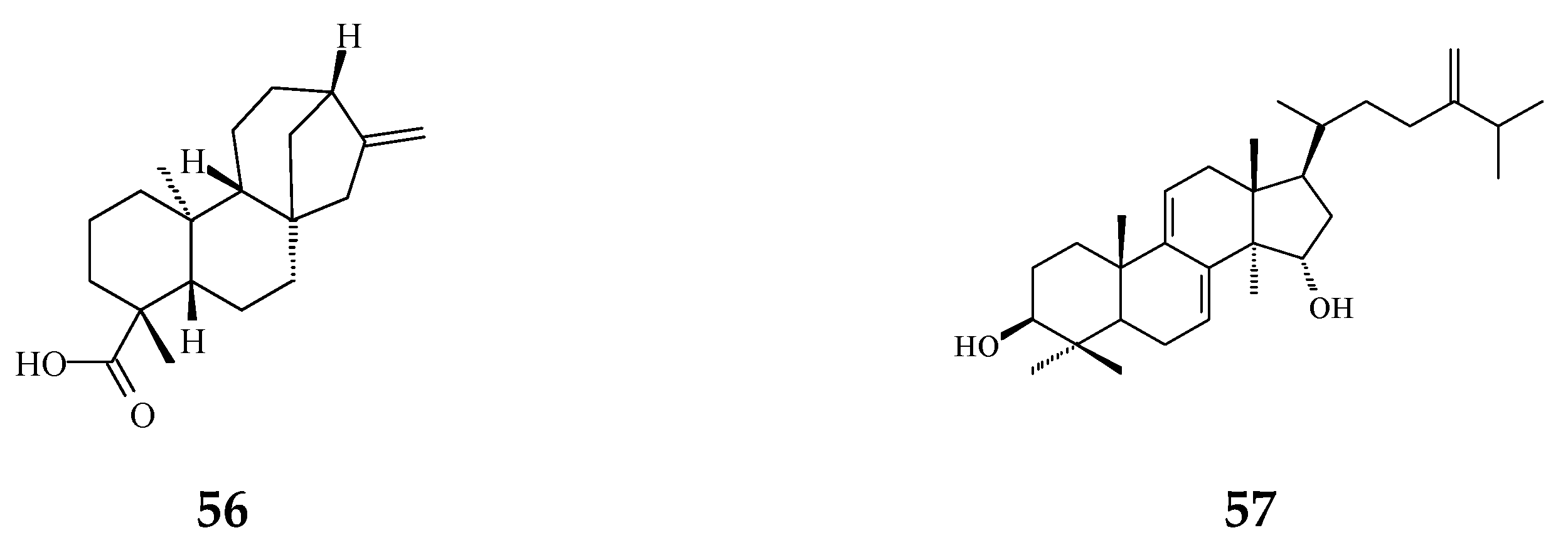
| ENT-kaur-16-en-19-oic acid (56) | Anti-viral [92] | 13.7 μg/mL | Anti-syncytium assay against HIV | |
| Triterpene | Suberosol (57) | Anti-viral [23] | 3 μg/mL | Inhibition of HIV replication in H9 lymphocytes |
| Cytotoxicity [93] | 34.30 μg/mL | SPC-A-1 | ||
| 15.02 μg/mL | SGC-7901 | |||
| 24-Methylenecycloartane-3β, 16β, 23β-triol (longitriol) (58) | Cytotoxicity [83] | 10.03–30.88 μg/mL | KB, MCF-7, A549, C33A | |
| Cytotoxicity [94] | 19.3–23 μM | MDA-MB-231 and SF-268 | ||
| 40.3 μM | MRC-5 | |||
| Apoptosis [94] | 40 μM | NCI-H460 | ||
| Triterpenoids | Friedelin (59) | Anti-bacterial [95] | 5 μg/mL | E. coli and M. tetragenus |
| Stigmast-4-ene-6α-ol-3-one (60) | Anti-bacterial [95] | 5 μg/mL | Same as above | |
| Flavonoids | Quercetin (61) | Anti-oxidant [54] | 1.56 μg/mL | Trolox equivalent antioxidant capacity (TEAC) assay |
| Quercetin-3-O-β-glucopyranoside (62) | Anti-oxidant [54] | 1.56 μg/mL | TEAC assay | |
| Rutin (63) | Anti-oxidant [54] | 1.56 μg/mL | TEAC assay | |
| Others | Crassalactones A (64) | Cytotoxicity [96] | 0.18–1.9 μg/mL | P-388, KB, Col-2, BCA-1, Lu-1, and ASK |
| Crassalactone B (65) | Cytotoxicity [96] | 3.8 μg/mL | P-388 | |
| Crassalactone D (66) | Cytotoxicity [96] | 1.1–4 μg/mL | P-388, KB, Col-2, BCA-1, and ASK | |
| Aristolactam AII (67) | Cytotoxicity [96] | 2.7 μg/mL | P-388 | |
| (+)-Tricinnamate (68) | Cytotoxicity [96] | 3.1 μg/mL | P-388 | |
| (+)-Rumphiin (69) | Cytotoxicity [39] | 63.2–187.6 μg/mL | SPC-A-1 and K562 | |
| α-Spinasterol (70) | Cytotoxicity [97] | 60.07 ± 7.10 nM/ml | Caco-2 | |
| Dehydrogoniothalamin (71) | Anti-inflammatory [65] | 11.6 ± 1.2 μM | Inhibition of fMLP/CB-induced superoxide anion production by human neutrophils | |
| 6.8 ± 0.9 μM | inhibition of elastase release by human neutrophils | |||
| Goniothalamin (72) | Anti-inflammatory [65] | 8.3 ± 1.4 μM | Inhibition of fMLP/CB-induced superoxide anion production by human neutrophils | |
| 15.4 ± 1.1 μM | inhibition of elastase release by human neutrophils | |||
| (−)-5-Hydroxy-goniothalamin (71) | Cytotoxicity [65] | 7.9 μM | A549 | |
| Anti-inflammatory [65] | 8.1 ± 2.3 μM | Inhibition of fMLP/CB-induced superoxide anion production by human neutrophils | ||
| 14.6 ± 0.7 μM | Inhibition of elastase release by human neutrophils | |||
| Octadeca-9,11,13-triynoic acid (72) | Anti-bacterial [19] | 6.25 μg/mL | M. tuberculosis | |
| Cytotoxicity [19] | 13 μg/mL | BC1 | ||
| α-Humulene (73) | Anti-bacterial [19] | 6.25 μg/mL | M. tuberculosis | |
| F2 peptide | Apoptosis [98] | 30 μg/mL | A549 and HeLa |
| Cell Type | Effective Dose | Effects | Effects on Signaling Pathway | Effects on Cell Cycle | References |
|---|---|---|---|---|---|
| Leukemia | |||||
| HL-60 | 10–30 μM | Apoptosis | Aurora B ↓ | [45] | |
| K562 | 10–30 μM | Apoptosis | Caspase-3 and -9 cleavage ↑ Aurora B, pPI3K, pAkt ↓ pJNK ↑ Survival signaling: FoxO3, FoxO4 ↑ Cell-cycle related proteins: p21 ↑ Cyclin A, cyclin B, CDK1, CDK2 ↓ | G2/M phase arrest | [99] |
| PRC2 complex: EZH2, Suz12 ↓ | |||||
| Head and neck cancer | |||||
| OECM1, SAS | 10–50 μM | Autophagy | LC3-II and beclin-1 ↑ Cyclin D1 ↓ | G0/G1 phase arrest (SAS cells) | [36] |
| Glioma | |||||
| N18, C6 | 3–10 μM | Autophagy | p-p38 MAPK, pERK1/2↑ | G0/G1 phase arrest | [34] |
| Apoptosis | Bad, Bax, and p53 ↑ ROS overproduction Inhibition of SOD, GSH, GST, GPx activities | ||||
| Colon cancer | Anti-migration | Rac1, cdc42, pFAK, and FAK ↓ | [53] | ||
| Caco-2 | 2.30 μM (48 h) | Apoptosis | cleavage of caspase-3, -8, and -9 ↑ Inhibition of growth factor-related signalling: Akt, PCNA ↓ cell cycle related proteins: p21 and p53 ↑ Inflammatory signalling: COX2, NF-κB ↓ | G2/M phase arrest | [72] |
| RCC | 10–40 μM | Anti-migration Anti-invasion anoikis | pFAK, FAK, pSrc, paxillin ↓, vimentin, vinculin, pNF-kB ↓, MMP2, MMP9, VEGF ↓ | G2/M phase arrest | [100] |
| 786-O, A-498 | |||||
| 10–40 μM | Apoptosis | pMEK1/2, pERK1/2, pAkt, pmTOR ↓ ROS overproduction, Cytochrome c release Caspase-3, PARP-1 cleavage ↑ cMyc, HIF-2α, HSP70, Bcl-2 ↓ Cyclin B1, cyclin D1, cyclin E, CDK1↓, CDK2, CDK4 ↓ FoxO3a, p21, p53 ↑ | [44] | ||
| Bladder cancer | 10–40 μM | Apoptosis | cyclin D1, CDK2 and CDK4 ↓ Increase of p21, p27Kip1 and p53 ↑ Caspase-3, PARP-1 cleavage, pH2A.X ↑ Cytochrome c release, ROS overproduction, Bcl-2↓ pEGFR, pMEK, pERK1/2, pAkt↓, pmTOR, p-P70S6K ↓ HIF-1α, cMyc, VEGF ↓ | G0/G1 phase arrest | [52] |
| T24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chia, Y.-C.; Huang, B.-M. Phytochemicals from Polyalthia Species: Potential and Implication on Anti-Oxidant, Anti-Inflammatory, Anti-Cancer, and Chemoprevention Activities. Molecules 2021, 26, 5369. https://doi.org/10.3390/molecules26175369
Chen Y-C, Chia Y-C, Huang B-M. Phytochemicals from Polyalthia Species: Potential and Implication on Anti-Oxidant, Anti-Inflammatory, Anti-Cancer, and Chemoprevention Activities. Molecules. 2021; 26(17):5369. https://doi.org/10.3390/molecules26175369
Chicago/Turabian StyleChen, Yung-Chia, Yi-Chen Chia, and Bu-Miin Huang. 2021. "Phytochemicals from Polyalthia Species: Potential and Implication on Anti-Oxidant, Anti-Inflammatory, Anti-Cancer, and Chemoprevention Activities" Molecules 26, no. 17: 5369. https://doi.org/10.3390/molecules26175369
APA StyleChen, Y.-C., Chia, Y.-C., & Huang, B.-M. (2021). Phytochemicals from Polyalthia Species: Potential and Implication on Anti-Oxidant, Anti-Inflammatory, Anti-Cancer, and Chemoprevention Activities. Molecules, 26(17), 5369. https://doi.org/10.3390/molecules26175369






