Scarlet Flax Linum grandiflorum (L.) In Vitro Cultures as a New Source of Antioxidant and Anti-Inflammatory Lignans
Abstract
1. Introduction
2. Results
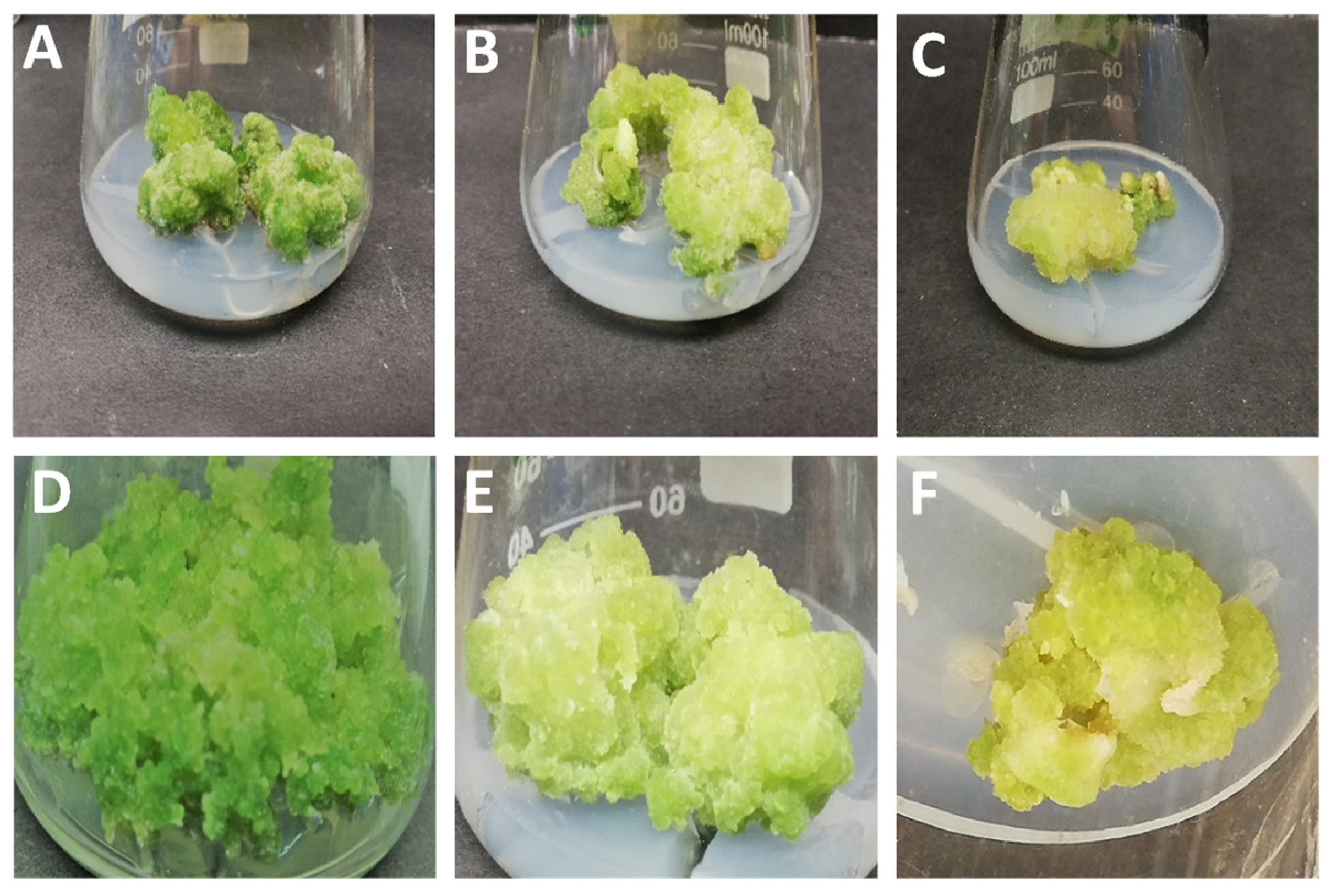
2.1. Callus Induction and Morphogenesis
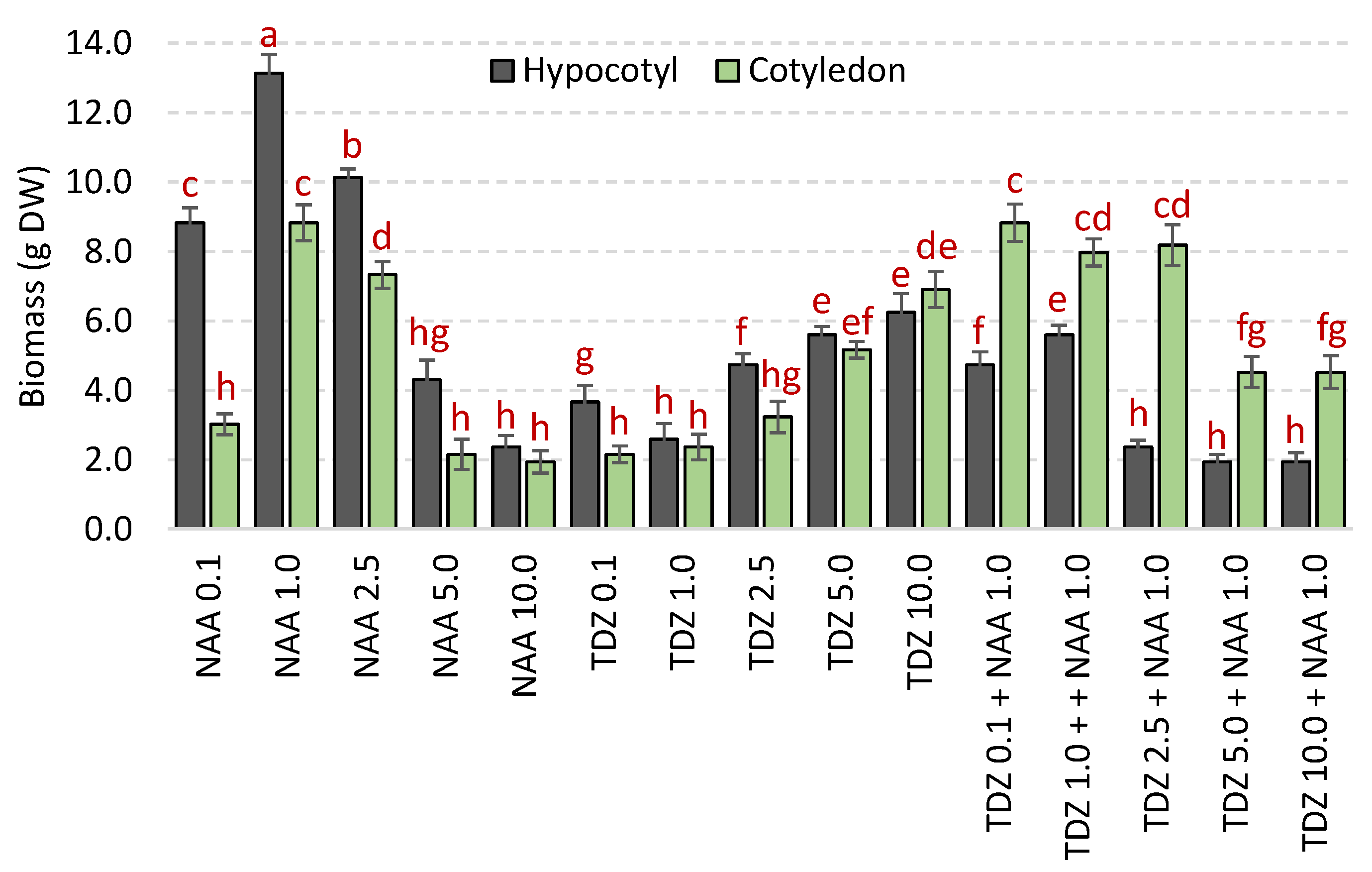
2.2. Biomass Accumulation
2.3. Total Phenolics and (Neo)lignans Accumulations
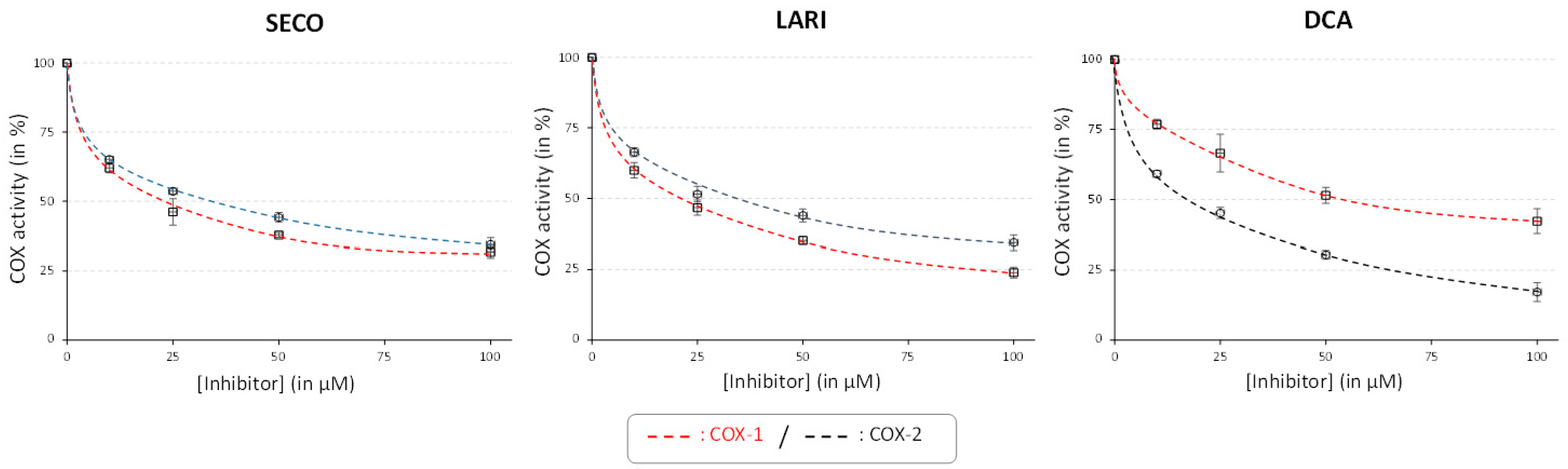
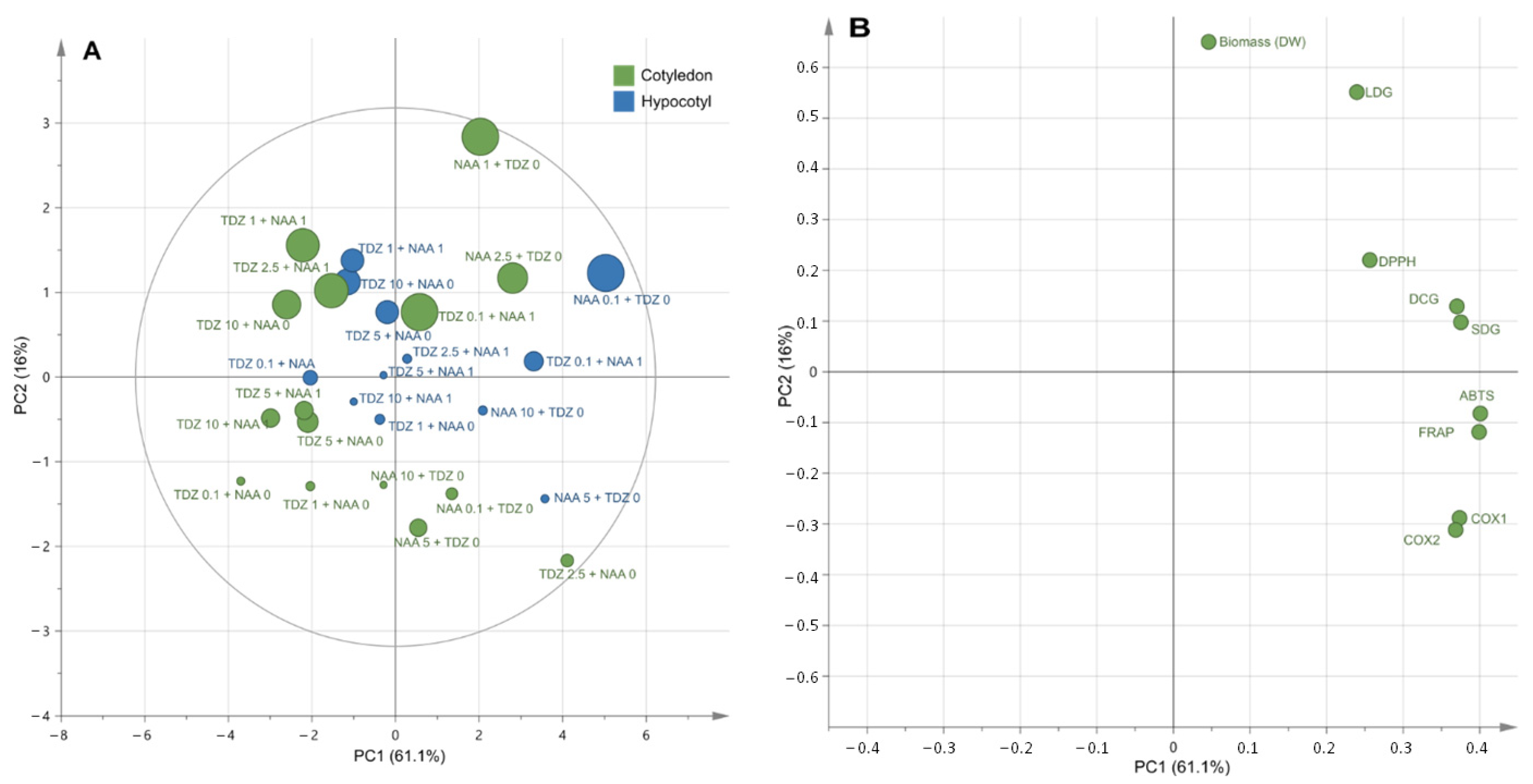
2.4. Antioxidant and Anti-Inflammatory Activities
3. Materials and Methods
3.1. Chemicals
3.2. Seed Germination
3.3. Establishment of Callus Cultures
3.4. Sample Extraction
3.5. Phytochemical Assays for Estimation of Secondary Metabolites
3.5.1. High-Performance Liquid Chromatography (HPLC) Analysis
3.5.2. Total Phenolic Contents
3.6. In Vitro Antioxidant Assays
3.6.1. Free Radical Scavenging Activity
3.6.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
3.6.3. ABTS Antioxidant Assay
3.7. Anti-Inflammatory COX-1 and COX-2 Inhibition Activities
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stojanoski, N. Development of health culture in Veles and its region from the past to the end of the 20th century. Veles Soc. Sci. Art 1999, 13, 34. [Google Scholar]
- Nadeem, M.; Abbasi, B.H.; Garros, L.; Drouet, S.; Zahir, A.; Ahmad, W.; Giglioli-Guivarc’h, N.; Hano, C. Yeast-extract improved biosynthesis of lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult. 2018, 135, 347–355. [Google Scholar] [CrossRef]
- Drouet, S.; Garros, L.; Hano, C.; Lainé, É. A critical view of different botanical, molecular, and chemical techniques used in authentication of plant materials for cosmetic applications. Cosmetics 2018, 5, 30. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medecines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.M.D.; Christensen, L.P.; Ibrahim, N.A.; Awad, N.E.; Zeid, I.F.; Pedersen, E.B. New acylated flavone and cyanogenic glycosides from Linum grandiflorum. Nat. Prod. Res. 2009, 23, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, J.L. Plants Used against Cancer: A Survey; Quaterman Publications, Inc.: Lawrence, MA, USA, 1982; ISBN 0880001305. [Google Scholar]
- Bown, D. The Royal Horticultural Society Encyclopedia of Herbs & Their Uses; Dorling Kindersley Limited: London, UK, 1995; ISBN 0751302031. [Google Scholar]
- Mohammed, M.M.D.; Christensen, L.P.; Ibrahim, N.A.; Awad, N.E.; Zeid, I.F.; Pedersen, E.B.; Jensen, K.B.; Colla, P.L. Anti-HIV-1 activities of the extracts from the medicinal plant Linum grandiflorum Desf.: In Proceedings of 4th Conference on Research and Development of Pharmaceutical Industries (Current Challenges). Med. Aromat. Plant Sci. Biotechnol. 2009, 3, 37–41. [Google Scholar]
- Schmidt, T.J.; Hemmati, S.; Klaes, M.; Konuklugil, B.; Mohagheghzadeh, A.; Ionkova, I.; Fuss, E.; Wilhelm Alfermann, A. Lignans in flowering aerial parts of Linum species—Chemodiversity in the light of systematics and phylogeny. Phytochemistry 2010, 71, 1714–1728. [Google Scholar] [CrossRef]
- Arroo, R.R.J.; Alfermann, A.W.; Medarde, M.; Petersen, M.; Pras, N.; Woolley, J.G. Plant cell factories as a source for anti-cancer lignans. Phytochem. Rev. 2002, 1, 27–35. [Google Scholar] [CrossRef]
- Lainé, E.; Hano, C.; Lamblin, F. Lignans. In Chemoprevention of Cancer and DNA Damage by Dietary Factors; Knasmüller, S., DeMarini, D.M., Johnson, I.T., Gerhäuser, C., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp. 555–577. [Google Scholar]
- Anjum, S.; Abbasi, B.H.; Doussot, J.; Favre-réguillon, A.; Hano, C.; Haider, B.; Doussot, J.; Favre-réguillon, A.; Hano, C. Effects of photoperiod regimes and ultraviolet-C radiations on biosynthesis of industrially important lignans and neolignans in cell cultures of Linum usitatissimum L. (Flax). J. Photochem. Photobiol. B Biol. 2017, 167, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Renouard, S.; Corbin, C.; Drouet, S.; Medvedec, B.; Doussot, J.; Colas, C.; Maunit, B.; Bhambra, A.S.; Gontier, E.; Jullian, N.; et al. Investigation of Linum flavum (L.) Hairy root cultures for the production of anticancer aryltetralin lignans. Int. J. Mol. Sci. 2018, 19, 990. [Google Scholar] [CrossRef]
- Hano, C.F.; Dinkova-Kostova, A.T.; Davin, L.B.; Cort, J.R.; Lewis, N.G. Lignans: Insights into their biosynthesis, metabolic engineering, analytical methods and health benefits. Front. Plant Sci. 2021, 11, 630327. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, R.; Khan, T.; Zaeem, A.; Garros, L.; Hano, C.; Abbasi, B.H. Biosynthesis of precious metabolites in callus cultures of Eclipta alba. Plant Cell Tissue Organ Cult. 2018, 135, 287–298. [Google Scholar] [CrossRef]
- Mikac, S.; Markulin, L.; Drouet, S.; Corbin, C.; Tungmunnithum, D.; Kiani, R.; Kabra, A.; Abbasi, B.H.; Renouard, S.; Bhambra, A.; et al. Bioproduction of anticancer podophyllotoxin and related aryltretralin-lignans in hairy root cultures of Linum flavum L. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–38. [Google Scholar]
- Khan, T.; Khan, T.; Hano, C.; Abbasi, B.H. Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Ind. Crops Prod. 2019, 129, 525–535. [Google Scholar] [CrossRef]
- Davies, K.M.; Deroles, S.C. Prospects for the use of plant cell cultures in food biotechnology. Curr. Opin. Biotechnol. 2014, 26, 133–140. [Google Scholar] [CrossRef]
- Gupta, S.K.; Liu, R.-B.; Liaw, S.-Y.; Chan, H.-S.; Tsay, H.-S. Enhanced tanshinone production in hairy roots of ‘Salvia miltiorrhiza Bunge’ under the influence of plant growth regulators in liquid culture. Bot Stud. 2011, 52, 435–443. [Google Scholar]
- Yamada, Y.; Sato, F. Production of berberine in cultured cells of Coptis japonica. Phytochemistry 1981, 20, 545–547. [Google Scholar] [CrossRef]
- Zhao, J.; Verpoorte, R. Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: From biochemical processing to metabolic engineering. Phytochem. Rev. 2007, 6, 435–457. [Google Scholar] [CrossRef]
- Khan, T.; Ullah, M.A.; Garros, L.; Hano, C.; Abbasi, B.H. Synergistic effects of melatonin and distinct spectral lights for enhanced production of anti-cancerous compounds in callus cultures of Fagonia indica. J. Photochem. Photobiol. B Biol. 2019, 190, 163–171. [Google Scholar] [CrossRef]
- Khurshid, R.; Ullah, M.A.; Tungmunnithum, D.; Drouet, S.; Shah, M.; Zaeem, A.; Hameed, S.; Hano, C.; Abbasi, B.H. Lights triggered differential accumulation of antioxidant and antidiabetic secondary metabolites in callus culture of Eclipta alba L. PLoS ONE 2020, 15, e0233963. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. J. Photochem. Photobiol. B Biol. 2018, 184, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Attoumbré, J.; Charlet, S.; Baltora-Rosset, S.; Hano, C.; Raynaud-Le Grandic, S.; Gillet, F.; Bensaddek, L.; Mesnard, F.; Fliniaux, M.A. High accumulation of dehydrodiconiferyl alcohol-4-β-D-glucoside in free and immobilized Linum usitatissimum cell cultures. Plant Cell Rep. 2006, 25, 859–864. [Google Scholar] [CrossRef]
- Corbin, C.; Decourtil, C.; Marosevic, D.; Bailly, M.; Lopez, T.; Renouard, S.; Doussot, J.; Dutilleul, C.; Auguin, D.; Giglioli-Guivarc’h, N.; et al. Role of protein farnesylation events in the ABA-mediated regulation of the Pinoresinol-Lariciresinol Reductase 1 (LuPLR1) gene expression and lignan biosynthesis in flax (Linum usitatissimum L.). Plant Physiol. Biochem. 2013, 72, 96–111. [Google Scholar] [CrossRef]
- Bose, S.; Munsch, T.; Lanoue, A.; Garros, L.; Tungmunnithum, D.; Messaili, S.; Destandau, E.; Billet, K.; St-Pierre, B.; Clastre, M.; et al. UPLC-HRMS analysis revealed the differential accumulation of antioxidant and anti-aging lignans and neolignans in in vitro cultures of Linum usitatissimum. Front. Plant Sci. 2020, 11, 1424. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radical chemistry. J. Gerontol. 1956, 11, 298–305. [Google Scholar] [CrossRef]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential production of phenylpropanoid metabolites in callus cultures of Ocimum basilicum L. with distinct in vitro antioxidant activities and in vivo protective effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Dziurka, M.; Warzecha, A.; Kubica, P.; Klimek-Szczykutowicz, M.; Ekiert, H. Targeted lignan profiling and anti-inflammatory properties of Schisandra rubriflora and Schisandra chinensis extracts. Molecules 2018, 23, 3103. [Google Scholar] [CrossRef]
- Borges, A.; Casoti, R.; e Silva, M.L.A.; da Cunha, N.L.; da Rocha Pissurno, A.P.; Kawano, D.F.; da Silva de Laurentiz, R. COX inhibition profiles and molecular docking studies of the lignan hinokinin and some synthetic derivatives. Mol. Inform. 2018, 37, 1800037. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Ullah, M.A.; Drouet, S.; Younas, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Interactive effects of light and melatonin on biosynthesis of silymarin and anti-inflammatory potential in callus cultures of Silybum marianum (L.) gaertn. Molecules 2019, 24, 1207. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Jan, H.; Drouet, S.; Tungmunnithum, D.; Shirazi, J.H.; Hano, C.; Abbasi, B.H. Chitosan elicitation impacts flavonolignan biosynthesis in Silybum marianum (L.) Gaertn cell suspension and enhances antioxidant and anti-inflammatory activities of cell extract. Molecules 2021, 26, 791. [Google Scholar] [CrossRef] [PubMed]
- Mohale, D.; Tripathi, A.; Wahane, J.; Chandewar, A. A pharmacological review on cyclooxygenase enzyme. Ind. J. Pharm. Pharmacol. 2014, 1, 46–58. [Google Scholar]
- Ullah, M.A.; Tungmunnithum, D.; Garros, L.; Hano, C.; Abbasi, B.H. Monochromatic lights-induced trends in antioxidant and antidiabetic polyphenol accumulation in in vitro callus cultures of Lepidium sativum L. J. Photochem. Photobiol. B Biol. 2019, 196, 111505. [Google Scholar] [CrossRef]
- Mathur, S.; Shekhawat, G.S. Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: An in vitro approach for production of stevioside. Acta Physiol. Plant. 2013, 35, 931–939. [Google Scholar] [CrossRef]
- Janowicz, J.; Niemann, J.; Wojciechowski, A. The effect of growth regulators on the regeneration ability of flax (Linum usitatissimum L.) hypocotyl explants in in vitro culture. Biotechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2012, 93, 135–138. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H.; Hano, C. Trends in accumulation of pharmacologically important antioxidant-secondary metabolites in callus cultures of Linum usitatissimum L. Plant Cell Tissue Organ. Cult. 2017, 129, 73–87. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Rashid, M.; Mahmood, T.; Fatima, N. Efficient regeneration and antioxidant potential in regenerated tissues of Piper nigrum L. Plant Cell Tissue Organ. Cult. 2010, 102, 129–134. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Thidiazuron-induced changes in biomass parameters, total phenolic content, and antioxidant activity in callus cultures of Artemisia absinthium L. Appl. Biochem. Biotechnol. 2014, 172, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Abbasi, B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 140, 223–227. [Google Scholar] [CrossRef]
- Lamblin, F.; Aimé, A.; Hano, C.; Roussy, I.; Domon, J.M.J.-M.; Van Droogenbroeck, B.; Lainé, E. The use of the phosphomannose isomerase gene as alternative selectable marker for Agrobacterium-mediated transformation of flax (Linum usitatissimum). Plant Cell Rep. 2007, 26, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Abbasi, B.H. Thidiazuron-enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L. Int. J. Nanomed. 2016, 11, 715. [Google Scholar]
- Cheng, H.; Yu, L.-J.; Hu, Q.-Y.; Chen, S.-C.; Sun, Y.-P. Establishment of callus and cell suspension cultures of Corydalis saxicola Bunting, a rare medicinal plant. Z. für Nat. C 2006, 61, 251–256. [Google Scholar] [CrossRef]
- Lisowska, K.; Wysokinska, H. In vitro propagation of Catalpa ovata G. Don. Plant Cell Tissue Organ Cult. 2000, 60, 171–176. [Google Scholar] [CrossRef]
- Rasool, R.; Ganai, B.A.; Kamili, A.N.; Akbar, S. Antioxidant potential in callus culture of Artemisia amygdalina Decne. Nat. Prod. Res. 2012, 26, 2103–2106. [Google Scholar] [PubMed]
- Fazal, H.; Abbasi, B.H.; Ahmad, N. Optimization of adventitious root culture for production of biomass and secondary metabolites in Prunella vulgaris L. Appl. Biochem. Biotechnol. 2014, 174, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Danya, U.; Udhayasankar, M.R.; Punitha, D.; Arumugasamy, K.; Suresh, S. In vitro regeneration of Tecomella undulata (Sm.) Seem-an endangered medicinal plant. Int. J. Plant Anim. Environ. Sci. 2012, 2, 44–49. [Google Scholar]
- Jain, P.; Rashid, A. Stimulation of shoot regeneration on Linum hypocotyl segments by thidiazuron and its response to light and calcium. Biol. Plant. 2001, 44, 611–613. [Google Scholar] [CrossRef]
- Kartnig, T.; Kögl, G.; Heydel, B. Production of flavonoids in cell cultures of Crataegus monogyna. Planta Med. 1993, 59, 537–538. [Google Scholar] [CrossRef]
- Chaâbani, G.; Tabart, J.; Kevers, C.; Dommes, J.; Khan, M.I.; Zaoui, S.; Chebchoub, L.; Lachaâl, M.; Karray-Bouraoui, N. Effects of 2, 4-dichlorophenoxyacetic acid combined to 6-Benzylaminopurine on callus induction, total phenolic and ascorbic acid production, and antioxidant activities in leaf tissue cultures of Crataegus azarolus L. var. aronia. Acta Physiol. Plant. 2015, 37, 16. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Mohamed, A.A.; Ali, S.I. Callus formation, phenolics content and related antioxidant activities in tissue culture of a medicinal plant colocynth (Citrullus colocynthis). Nov. Biotechnol. 2010, 10, 79–94. [Google Scholar]
- Markulin, L.; Makhno, Y.; Drouet, S.; Zare, S.; Sumaira; Anjum; Tungmunnithum, D.; Sabzalian, M.R.; Abbasi, B.H.; Lainé, E.; et al. On “the most useful” oleaginous seeds: Linum usitatissimum L., a genomic view with emphasis on important flax seed storage compounds. In Oil Crop Genomics; Tombuloglu, H., Unver, T., Tombuloglu, G., Hakeem, K.R., Eds.; Springer Nature: Cham, Switzerland, 2021; in press. [Google Scholar]
- Attoumbre, J.; Hano, C.; Mesnard, F.; Lamblin, F.; Bensaddek, L.; Raynaud-Le Grandic, S.; Lainé, E.; Fliniaux, M.A.; Baltora-Rosset, S. Identification by NMR and accumulation of a neolignan, the dehydrodiconiferyl alcohol-4-β-d-glucoside, in Linum usitatissimum cell cultures. Comptes Rendus Chim. 2006, 9, 420–425. [Google Scholar] [CrossRef]
- Hano, C.; Addi, M.; Bensaddek, L.; Crônier, D.; Baltora-Rosset, S.; Doussot, J.; Maury, S.; Mesnard, F.; Chabbert, B.; Hawkins, S.; et al. Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 2006, 223, 975–989. [Google Scholar] [CrossRef]
- Beejmohun, V.; Fliniaux, O.; Grand, É.; Lamblin, F.; Bensaddek, L.; Christen, P.; Kovensky, J.; Fliniaux, M.-A.; Mesnard, F. Microwave-assisted extraction of the main phenolic compounds in flaxseed. Phytochem. Anal. 2007, 18, 275–282. [Google Scholar] [CrossRef]
- Hano, C.; Addi, M.; Fliniaux, O.; Bensaddek, L.; Duverger, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Molecular characterization of cell death induced by a compatible interaction between Fusarium oxysporum f. sp. linii and flax (Linum usitatissimum) cells. Plant Physiol. Biochem. 2008, 46, 590–600. [Google Scholar] [CrossRef]
- Corbin, C.; Renouard, S.; Lopez, T.; Lamblin, F.; Lainé, E.; Hano, C. Identification and characterization of cis-acting elements involved in the regulation of ABA-and/or GA-mediated LuPLR1 gene expression and lignan biosynthesis in flax (Linum usitatissimum L.) cell cultures. J. Plant Physiol. 2013, 170, 516–522. [Google Scholar] [CrossRef]
- Gabr, A.M.; Mabrok, H.B.; Ghanem, K.Z.; Blaut, M.; Smetanska, I. Lignan accumulation in callus and Agrobacterium rhizogenes-mediated hairy root cultures of flax (Linum usitatissimum). Plant Cell Tissue Organ Cult. 2016, 126, 255–267. [Google Scholar] [CrossRef]
- Nadeem, M.; Ahmad, W.; Zahir, A.; Hano, C.; Abbasi, B.H. Salicylic acid-enhanced biosynthesis of pharmacologically important lignans and neo lignans in cell suspension culture of Linum ussitatsimum L. Eng. Life Sci. 2019, 19, 168–174. [Google Scholar] [CrossRef]
- Ahmad, W.; Zahir, A.; Nadeem, M.; Garros, L.; Drouet, S.; Renouard, S.; Doussot, J.; Guivarc’h-Giglioli, N.; Hano, C. Abbasi, B.H. Enhanced production of lignans and neolignans in chitosan-treated flax (Linum usitatissimum L.) cell cultures. Process. Biochem. 2019, 79, 155–165. [Google Scholar] [CrossRef]
- Markulin, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Garros, L.; Renouard, S.; Lopez, T.; Mogelard, G.; Gutierrez, L.; Auguin, D.; et al. The control exerted by ABA on lignan biosynthesis in flax (Linum usitatissimum L.) is modulated by a Ca2+ signal transduction involving the calmodulin-like LuCML15b. J. Plant Physiol. 2019, 236, 74–87. [Google Scholar] [CrossRef]
- Zaeem, A.; Drouet, S.; Anjum, S.; Khurshid, R.; Younas, M.; Blondeau, J.P.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Effects of biogenic zinc oxide nanoparticles on growth and oxidative stress response in flax seedlings vs. in vitro cultures: A comparative analysis. Biomolecules 2020, 10, 918. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Komal, A.; Drouet, S.; Kausar, H.; Hano, C.; Abbasi, B.H. Feasible production of lignans and neolignans in root-derived in vitro cultures of flax (Linum usitatissimum L.). Plants 2020, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.D.; Lynn, D.G. Biosynthesis of dehydrodiconiferyl alcohol glucosides: Implications for the control of tobacco cell growth. Plant Physiol. 1992, 98, 343–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Hano, C.; Corbin, C.; Drouet, S.; Quéro, A.; Rombaut, N.; Savoire, R.; Molinié, R.; Thomasset, B.; Mesnard, F.; Lainé, E. The lignan (+)-secoisolariciresinol extracted from flax hulls is an effective protectant of linseed oil and its emulsion against oxidative damage. Eur. J. Lipid Sci. Technol. 2017, 119, 1600219. [Google Scholar] [CrossRef]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Socrier, L.; Quéro, A.; Verdu, M.; Song, Y.; Molinié, R.; Mathiron, D.; Pilard, S.; Mesnard, F.; Morandat, S. Flax phenolic compounds as inhibitors of lipid oxidation: Elucidation of their mechanisms of action. Food Chem. 2019, 274, 651–658. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of traditional medicinal plants in Alexandria. Evidence-Based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef]
- Bauer, R.; Tittel, G. Quality assessment of herbal preparations as a precondition of pharmacological and clinical studies. Phytomedicine 1996, 2, 193–198. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Usman, H.; Ullah, M.A.; Jan, H.; Siddiquah, A.; Drouet, S.; Anjum, S.; Giglioli-Guviarc’h, N.; Hano, C.; Abbasi, B.H. Interactive effects of wide-spectrum monochromatic lights on phytochemical production, antioxidant and biological activities of Solanum xanthocarpum callus cultures. Molecules 2020, 25, 2201. [Google Scholar] [CrossRef]
- Shah, M.; Nawaz, S.; Jan, H.; Uddin, N.; Ali, A.; Anjum, S.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Synthesis of bio-mediated silver nanoparticles from Silybum marianum and their biological and clinical activities. Mater. Sci. Eng. C 2020, 112, 110889. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Patrono, C. Selective cyclooxygenase 2 inhibitors, aspirin, and cardiovascular disease: A reappraisal. Arthritis Rheum. 2003, 48, 12–20. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nazir, S.; Jan, H.; Tungmunnithum, D.; Drouet, S.; Zia, M.; Hano, C.; Abbasi, B.H. Callus culture of Thai basil is an effective biological system for the production of antioxidants. Molecules 2020, 25, 4859. [Google Scholar] [CrossRef] [PubMed]
| PRGs (mg/L) | SECO | LARI | DCA | |||
|---|---|---|---|---|---|---|
| Hypocotyl | Cotyledon | Hypocotyl | Cotyledon | Hypocotyl | Cotyledon | |
| NAA 0.1 | 3.4 ± 0.2 bc | 1.7 ± 0.2 d | 2.5 ± 0.2 c | 1.2 ± 0.1 ef | 43.9 ± 3.6 bc | 22.7 ± 2.4 ef |
| NAA 1.0 | 3.7 ± 0.4 b | 1.8 ± 0.3 de | 4.4 ± 0.3 a | 3.2 ± 0.1 b | 48.0 ± 4.7 b | 26.8 ± 1.4 e |
| NAA 2.5 | 3.5 ± 0.2 b | 1.9 ± 0.2 d | 4.1 ± 0.1 a | 3.1 ± 0.3 bc | 45.9 ± 2.1 b | 26.0 ± 3.4 e |
| NAA 5.0 | 1.5 ± 0.3 de | 0.6 ± 0.3 f | 2.7 ± 0.2 c | 1.3 ± 0.2 ef | 16.6 ± 6.0 fg | 7.9 ± 3.6 gh |
| NAA 10.0 | 2.5 ± 0.2 c | 1.3 ± 0.1 ef | 2.2 ± 0.3 cd | 1.4 ± 0.2 e | 31.4 ± 5.0 de | 16.9 ± 2.5 fg |
| TDZ 0.1 | 1.1 ± 0.4 ef | 0.4 ± 0.1 f | 1.7 ± 0.1 de | 0.9 ± 0.1 f | 13.1 ± 2.1 g | 5.2 ± 1.9 h |
| TDZ 1.0 | 1.6 ± 0.3 de | 0.7 ± 0.2 f | 2.0 ± 0.3 d | 1.0 ± 0.2 ef | 15.3 ± 2.5 g | 11.0 ± 0.8 g |
| TDZ 2.5 | 5.3 ± 0.1 a | 2.6 ± 0.3 c | 3.1 ± 0.2 b | 1.5 ± 0.3 de | 67.6 ± 2.9 a | 32.7 ± 2.7 de |
| TDZ 5.0 | 1.5 ± 0.1 de | 0.8 ± 0.1 f | 2.3 ± 0.1 c | 1.3 ± 0.1 e | 21.0 ± 1.8 f | 9.4 ± 1.3 h |
| TDZ 10.0 | 1.2 ± 0.3 def | 0.6 ± 0.2 f | 2.6 ± 0.4 cd | 2.1 ± 0.2 d | 15.3 ± 6.7 fg | 7.9 ± 1.3 h |
| TDZ 0.1 + NAA 1.0 | 2.8 ± 0.3 c | 1.5 ± 0.1 e | 2.4 ± 0.3 cd | 1.9 ± 0.1 d | 35.6 ± 3.9 cd | 16.6 ± 0.8 g |
| TDZ 1.0 + + NAA 1.0 | 1.2 ± 0.2 def | 0.7 ± 0.2 ef | 2.4 ± 0.2 cd | 2.1 ± 0.1 d | 14.0 ± 3.0 g | 7.9 ± 1.5 h |
| TDZ 2.5 + NAA 1.0 | 1.6 ± 0.1 de | 0.8 ± 0.3 ef | 2.0 ± 0.1 d | 1.3 ± 0.2 e | 26.9 ± 1.9 e | 9.4 ± 3.1 gh |
| TDZ 5.0 + NAA 1.0 | 1.5 ± 0.1 de | 0.9 ± 0.2 f | 2.0 ± 0.1 d | 1.0 ± 0.2 ef | 19.6 ± 1.5 fg | 9.0 ± 1.7 h |
| TDZ 10.0 + NAA 1.0 | 1.4 ± 0.2 de | 0.4 ± 0.3 f | 1.6 ± 0.2 de | 0.8 ± 0.2 f | 18.3 ± 3.6 fg | 7.6 ± 2.6 gh |
| PRGs (mg/L) | DPPH 1 | ABTS 2 | FRAP 2 | |||
|---|---|---|---|---|---|---|
| Hypocotyl | Cotyledon | Hypocotyl | Cotyledon | Hypocotyl | Cotyledon | |
| NAA 0.1 | 80.9 ± 3.2 bc | 73.0 ± 2.2 d | 326.9 ± 26.0 cd | 269.2 ± 17.3 d | 582.5 ± 40.8 c | 407.8 ± 33.4 de |
| NAA 1.0 | 90.5 ± 4.0 a | 84.1 ± 3.8 ab | 423.1 ± 19.2 b | 336.5 ± 21.2 cd | 737.9 ± 68.0 d | 504.8 ± 56.3 cd |
| NAA 2.5 | 80.9 ± 1.9 c | 77.8 ± 2.9 c | 346.2 ± 13.5 c | 259.6 ± 18.0 d | 601.9 ± 31.1 c | 427.2 ± 42.7 de |
| NAA 5.0 | 76.2 ± 3.2 cd | 66.7 ± 4.1 de | 365.4 ± 20.2 c | 288.5 ± 25.3 d | 679.6 ± 52.4 bc | 330.1 ± 52.4 e |
| NAA 10.0 | 71.4 ± 2.4 d | 60.3 ± 2.4 e | 230.8 ± 17.3 de | 201.9 ± 15.2 e | 427.2 ± 40.8 d | 310.7 ± 27.2 e |
| TDZ 0.1 | 52.4 ± 3.5 ef | 42.9 ± 1.7 g | 96.2 ± 19.2 gh | 48.1 ± 10.6 h | 194.2 ± 62.1 f | 97.1 ± 25.2 fg |
| TDZ 1.0 | 55.6 ± 3.3 ef | 50.8 ± 2.7 f | 153.9 ± 22.1 fg | 115.4 ± 16.8 g | 388.4 ± 56.3 de | 291.3 ± 40.8 ef |
| TDZ 2.5 | 69.8 ± 2.4 d | 60.3 ± 3.3 e | 490.4 ± 18.3 a | 355.8 ± 24.0 c | 970.9 ± 20.8 a | 679.6 ± 48.5 bc |
| TDZ 5.0 | 54.0 ± 1.7 ef | 41.3 ± 1.8 g | 163.5 ± 10.6 h | 117.3 ± 16.7 g | 330.1 ± 21.8 e | 291.3 ± 25.2 e |
| TDZ 10.0 | 46.0 ± 4.0 f | 39.7 ± 3.8 g | 135.6 ± 19.7 fg | 86.5 ± 23.2 gh | 271.8 ± 60.2 ef | 135.9 ± 33.0 fg |
| TDZ 0.1 + NAA 1.0 | 69.8 ± 2.7 d | 58.7 ± 4.0 e | 269.2 ± 17.1 d | 214.4 ± 21.2 e | 582.5 ± 44.7 c | 427.2 ± 15.5 d |
| TDZ 1.0 + + NAA 1.0 | 74.6 ± 2.1 cd | 63.5 ± 2.9 de | 115.4 ± 14.5 g | 94.2 ± 18.7 gh | 213.6 ± 34.9 f | 116.5 ± 42.7 fg |
| TDZ 2.5 + NAA 1.0 | 85.7 ± 1.4 b | 76.2 ± 4.3 bcd | 153.9 ± 8.7 f | 120.2 ± 23.8 fg | 252.4 ± 15.5 e | 213.6 ± 64.1 ef |
| TDZ 5.0 + NAA 1.0 | 77.8 ± 1.6 c | 61.9 ± 3.3 e | 144.2 ± 6.7 f | 101.9 ± 20.9 fg | 291.3 ± 21.4 e | 194.2 ± 41.2 fg |
| TDZ 10.0 + NAA 1.0 | 71.4 ± 1.9 d | 60.3 ± 3.5 e | 134.6 ± 11.0 f | 67.3 ± 19.6 h | 271.8 ± 31.1 ef | 174.8 ± 52.4 fg |
| Biological Assay | SECO | LARI | DCA | TPC |
|---|---|---|---|---|
| DPPH | 0.523 * | 0.646 *** | 0.555 * | 0.639 *** |
| ABTS | 0.837 *** | 0.650 ** | 0.833 *** | 0.713 *** |
| FRAP | 0.872 *** | 0.627 * | 0.890 *** | 0.685 *** |
| COX-1 | 0.670 *** | 0.344 ** | 0.683 ns | 0.470 ** |
| COX-2 | 0.679 ** | 0.352 ** | 0.696 *** | 0.474 ** |
| PRGs (mg/L) | COX-1 | COX-2 | ||
|---|---|---|---|---|
| Hypocotyl | Cotyledon | Hypocotyl | Cotyledon | |
| NAA 0.1 | 31.1 ± 2.3 bc | 23.1 ± 2.1 de | 34.2 ± 2.4 bc | 27.2 ± 2.1 cd |
| NAA 1.0 | 12.3 ± 1.1 fg | 10.1 ± 0.5 g | 14.4 ± 1.2 fg | 13.7 ± 1.1 g |
| NAA 2.5 | 33.0 ± 2.3 bc | 28.1 ± 2.2 cd | 37.1 ± 2.4 b | 31.7 ± 2.2 c |
| NAA 5.0 | 34.2 ± 2.3 bc | 30.0 ± 2.3 c | 38.4 ± 2.6 b | 32.3 ± 2.3 c |
| NAA 10.0 | 21.5 ± 1.5 de | 18.1 ± 1.5 e | 24.6 ± 1.9 de | 20.1 ± 1.9 e |
| TDZ 0.1 | 8.9 ± 0.4 gh | 7.6 ± 0.3 h | 10.8 ± 0.6 h | 9.8 ± 0.4 h |
| TDZ 1.0 | 14.1 ± 1.2 f | 11.2 ± 1.1 g | 17.0 ± 1.5 ef | 14.4 ± 1.3 fg |
| TDZ 2.5 | 47.4 ± 2.8 a | 35.9 ± 2.5 b | 51.1 ± 2.9 a | 39.8 ± 2.6 b |
| TDZ 5.0 | 13.2 ± 1.1 fg | 11.0 ± 0.9 g | 16.7 ± 1.4 ef | 14.9 ± 1.4 fg |
| TDZ 10.0 | 10.0 ± 0.7 g | 9.4 ± 0.7 g | 13.4 ± 1.1 g | 12.7 ± 1.2 g |
| TDZ 0.1 + NAA 1.0 | 24.1 ± 1.6 d | 19.3 ± 1.8 e | 28.1 ± 2.1 cd | 22.7 ± 2.0 de |
| TDZ 1.0 + + NAA 1.0 | 9.1 ± 0.3 g | 8.1 ± 0.3 h | 12.6 ± 1.1 g | 12.1 ± 1.1 gh |
| TDZ 2.5 + NAA 1.0 | 13.3 ± 1.3 fg | 10.2 ± 0.9 g | 17.0 ± 1.4 ef | 14.4 ± 1.4 fg |
| TDZ 5.0 + NAA 1.0 | 11.0 ± 1.1 g | 10.0 ± 0.5 g | 15.6 ± 1.2 f | 13.6 ± 1.2 fg |
| TDZ 10.0 + NAA 1.0 | 9.9 ± 0.5 g | 8.3 ± 0.3 h | 11.2 ± 0.8 gh | 10.1 ± 0.8 gh |
| Compound | COX-1 IC50 | COX-2 IC50 | Specificity (COX-1/COX2 IC50 Values) | ||
|---|---|---|---|---|---|
| (in µM) | (in µg/mL) | (in µM) | (in µg/mL) | ||
| SECO | 21.7± 1.9 a | 59.9 ± 5.2 a | 32.7 ± 0.8 b | 90.2 ± 2.2 b | 0.67 ± 0.01 b |
| LARI | 24.2 ± 6.9 a | 67.1 ± 19.1 a | 34.6 ± 2.9 b | 96.0 ± 8.0 b | 0.70 ± 0.04 b |
| DCA | 51.3 ± 0.3 b | 144.0 ± 0.8 b | 19.2± 2.5 a | 53.6 ± 7.0 a | 2.67 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asad, B.; Khan, T.; Gul, F.Z.; Ullah, M.A.; Drouet, S.; Mikac, S.; Garros, L.; Ferrier, M.; Bose, S.; Munsch, T.; et al. Scarlet Flax Linum grandiflorum (L.) In Vitro Cultures as a New Source of Antioxidant and Anti-Inflammatory Lignans. Molecules 2021, 26, 4511. https://doi.org/10.3390/molecules26154511
Asad B, Khan T, Gul FZ, Ullah MA, Drouet S, Mikac S, Garros L, Ferrier M, Bose S, Munsch T, et al. Scarlet Flax Linum grandiflorum (L.) In Vitro Cultures as a New Source of Antioxidant and Anti-Inflammatory Lignans. Molecules. 2021; 26(15):4511. https://doi.org/10.3390/molecules26154511
Chicago/Turabian StyleAsad, Bushra, Taimoor Khan, Faiza Zareen Gul, Muhammad Asad Ullah, Samantha Drouet, Sara Mikac, Laurine Garros, Manon Ferrier, Shankhamala Bose, Thibaut Munsch, and et al. 2021. "Scarlet Flax Linum grandiflorum (L.) In Vitro Cultures as a New Source of Antioxidant and Anti-Inflammatory Lignans" Molecules 26, no. 15: 4511. https://doi.org/10.3390/molecules26154511
APA StyleAsad, B., Khan, T., Gul, F. Z., Ullah, M. A., Drouet, S., Mikac, S., Garros, L., Ferrier, M., Bose, S., Munsch, T., Tungmunnithum, D., Lanoue, A., Giglioli-Guivarc’h, N., Hano, C., & Abbasi, B. H. (2021). Scarlet Flax Linum grandiflorum (L.) In Vitro Cultures as a New Source of Antioxidant and Anti-Inflammatory Lignans. Molecules, 26(15), 4511. https://doi.org/10.3390/molecules26154511










