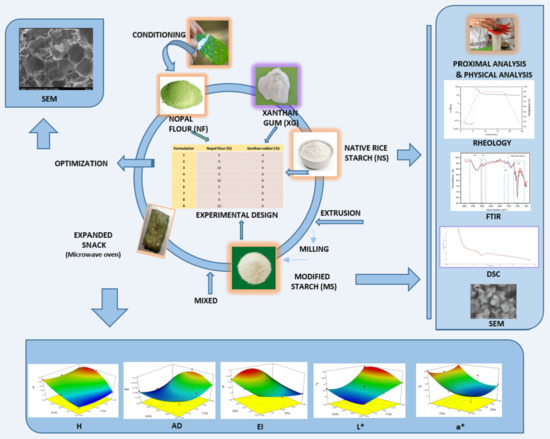
Development of a Third Generation Snack of Rice Starch Enriched with Nopal Flour (Opuntia ficus indica)
Abstract
1. Introduction
2. Results and Discussions
2.1. Proximal Analysis
2.1.1. Native Rice Starch and Modified Starch by Extrusion
2.1.2. Nopal Flour
2.2. Physicochemical Analysis of the Raw Material
2.2.1. Absorption Index (WAI) and Water Solubility (WSI)
2.2.2. pH
2.2.3. Color
2.2.4. Particle Size
2.3. Characterization of Native Rice Starch and Extrusion Modified Starch
2.3.1. Degree of Substitution (DS)
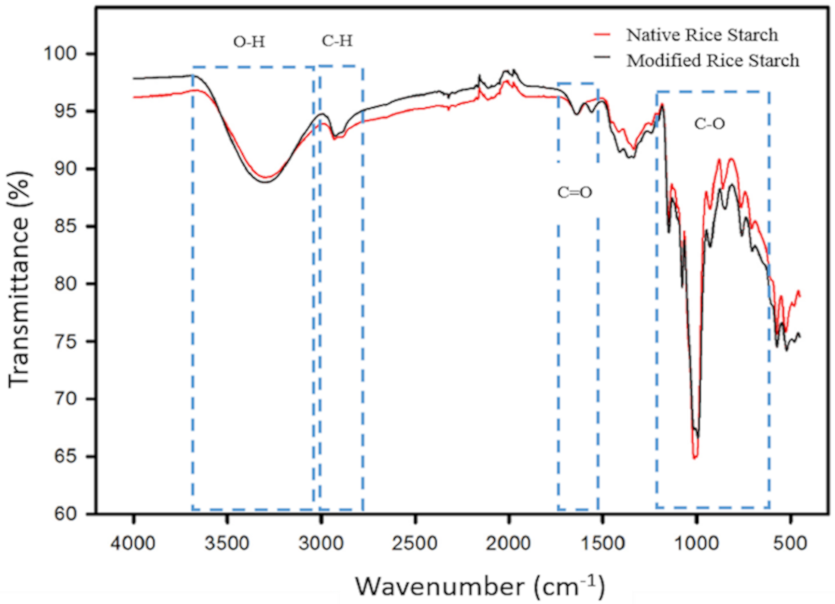
2.3.2. Fourier Transform Infrared Spectrometry (FTIR)
2.3.3. Differential Scanning Calorimetry (DSC)
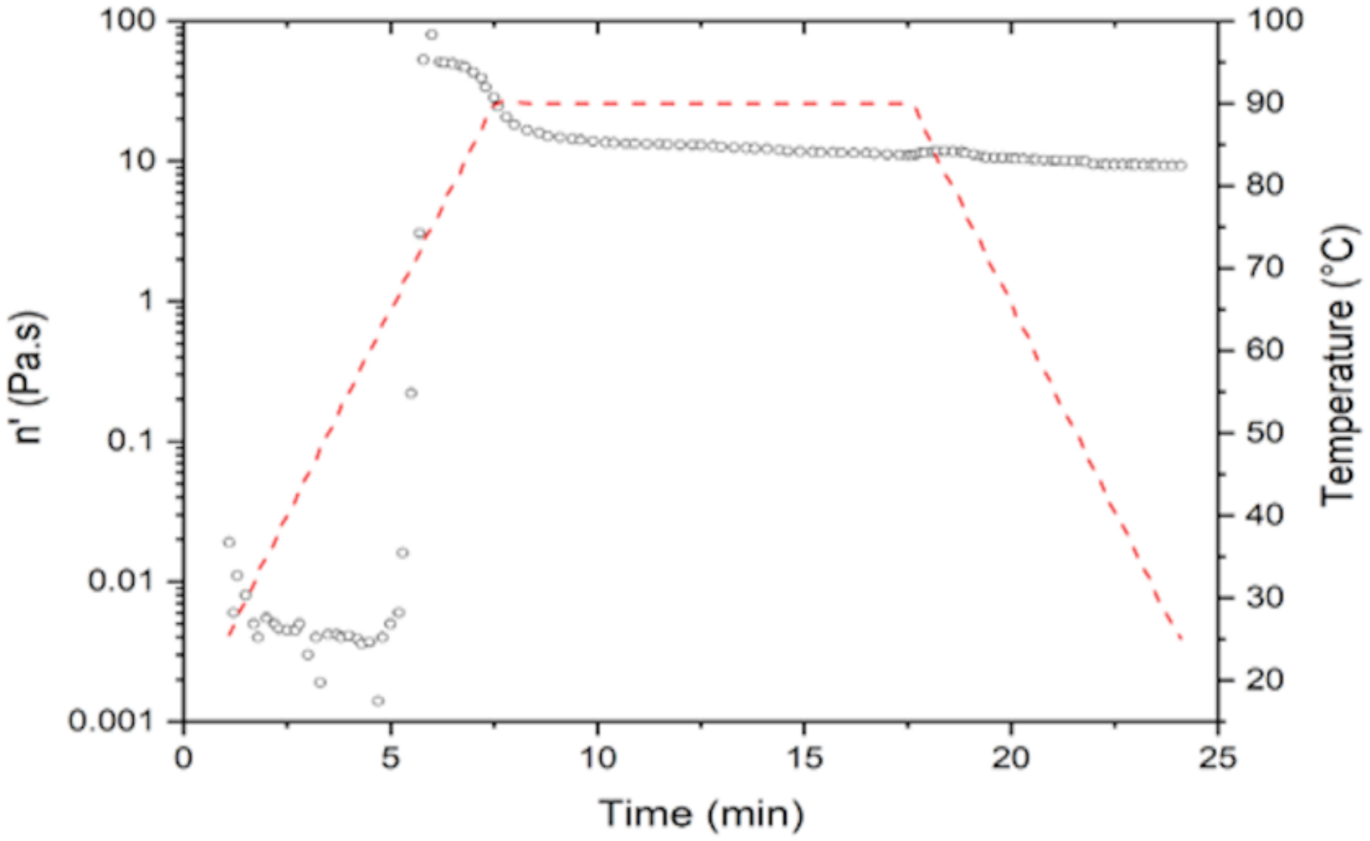
2.3.4. Rheology
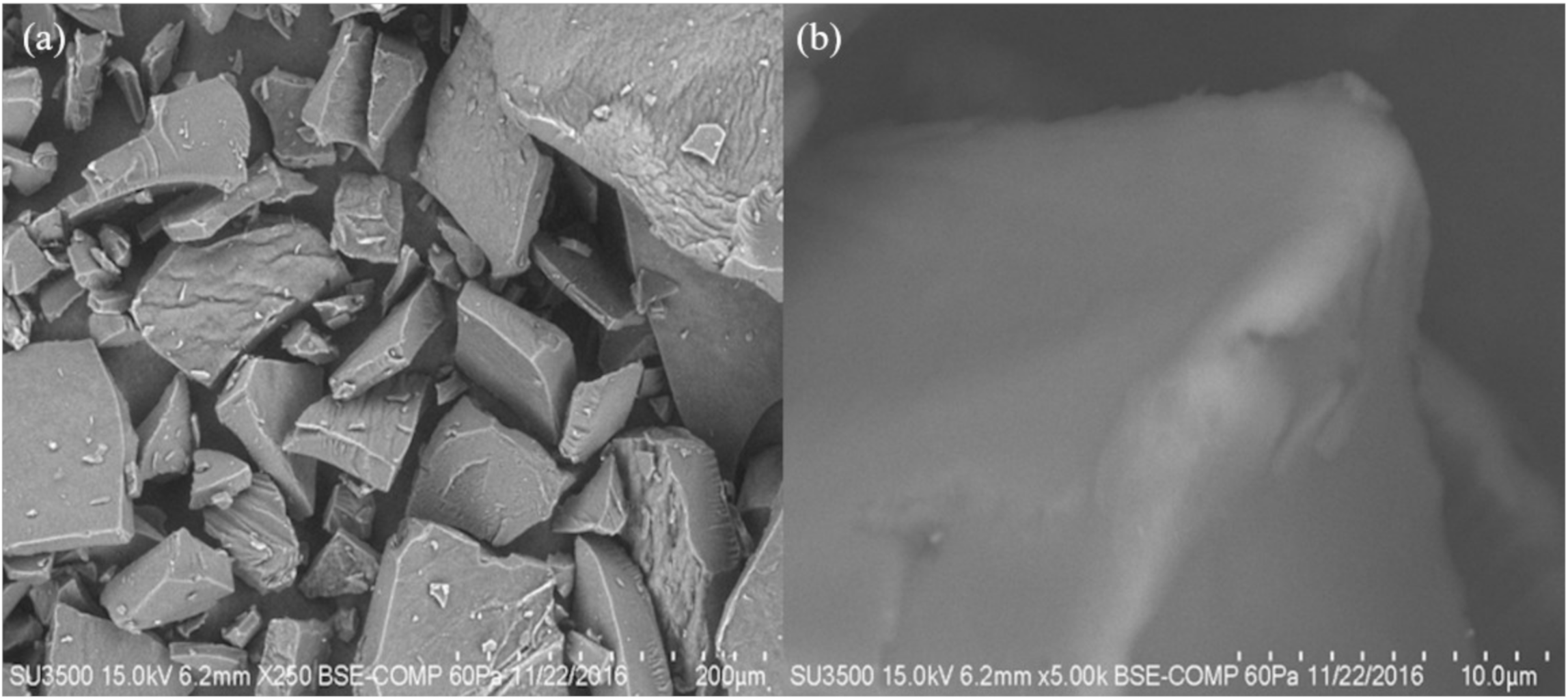
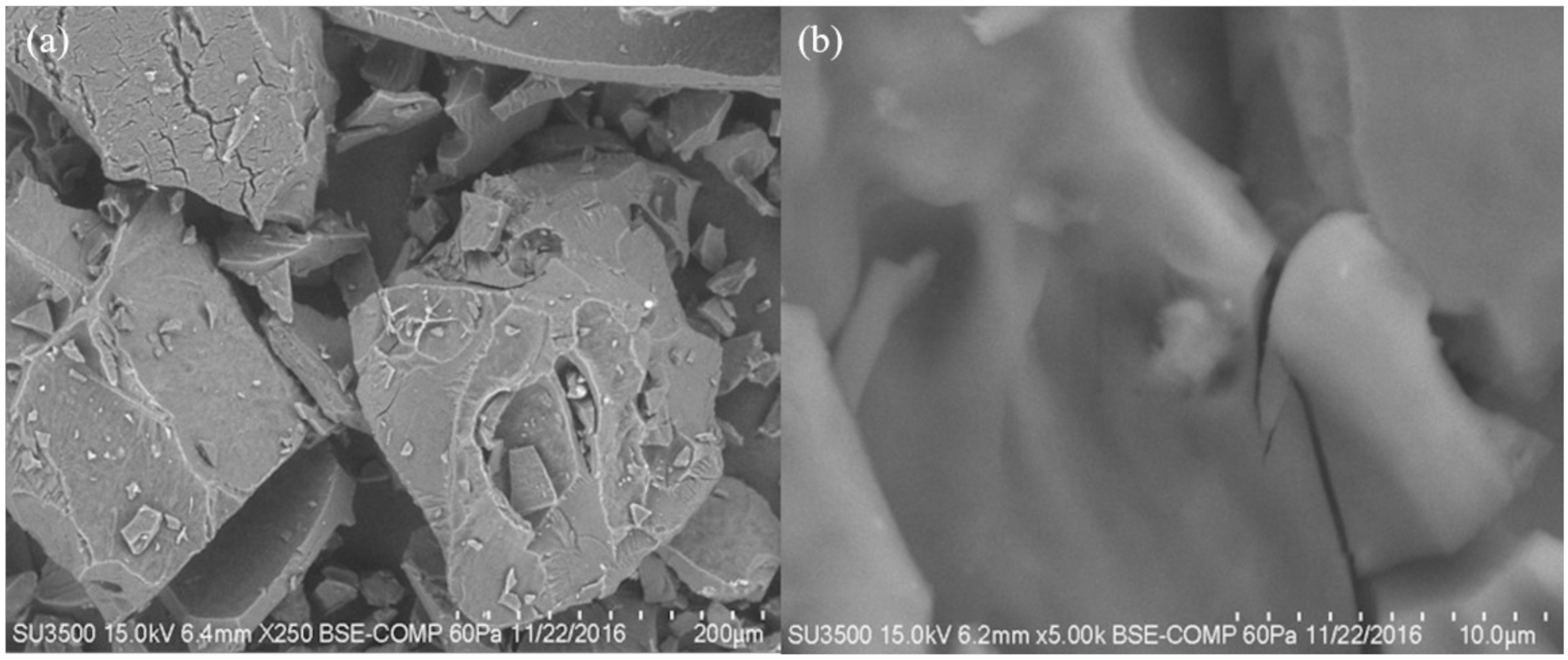
2.3.5. Scanning Electron Microscopy (SEM)
2.4. Characterization of the Expanded Product
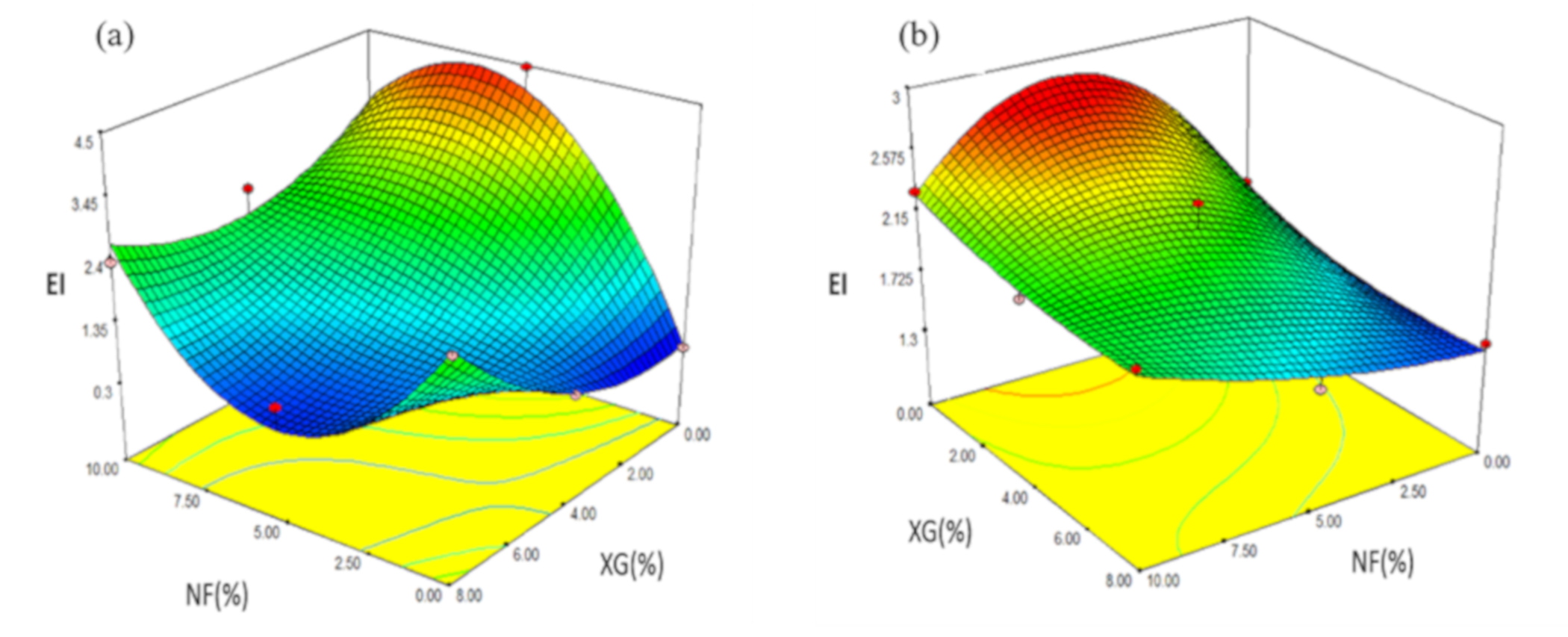
2.4.1. Expansion Index (EI)
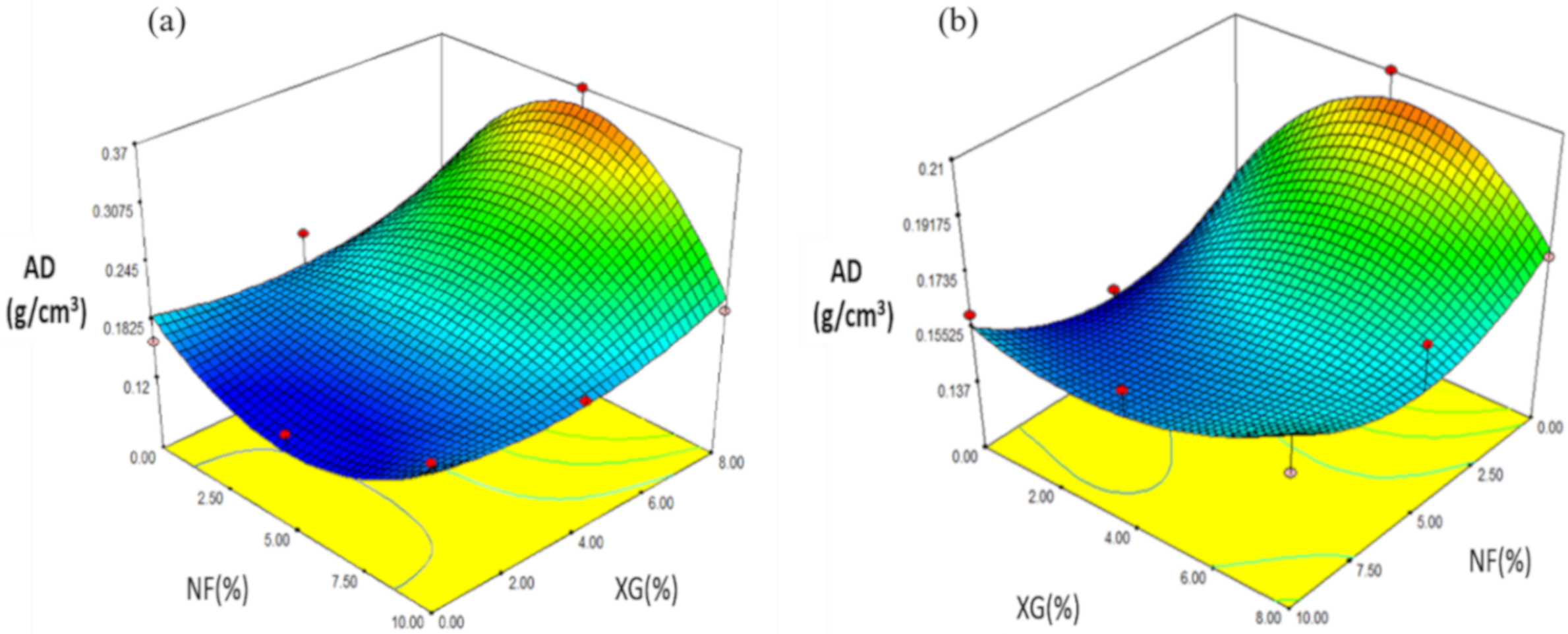
2.4.2. Apparent Density (AD)
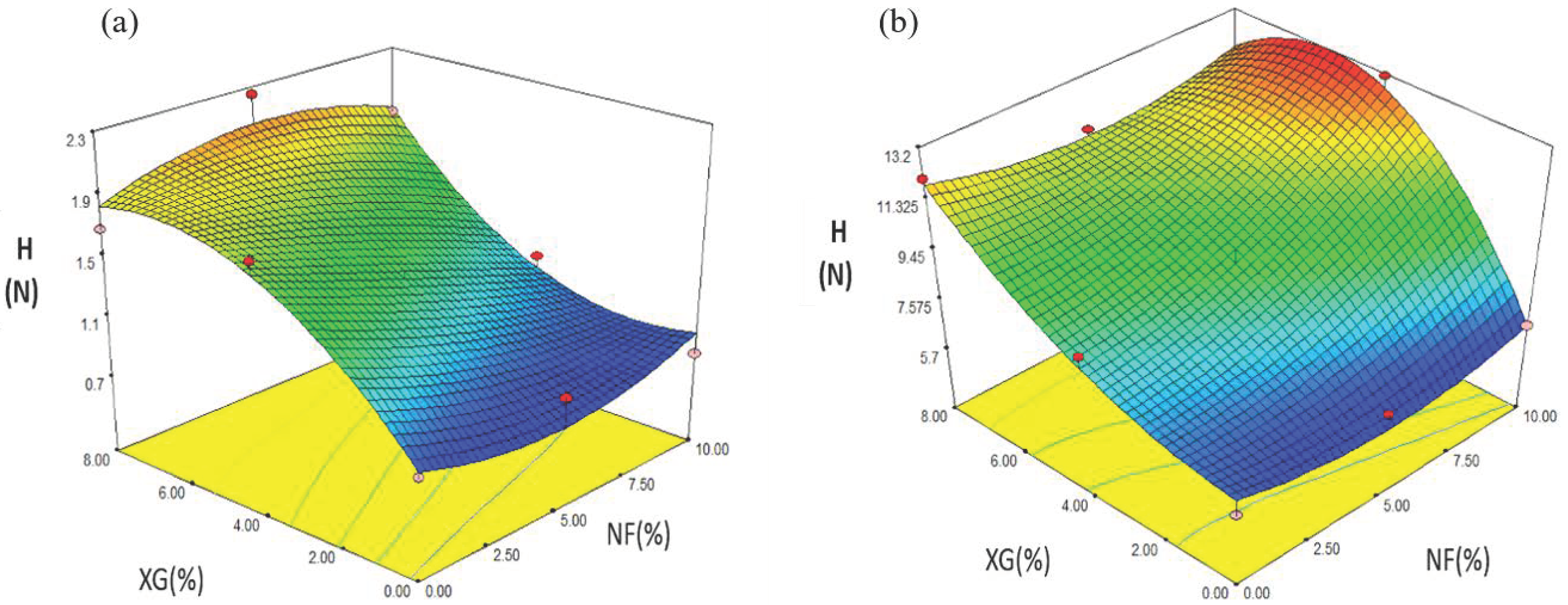
2.4.3. Hardness (H)
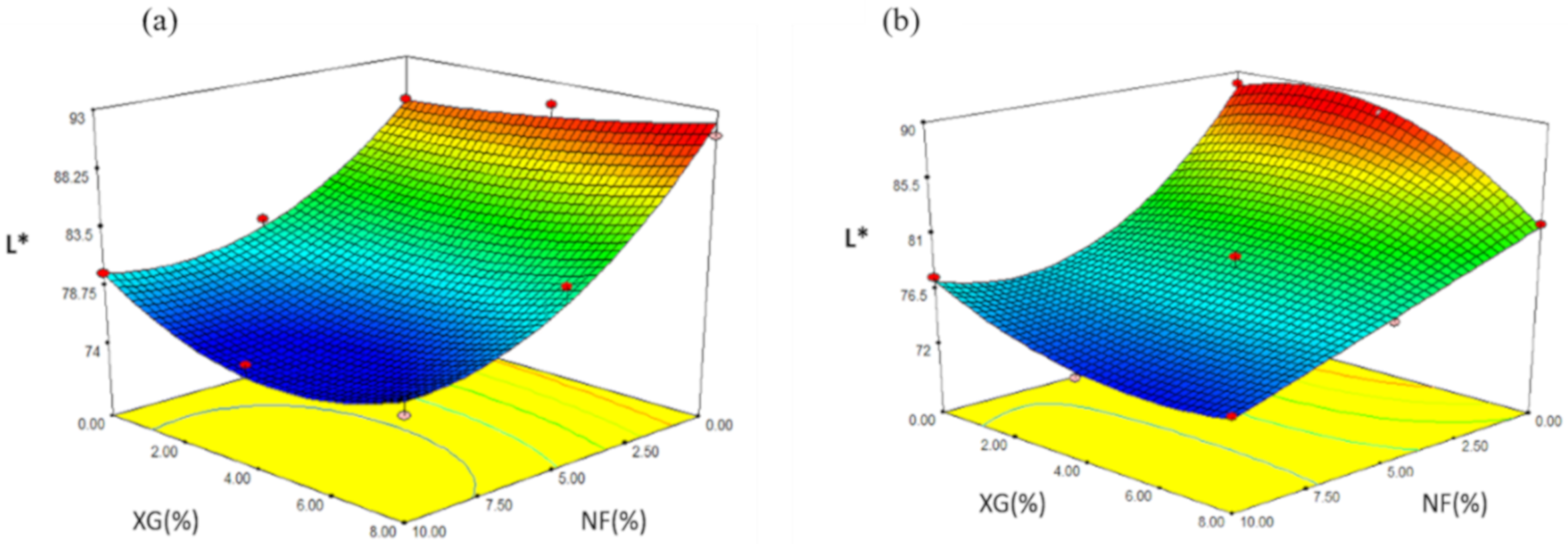
2.4.4. Brightness (L*)
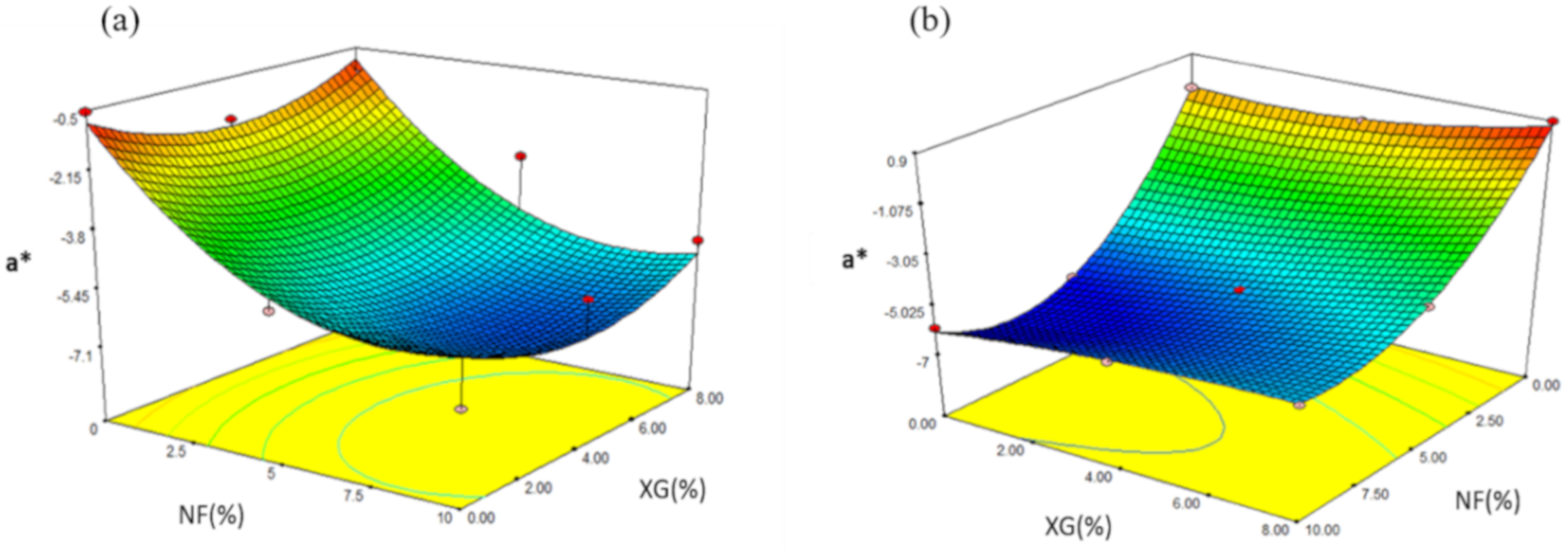
2.4.5. Parameter a* (Trend from –Green to + Red)
2.4.6. Optimization of the Third-Generation Snack
2.5. Scanning Electron Microscopy (SEM)
3. Materials and Methods
3.1. Raw Material
3.2. Starch Acetylation
3.3. Nopal Conditioning
3.4. Technological Process
3.5. Characterization of Native Rice Starch, Modified by Extrusion and Nopal Flour
3.5.1. Proximal Analysis
3.5.2. pH
3.5.3. Determination of Particle Size
3.5.4. Water Absorption Index (WAI) and Water Solubility Index (WSI)
3.5.5. Color
3.5.6. Degree of Substitution (DS)
3.5.7. Rheology
3.5.8. Differential Scanning Calorimetry (DSC)
3.5.9. Fourier Transform Infrared Spectrometry (FTIR)
3.5.10. Scanning Electron Microscopy (SEM)
3.6. Expanded Product Characterization
3.6.1. Color
3.6.2. Hardness (H)
3.6.3. Expansion Index (EI) and Apparent Density (AD)
3.6.4. Scanning Electron Microscopy (SEM)
3.7. Experimental Design and Results Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Espinosa, A.M. La consolidación del ambiente obesogénico en México. Estud. Soc. 2017, 27, 32. [Google Scholar] [CrossRef][Green Version]
- Ciappini, C.; Gatti, B.; Cabreriso, S.; Chaín, P. Modificaciones fisicoquímicas y sensoriales producidas durante las frituras domésticas sobre aceite de girasol refinado y aceite de oliva extra. Invenio 2016, 19, 153–161. [Google Scholar]
- Morales, L.E. Morfometría de los granos de arroz (Oryza sativa L.) y caracterización fisicoquímica, estructural y reológica de las harinas integrales de las variedades Morelos A-92 y Koshihikari y de seis líneas provenientes de estas. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, México, June 2012. [Google Scholar]
- Olatunde, G.O.; Arogundade, L.K.; Orija, O.I. Chemical, functional and pasting properties of banana and plantain starches modified by pre-gelatinization, oxidation and acetylation. Cogent Food Agric. 2017, 3, 12. [Google Scholar] [CrossRef]
- Alvarez, C.C.Y. Efecto de la acetilación en las propiedades fisicoquímicas y funcionales de almidón de Quinua (Chenopodium quinoa Willd). Master’s Thesis, Universidad Nacional San Antonio Abad, Cucso city, Perú, January 2016. [Google Scholar]
- El Halal, S.L.M.; Colussi, R.; Pinto, V.Z.; Bartz, J.; Radunz, M.; Carreño, N.L.V.; Dias, A.R.G.; Zavareze, E.D.R. Structure, morphology and functionality of acetylated and oxidised barley starches. Food Chem. 2015, 168, 247–256. [Google Scholar] [CrossRef]
- Jarrillo, G.C.; Marín, M.L.R.; Román-Gutiérrez, A.D.; Ortíz, F.A.G. Evaluación de las propiedades fisicoquímicas de almidones de diferentes cereales. PÄDI Boletín Científico de Ciencias Básicas e Ingenierías del ICBI 2016, 3, 5. [Google Scholar] [CrossRef]
- Ruiz-Saenz, T.S.; Galicia-García, T.; Estrada-Moreno, I.A.; Mendoza-Duarte, M.E.; Márquez-Meléndez, R.; Márquez-Goméz, M.; Ruiz-Gutiérrez, M.G.; Quintero-Ramos, A.; Portillo-Arroyo, B.; Soto-Figueroa, C. Effect of the Extraction, Chemical Modification and Extrusion of Tritical Starch (Triticosecale) in its Functional Properties. Biotecnia 2020, 22, 153–159. [Google Scholar] [CrossRef]
- Vargas, G.; Velezmoro, C.; Martinez, P. Functional properties of potato (Solanum tuberosum) starch and its chemical modification by acetylation. Sci. Agropecu. 2016, 7, 223–230. [Google Scholar] [CrossRef][Green Version]
- Astello Garcia, M.G.; Cervantes, I.; Nair, V.; Santos Díaz, M.d.S.; Reyes Agüero, A.; Guéraud, F.; Negre Salvayre, A.; Rossignol, M.; Cisneros Zevallos, L.; Barba de la Rosa, A.P. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Ali, A.; Wani, T.A.; Wani, I.A.; Masoodi, F.A. Comparative study of the physico-chemical properties of rice and corn starches grown in Indian temperate climate. J. Saudi Soc. Agric. Sci. 2016, 15, 75–82. [Google Scholar] [CrossRef]
- Chávez González, J.L. Estudios in vivo para evaluar el efecto fisiológico de la ingesta de tortilla de maíz adicionada con harina de nopal. Master’s Thesis, Universidad Michoacana de San Nicolas de Hidalgo, Morelia, Michoacan, México, April 2015. [Google Scholar]
- Dick, M.; Limberger, C.; Thys, R.C.S.; Rios, A.D.O.; Flôres, S.H. Mucilage and cladode flour from cactus (Opuntia monacantha) as alternative ingredients in gluten-free crackers. Food Chem. 2020, 314, 126178. [Google Scholar] [CrossRef]
- Márquez-Gómez, M. Utilización de agregados esféricos de almidón en la microencapsulación de aceites esenciales. Master’s Thesis, Universidad Autónoma de Chihuahua, Chihuahua, Chihuahua, México, August 2016. [Google Scholar]
- Guevara-Arauza, J.C.; Bárcenas, D.G.; Ortega-Rivas, E.; Pérez-Martínez, J.; Reyes-Hernández, J.; Ornelas-Paz, J.D.J. Effect of fiber fractions of prickly pear cactus (nopal) on quality and sensory properties of wheat bread rolls. J. Food Sci. Technol. 2014, 52, 2990–2997. [Google Scholar] [CrossRef] [PubMed]
- Colussi, R.; El Halal, S.L.M.; Pinto, V.; Bartz, J.; Gutkoski, L.C.; Zavareze, E.D.R.; Dias, A.R.G. Acetylation of rice starch in an aqueous medium for use in food. LWT 2015, 62, 1076–1082. [Google Scholar] [CrossRef]
- Castellanos-Gallo, L.; Galicia-García, T.; Estrada-Moreno, I.A.; Mendoza-Duarte, M.; Márquez-Meléndez, R.; Portillo-Arroyo, B.; Soto-Figueroa, C.; Leal-Ramos, M.Y.; Aldana, D.S. Development of an Expanded Snack of Rice Starch Enriched with Amaranth by Extrusion Process. Molecules 2019, 24, 2430. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Mendoza, J.G.; Rodríguez-Lora, M.C.; Figueroa-Flórez, J.A. Effect of acetylation on structural and functional properties starches from cassava (Manihot esculenta Crantz) and yam (Dioscorea alatacv. Diamante 22). Revista Mexicana de Ingeniería Química 2016, 15, 787–796. [Google Scholar]
- Tupa, M.V. Desarrollo de una metodología sostenible de síntesis de almidones acetilados. Master’s Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, April 2015. [Google Scholar]
- Nawaz, M.A.; Fukai, S.; Prakash, S.; Bhandari, B. Effect of starch modification in the whole white rice grainson physicochemical properties of two contrasting rice varieties. J. Cereal Sci. 2018, 80, 143–149. [Google Scholar] [CrossRef]
- Anuntagool, J.; Alvarez, G.; Flick, D. Predictive model for viscosity development of modified rice starch suspension under unsteady temperature change. J. Food Eng. 2017, 209, 45–51. [Google Scholar] [CrossRef]
- Cardoso, F.F.; Ascheri, D.P.R.; Carvalho, C.W.P. Propiedades reológicas y de adsorción de agua de harina extrudida de arroz y bagazo de cebada. Rev. Ceres 2014, 61, 313–322. [Google Scholar] [CrossRef]
- Ali, S.; Singh, B.; Sharma, S. Effect of processing temperature on morphology, crystallinity, functional properties, and in vitro digestibility of extruded corn and potato starches. J. Food Process. Preserv. 2020, 44, 14531. [Google Scholar] [CrossRef]
- Korkerd, S.; Wanlapa, S.; Puttanlek, C.; Uttapap, D.; Rungsardthong, V. Expansion and functional properties of extruded snacks enriched with nutrition sources from food processing by-products. J. Food Sci. Technol. 2016, 53, 561–570. [Google Scholar] [CrossRef]
- Guerrero, B.G.; Montero-Montero, J.C.; Fernández-Quintero, A.; Rivera-Agredo, Y.J.; Patiño, B.O.; Gallego, S. Assessing the effect of adding maize and rice brans in the development by twin-screw extrusion of a ready-to-eat cereal formulated with flours of quality protein maize and zinc biofortified rice. DYNA 2019, 86, 298–303. [Google Scholar] [CrossRef]
- Gómez-López, P.; Aquino-Bolaños, F.; Martínez-Bustos, I.; Los Alimentos Extrudidos Están Por Todos Lados. La Ciencia y el Hombre. Revista de divulgación científica y tecnológica de la Universidad Veracruzana. Available online: https://www.uv.mx/cienciahombre/revistae/vol26num1/articulos/los-alimentos-extruidos.html26 (accessed on 13 October 2020).
- Delgado-Nieblas, C.I.; Zazueta-Morales, J.D.J.; Gallegos-Infante, J.; Aguilar-Palazuelos, E.; Camacho-Hernández, I.; Ordorica-Falomir, C.; De Melo, M.P.; Carrillo-Lopez, A. Elaboration of functional snack foods using raw materials rich in carotenoids and dietary fiber: Effects of extrusion processing. Food 2014, 13, 69–79. [Google Scholar] [CrossRef]
- Félix-Medina, J.; Montes-Ávila, J.; Reyes-Moreno, C.; Perales-Sánchez, J.X.K.; Gómez-Favela, M.A.; Aguilar-Palazuelos, E.; Gutiérrez-Dorado, R. Second-generation snacks with high nutritional and antioxidant value produced by an optimized extrusion process from corn/common bean flours mixtures. LWT 2020, 124, 109172. [Google Scholar] [CrossRef]
- Design-Expert; Version 9.0.0.; Stat-Ease Inc.: Minneapolis, MN, USA, 2014.
- Delgado-Nieblas, C.; Aguilar-Palazuelos, E.; Gallegos-Infante, A.; Rocha-Guzmán, N.; Zazueta-Morales, J.; Caro-Corrales, J. Characterization and Optimization of Extrusion Cooking for the Manufacture of Third-Generation Snacks with Winter Squash (Cucurbita moschata D.) Flour. Cereal Chem. J. 2012, 89, 65–72. [Google Scholar] [CrossRef]
- Molina-Rosell, C. Alimentos sin gluten derivados de cereales. Enfermedad celíaca y sensibilidad al gluten no celíaca. OmniaScience. Barcelona 2013, 4, 447–461. [Google Scholar]
- Encina-Zelada, C.R.; Cadavez, V.; Monteiro, F.; Teixeira, J.A.; Gonzales-Barron, U. Combined effect of xanthan gum and water content on physicochemical and textural properties of gluten-free batter and bread. Food Res. Int. 2018, 111, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Mechanical and thermal properties of yam starch films. Food Hydrocoll. 2005, 19, 157–164. [Google Scholar] [CrossRef]
- Boukid, F.; Boukid, Z.; Mejri, M. Opuntia cladodes: Physicochemical parameters, functional properties and application in formulation of rolled cake of cladode flour fabric (Part 2). Int. J. Adv. Trends Comput. Sci. Eng. 2015, 1, 30–34. [Google Scholar]
- Penna, R.S.M. Formulación y Elaboración de un “Snack” Frito Con Incorporación de un Ingrediente Funcional. Master’s Thesis, Universidad de Chile, Santiago, Chile, January 2016. [Google Scholar]
- Association-of-Official-Analytical-Chemist-(AOAC). Official Methods of Analysis; Association-of-Official-Analytical-Chemist-(AOAC): Gaithersburg, MA, USA, 2016. [Google Scholar]
- Badui, D.S. Química de los Alimentos. Available online: http://depa.fquim.unam.mx/amyd/archivero/Libro-Badui2006_26571.pdf (accessed on 18 November 2020).
- American-Association-of-Cereal-Chemists-(AACC). Approved methods of the American Association of Cereal Chemists.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Grajeda Nieto, P. Desarrollo de películas a base de almidón, mucilago de chia (Salvia hispánica) y nopal (Opuntia ficus-indica) por el método de vaciado en placa. Master’s Thesis, Universidad Autónoma de Chihuahua, Chihuahua, México, June 2017. [Google Scholar]
- American-Society-for-Testing-and-Materials-(ASTM). Standard Test Method for Color Determination of Plastic Pellets. ASTM D6290 International; ASTM: West Conshonhocken, PA, USA, 2013. [Google Scholar]
- Sulbarán, A.; Matiz, G.E.; Baena, Y. Acetilación del almidón de millo (Pennisetum glaucum) y evaluación de su aplicación como posible excipiente. Revista Colombiana de Ciencias Químico-Farmacéuticas 2018, 47, 255–276. [Google Scholar]
- López-Castejón, M.L.; Bengoechea, C.; Espinosa, S.; Carrera, C. Caracterización de emulsiones prebióticas estabilizadas con inulina y b-lactoglobulina. Afinidad 2019, 76, 12–22. [Google Scholar]
- Wang, Y.; Zhan, J.; Lu, H.; Chang, R.; Qiu, L.; Tian, Y. Amylopectin crystal seeds: Characterization and their effect on amylopectin retrogradation. Food Hydrocoll. 2021, 111, 106409. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; Contreras-Jiménez, B.; Hernández-Becerra, E.; Rodriguez-Garcia, M.E. Physicochemical changes of starch during malting process of sorghum grain. J. Cereal Sci. 2020, 95, 103069. [Google Scholar] [CrossRef]
- Fiorda, F.A.; Soares, M.S.; Da Silva, F.A.; De Moura, C.M.A.; Grossmann, M.V.E. Physical quality of snacks and technological properties of pre-gelatinized flours formulated with cassava starch and dehydrated cassava bagasse as a function of extrusion variables. LWT 2015, 62, 1112–1119. [Google Scholar] [CrossRef]
- Limón-Valenzuela, V.; Aguilar-Palazuelos, E.; Zazueta-Morales, J.D.J.; Martínez-Bustos, F. Propiedades microestructurales y de formación de pasta de pellets extrudidos elaborados a partir de almidón de maíz enriquecidos con MCP y concentrado proteínico de leche. Revista Mexicana de Ingeniería Química 2017, 16, 193–205. [Google Scholar]




| Chemical Properties | NS (%) | MS (%) | NF (%) |
|---|---|---|---|
| Protein | 0.60 ± 0.01 a | 0.45 ± 0.02 b | 13.23 ± 0.58 c |
| Fat | 0.32 ± 0.04 a | 0.13 ± 0.00 b | 2.12 ± 0.13 c |
| Ashes | 0.14 ± 0.00 a | 0.13 ± 0.01 a | 16.69 ± 0.12 b |
| Humidity | 10.01 ± 0.04 a | 10.33 ± 0.30 b | 4.70 ± 0.15 c |
| Fiber | 0 | 0 | 9.63 ± 0.04 |
| Physicochemical Property | NS | MS | NF |
|---|---|---|---|
| WAI | 1.83 ± 0.04 a | 4.69 ± 0.04 b | 4.70 ± 0.16 b |
| WSI | 0.47 ± 0.02 a | 12.61 ± 0.10 b | 24.98 ± 0.28 c |
| pH | 6.55 ± 0.05 a | 8.70 ± 0.01 b | 4.35 ± 0.07 c |
| L* | 98.06 ± 0.42 a | 97.80 ± 0.38 b | 60.73 ± 0.008 c |
| a* | 0.016 ± 0.013 a | 0.06 ± 0.01 a | −6.51 ± 0.004 b |
| b* | −0.36 ± 0.052 a | −2.09 ± 0.01 b | 28.55 ± 0.02 c |
| To (°C) | Tp (°C) | Tf (°C) | ΔH (mW) | |
|---|---|---|---|---|
| NS | 65.40 ± 0.09 a | 71.58 ± 0.10 a | 81.85 ± 0.10 a | 24.03 ± 0.09 a |
| MS | 65.56 ± 0.00 b | 65.92 ± 0.00 b | 66.60 ± 0.00 b | 23.78 ± 0.18 a |
| n’ (Pa.s) | Tg (°C) | Retrogradation (Pa.s) | |
|---|---|---|---|
| NS | 79.745 ± 0.07 a | 75 ± 0.33 a | 0.36 ± 0.03 a |
| MS | 81.09 ± 0.94 a | 65.85 ± 0.20 b | 3.35 ± 0.04 b |
| Formulation | NF (%) | XG (%) | EI | AD | H | L* | a* |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 4 | 1.08 | 0.17 | 1.19 | 76.58 | −7.01 |
| 2 | 0 | 0 | 0.32 | 0.16 | 0.84 | 89.22 | −0.54 |
| 3 | 10 | 4 | 2.61 | 0.18 | 1.12 | 75.92 | −5.15 |
| 4 | 0 | 8 | 2.65 | 0.21 | 1.68 | 90.97 | −1.32 |
| 5 | 10 | 8 | 2.38 | 0.20 | 1.85 | 76.00 | −4.80 |
| 6 | 5 | 8 | 0.84 | 0.37 | 2.25 | 82.15 | −3.12 |
| 7 | 5 | 0 | 4.47 | 0.14 | 0.90 | 81.35 | −5.04 |
| 8 | 0 | 4 | 0.73 | 0.21 | 1.80 | 91.09 | −1.73 |
| 9 | 10 | 0 | 2.97 | 0.20 | 0.78 | 79.88 | −6.58 |
| Coefficients | YEI | YAD | YH | YL* | Ya* |
|---|---|---|---|---|---|
| Intercept | 1.47 | 0.21 | 1.38 | 78.65 | −5.77 |
| Linear | |||||
| β1 | 0.71 ** | −0.34 * | −7.59 ** | −2.16 *** | |
| β2 | −1.81 ** | 0.12 *** | 0.68 *** | ||
| Quadratic | |||||
| β11 | −0.033 ns | 3.82 * | 1.7 * | ||
| Β22 | 0.80 ns | 0.027 ns | 2.06 ns | 1.06 ns | |
| Interaction | |||||
| β1 * β2 | −0.73 * | −1.41ns | |||
| β11 * β2 | 2.25 ** | −0.10 ** | −0.20 ns | ||
| β1 * β22 | 0.37 * | 1.51 ns | |||
| R2 | 0.9431 | 0.8672 | 0.9227 | 0.9653 | 0.8215 |
| p≤ | 0.0438 | 0.0482 | 0.0170 | 0.0213 | 0.0256 |
| Formulation | NF | XG (%) | EI | H | AD | L* | a* |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 4 | 2.35 | 0.14 | 8.71 | 79.31 | −5.10 |
| 2 | 0 | 0 | 1.78 | 0.15 | 6.05 | 89.01 | −0.49 |
| 3 | 10 | 4 | 2.02 | 0.16 | 13.12 | 72.71 | −5.33 |
| 4 | 0 | 8 | 1.53 | 0.17 | 12.09 | 82.0 | 0.87 |
| 5 | 10 | 8 | 2.08 | 0.16 | 11.43 | 73.55 | −4.98 |
| 6 | 5 | 8 | 1.58 | 0.17 | 11.21 | 77.26 | −3.97 |
| 7 | 5 | 0 | 2.76 | 0.14 | 6.38 | 76.91 | −6.32 |
| 8 | 0 | 4 | 1.40 | 0.21 | 8.6 | 88.57 | −0.48 |
| 9 | 10 | 0 | 2.29 | 0.16 | 6.58 | 77.48 | −5.90 |
| Coefficients | YEI | YAD | YH | YL* | Ya* |
|---|---|---|---|---|---|
| Intercept | 2.17 | 0.16 | 9.56 | 78.38 | −5.24 |
| Linear | |||||
| β1 | 0.28 ** | −0.025 ns | 2.26 ** | −7.93 *** | −2.42 *** |
| β2 | −0.59 ** | 0.015 ns | 2.62 *** | 1.17 ** | |
| Quadratic | |||||
| β11 | −0.38 ** | 0.018 ns | 0.88 ns | 2.73 * | 2.41 *** |
| Β22 | 0.083 ns | −0.012 ns | −1.18 ns | −0.83 ns | 0.17 ns |
| Interaction | |||||
| β1 * β2 | 0.77 ns | ||||
| β11 *β2 | 0.47 ** | −0.001 ns | −2.74 ** | −0.60 * | |
| β1 * β22 | 0.025 ns | −2.29 * | 2.94 * | 0.39 ns | |
| R2 | 0.95 | 0.77 | 0.96 | 0.99 | 0.99 |
| p≤ | 0.0313 | 0.5401 | 0.0226 | 0.0217 | 0.0048 |
| Snacks | EI | AD | H | L* | a* |
|---|---|---|---|---|---|
| TS | 2.36 ± 0.26a | 0.13 ± 0.01 a | 4.83 ± 1.26 a | 81.33 ± 0.52 a | 7.9 ± 0.28 a |
| M | 0.15 ± 0.01 a | 5.4 ± 0.65 b | 72.27 ± 0.62 b | 10.37 ± 0.23 b | |
| VB | 0.12 ± 0.006 a | 4.63 ± 0.32 a | 66.76 ± 0.79 c | 9.8 ± 0.48 b |
| Formulation | Nopal Flour (NF) | Xanthan Rubber (XG) |
|---|---|---|
| 1 | 5 | 4 |
| 2 | 0 | 0 |
| 3 | 10 | 4 |
| 4 | 0 | 8 |
| 5 | 10 | 8 |
| 6 | 5 | 8 |
| 7 | 5 | 0 |
| 8 | 0 | 4 |
| 9 | 10 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anchondo-Trejo, C.; Loya-Carrasco, J.A.; Galicia-García, T.; Estrada-Moreno, I.; Mendoza-Duarte, M.; Castellanos-Gallo, L.; Márquez-Meléndez, R.; Portillo-Arroyo, B.; Soto-Figueroa, C. Development of a Third Generation Snack of Rice Starch Enriched with Nopal Flour (Opuntia ficus indica). Molecules 2021, 26, 54. https://doi.org/10.3390/molecules26010054
Anchondo-Trejo C, Loya-Carrasco JA, Galicia-García T, Estrada-Moreno I, Mendoza-Duarte M, Castellanos-Gallo L, Márquez-Meléndez R, Portillo-Arroyo B, Soto-Figueroa C. Development of a Third Generation Snack of Rice Starch Enriched with Nopal Flour (Opuntia ficus indica). Molecules. 2021; 26(1):54. https://doi.org/10.3390/molecules26010054
Chicago/Turabian StyleAnchondo-Trejo, Cecilia, Jaime Alonso Loya-Carrasco, Tomás Galicia-García, Iván Estrada-Moreno, Mónica Mendoza-Duarte, Lilisbet Castellanos-Gallo, Rubén Márquez-Meléndez, Beatriz Portillo-Arroyo, and Cesar Soto-Figueroa. 2021. "Development of a Third Generation Snack of Rice Starch Enriched with Nopal Flour (Opuntia ficus indica)" Molecules 26, no. 1: 54. https://doi.org/10.3390/molecules26010054
APA StyleAnchondo-Trejo, C., Loya-Carrasco, J. A., Galicia-García, T., Estrada-Moreno, I., Mendoza-Duarte, M., Castellanos-Gallo, L., Márquez-Meléndez, R., Portillo-Arroyo, B., & Soto-Figueroa, C. (2021). Development of a Third Generation Snack of Rice Starch Enriched with Nopal Flour (Opuntia ficus indica). Molecules, 26(1), 54. https://doi.org/10.3390/molecules26010054