Properties of High-Swelling Native Starch Treated by Heat–Moisture Treatment with Different Holding Times and Iterations
Abstract
1. Introduction
2. Results and Discussion
2.1. Chain Length Distribution
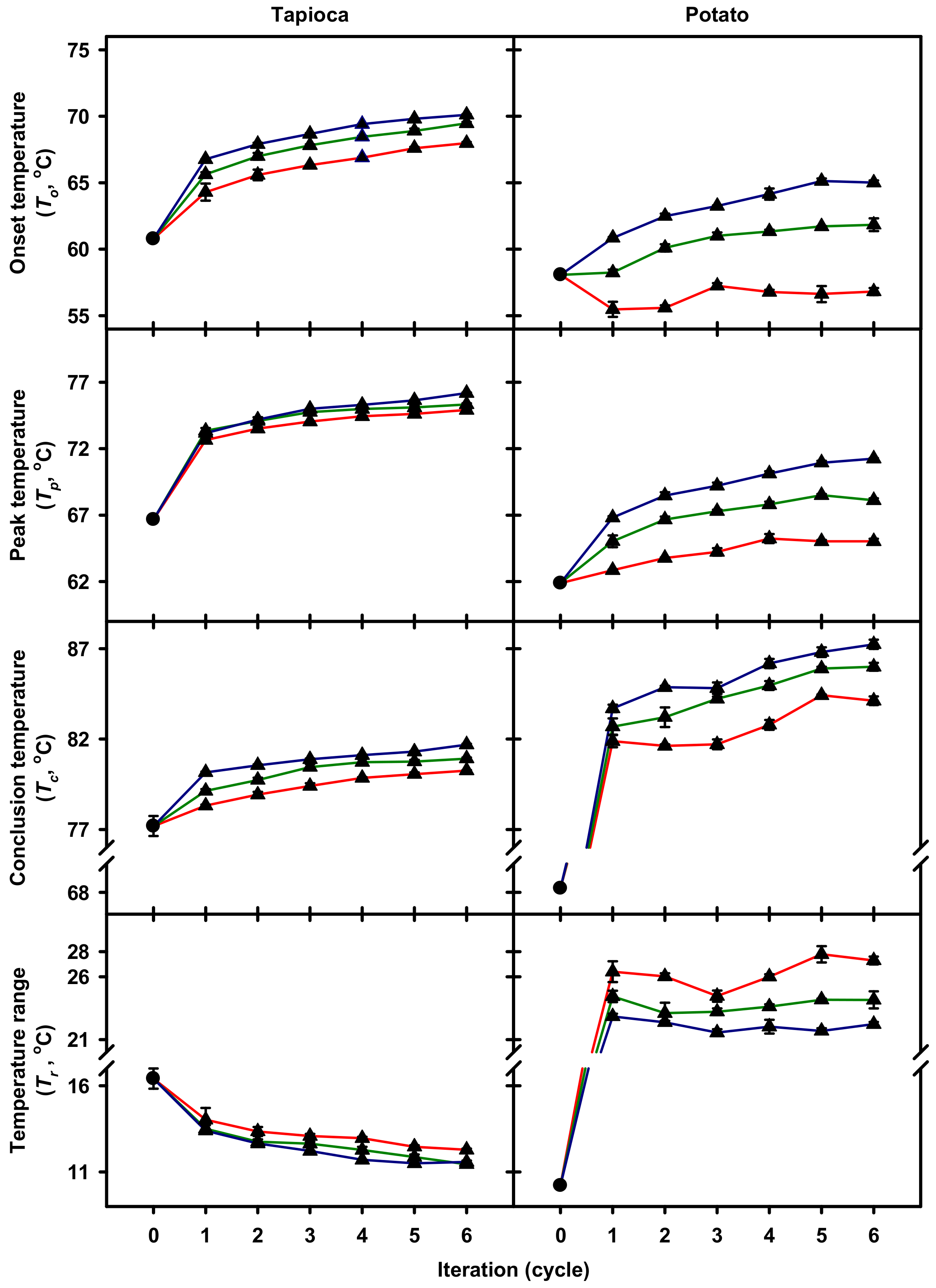
2.2. Gelatinization Thermal Properties
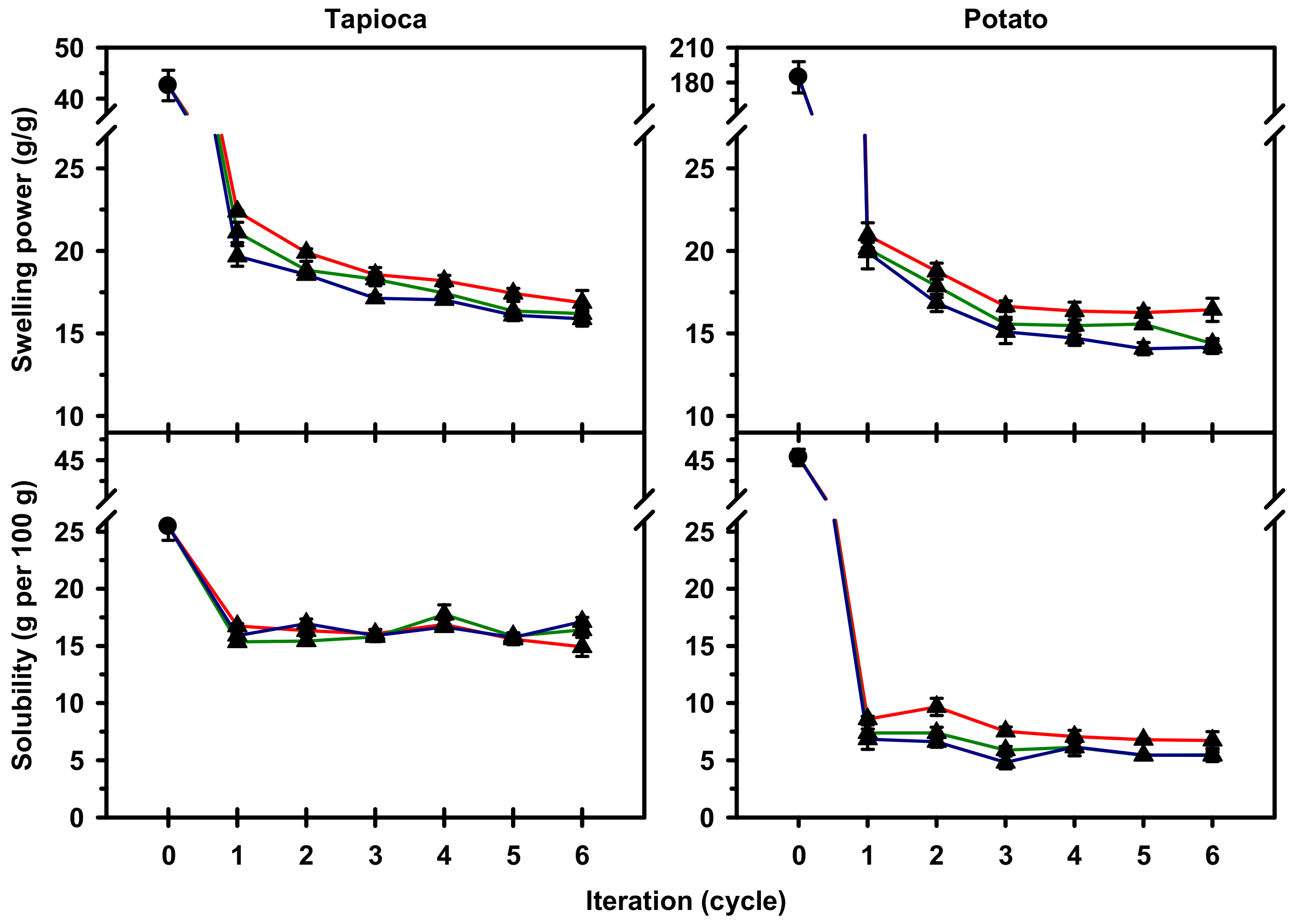
2.3. Swelling Power and Solubility
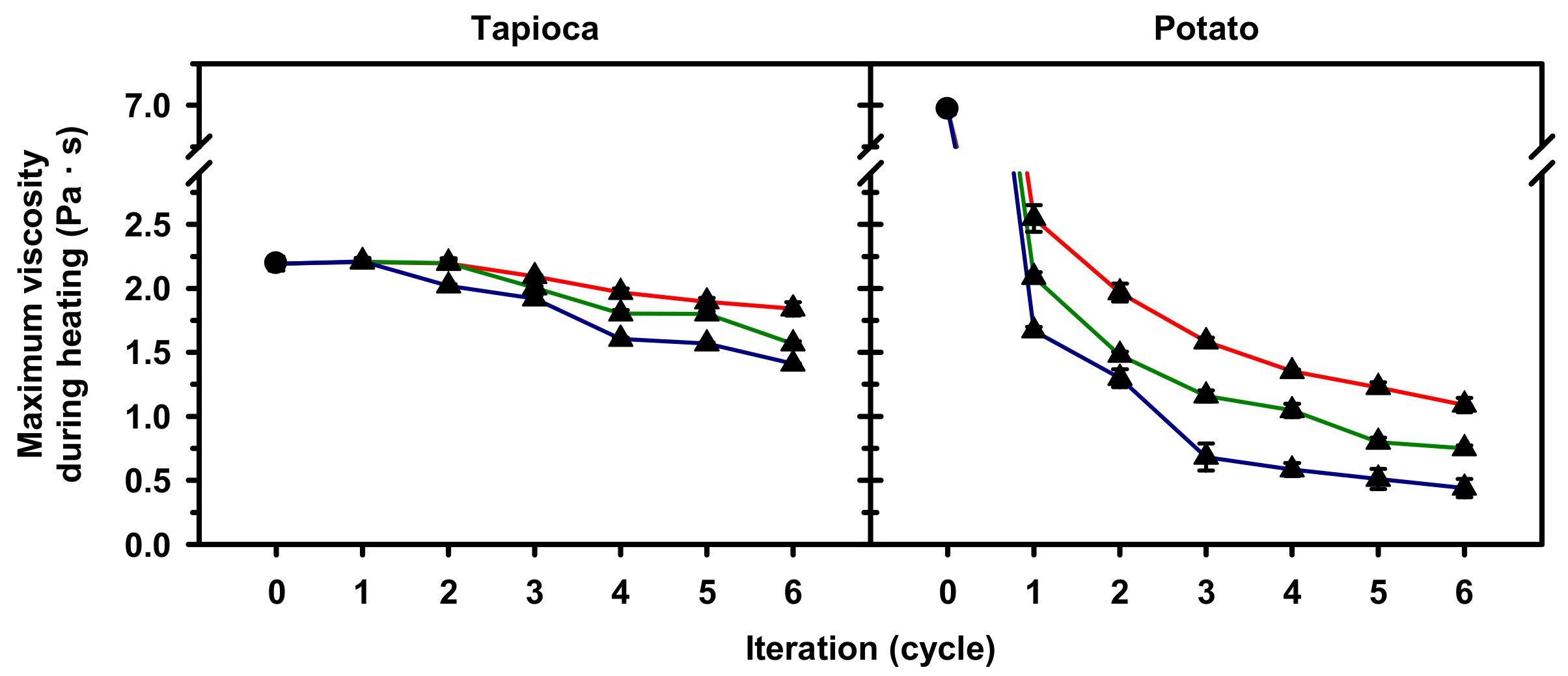
2.4. Pasting Properties
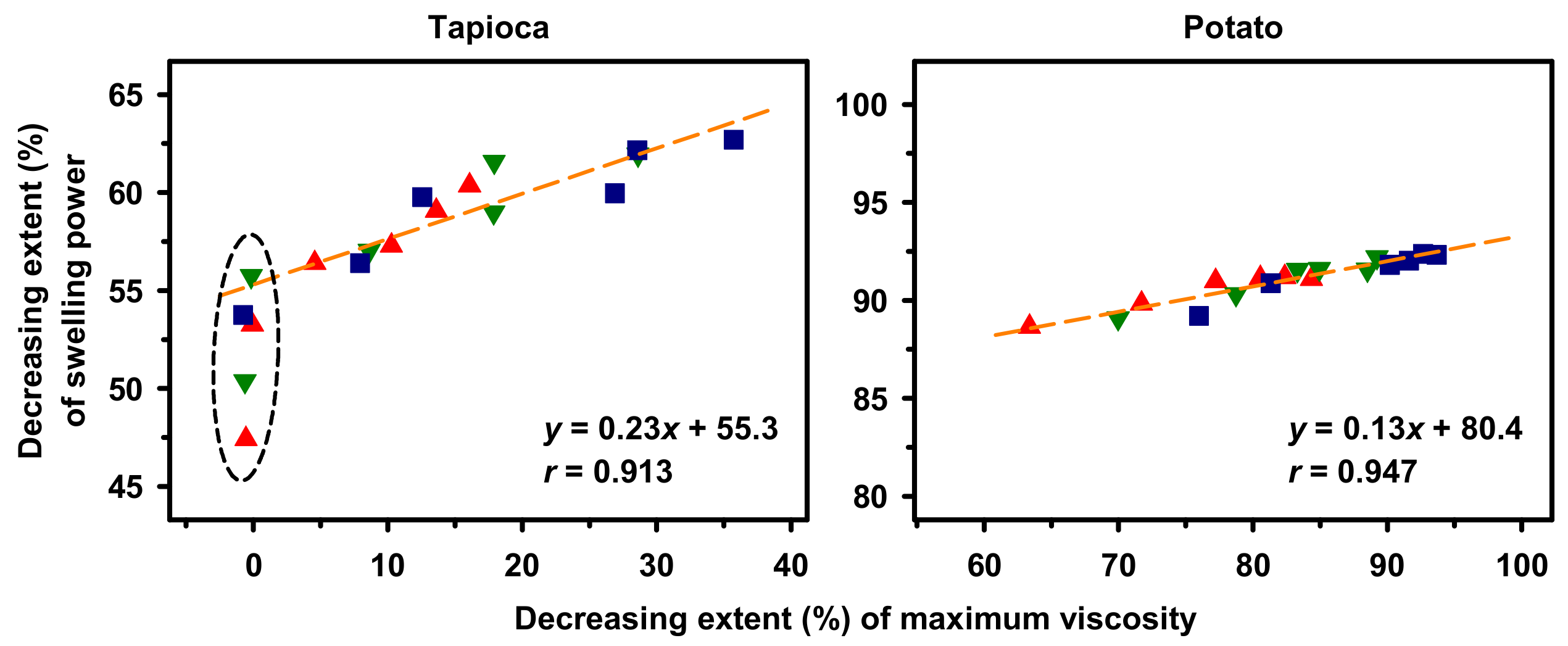
2.5. Correlation between Swelling Power and Maximum Viscosity
3. Materials and Methods
3.1. Materials
Heat–Moisture Treatment
3.2. Methods
3.2.1. Chain Length Distribution
3.2.2. Gelatinization Thermal Properties
3.2.3. Swelling Power and Solubility
3.2.4. Pasting Properties
3.2.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoover, R. The impact of heat–moisture treatment on molecular structures and properties of starches isolated from different botanical sources. Crit. Rev. Food Sci. Nutr. 2010, 50, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.; Hoover, R. Effect of heat–moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Jiranuntakul, W.; Puttanlek, C.; Rungsardthong, V.; Puncha-Arnon, S.; Uttapap, D. Microstructural and physicochemical properties of heat-moisture treated waxy and normal starches. J. Food Eng. 2011, 104, 246–258. [Google Scholar] [CrossRef]
- Sui, Z.; Yao, T.; Zhao, Y.; Ye, X.; Kong, X.; Ai, L. Effects of heat–moisture treatment reaction conditions on the physicochemical and structural properties of maize starch: Moisture and length of heating. Food Chem. 2015, 173, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Li, X.; Chen, L.; Zhang, B. Multi-scale structure, pasting and digestibility of heat moisture treated red adzuki bean starch. Int. J. Biol. Macromol. 2017, 102, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Vermeylen, R.; Goderis, B.; Delcour, J.A. An X-ray study of hydrothermally treated potato starch. Carbohydr. Polym. 2006, 64, 364–375. [Google Scholar] [CrossRef]
- Huang, T.-T.; Zhou, D.-N.; Jin, Z.-Y.; Xu, X.-M.; Chen, H.-Q. Effect of repeated heat-moisture treatments on digestibility, physicochemical and structural properties of sweet potato starch. Food Hydrocoll. 2016, 54, 202–210. [Google Scholar] [CrossRef]
- Suriya, M.; Reddy, C.K.; Haripriya, S. Functional and thermal behaviors of heat-moisture treated elephant foot yam starch. Int. J. Biol. Macromol. 2019, 137, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Xu, M.; Li, B.; Wu, H.; Liu, Y.; Zhang, G.; Ouyang, S.; Li, W. Repeated heat-moisture treatment exhibits superiorities in modification of structural, physicochemical and digestibility properties of red adzuki bean starch compared to continuous heat-moisture way. Food Res. Int. 2017, 102, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Wu, H.; Saleh, A.S.M.; Jing, L.; Liu, Y.; Zhao, K.; Su, C.; Zhang, B.; Jiang, H.; Li, W. Effects of repeated and continuous dry heat treatments on properties of sweet potato starch. Int. J. Biol. Macromol. 2019, 129, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Srichuwong, S.; Jane, J.-l. Physicochemical properties of starch affected by molecular composition and structures: A review. Food Sci. Biotechnol. 2007, 16, 663–674. [Google Scholar]
- Lin, C.-L.; Lin, J.-H.; Lin, J.-J.; Chang, Y.-H. Progressive alterations in crystalline structure of starches during heat-moisture treatment with varying iterations and holding times. Int. J. Biol. Macromol. 2019, 135, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Zavareze, E.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Perera, C.; Shen, J.; Ye, L.; Suh, D.-S.; Jane, J.-L. Molecular structure of selected tuber and root starches and effect of amylopectin structure on their physical properties. J. Agric. Food Chem. 2009, 57, 1556–1564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varatharajan, V.; Hoover, R.; Li, J.; Vasanthan, T.; Nantanga, K.K.M.; Seetharaman, K.; Liu, Q.; Donner, E.; Jaiswal, S.; Chibbar, R.N. Impact of structural changes due to heat-moisture treatment at different temperatures on the susceptibility of normal and waxy potato starches towards hydrolysis by porcine pancreatic alpha amylase. Food Res. Int. 2011, 44, 2594–2606. [Google Scholar] [CrossRef]
- Chung, H.-J.; Hoover, R.; Liu, Q. The impact of single and dual hydrothermal modifications on the molecular structure and physicochemical properties of normal corn starch. Int. J. Biol. Macromol. 2009, 44, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Hizukuri, S.; Abe, J.; Hanashiro, I. Starch: Analytical aspects. In Carbohydrates in Food; Eliasson, A.-C., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 305–390. [Google Scholar]
- Chang, Y.-H.; Lin, C.-L.; Chen, J.-C. Characteristics of mung bean starch isolated by using lactic acid fermentation solution as the steeping liquor. Food Chem. 2006, 99, 794–802. [Google Scholar] [CrossRef]
- Lin, J.-H.; Kao, W.-T.; Tsai, Y.-C.; Chang, Y.-H. Effect of granular characteristics on pasting properties of starch blends. Carbohydr. Polym. 2013, 98, 1553–1560. [Google Scholar] [CrossRef] [PubMed]



| Tapioca | Potato | |
|---|---|---|
| Amylose | ||
| % 2 | 21.3 ± 0.5 | 21.2 ± 0.3 |
| DPw 3 | 9944 ± 925 | 9234 ± 123 |
| Amylopectin | ||
| B2+ chains | ||
| % | 21.3 ± 0.4 | 29.6 ± 0.2 |
| DPw | 57.8 ± 1.4 | 63.1 ± 0.2 |
| B1 chains | ||
| % | 29.9 ± 0.5 | 27.6 ± 0.0 |
| DPw | 23.4 ± 0.1 | 23.7 ± 0.0 |
| A chains | ||
| % | 27.4 ± 0.9 | 21.6 ± 0.2 |
| DPw | 11.6 ± 0.1 | 12.4 ± 0.0 |
| S/L ratio 4 | 2.69 ± 0.08 | 1.66 ± 0.01 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-L.; Lin, J.-H.; Lin, J.-J.; Chang, Y.-H. Properties of High-Swelling Native Starch Treated by Heat–Moisture Treatment with Different Holding Times and Iterations. Molecules 2020, 25, 5528. https://doi.org/10.3390/molecules25235528
Lin C-L, Lin J-H, Lin J-J, Chang Y-H. Properties of High-Swelling Native Starch Treated by Heat–Moisture Treatment with Different Holding Times and Iterations. Molecules. 2020; 25(23):5528. https://doi.org/10.3390/molecules25235528
Chicago/Turabian StyleLin, Chia-Long, Jheng-Hua Lin, Jia-Jing Lin, and Yung-Ho Chang. 2020. "Properties of High-Swelling Native Starch Treated by Heat–Moisture Treatment with Different Holding Times and Iterations" Molecules 25, no. 23: 5528. https://doi.org/10.3390/molecules25235528
APA StyleLin, C.-L., Lin, J.-H., Lin, J.-J., & Chang, Y.-H. (2020). Properties of High-Swelling Native Starch Treated by Heat–Moisture Treatment with Different Holding Times and Iterations. Molecules, 25(23), 5528. https://doi.org/10.3390/molecules25235528





