Multi-Targeting Bioactive Compounds Extracted from Essential Oils as Kinase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
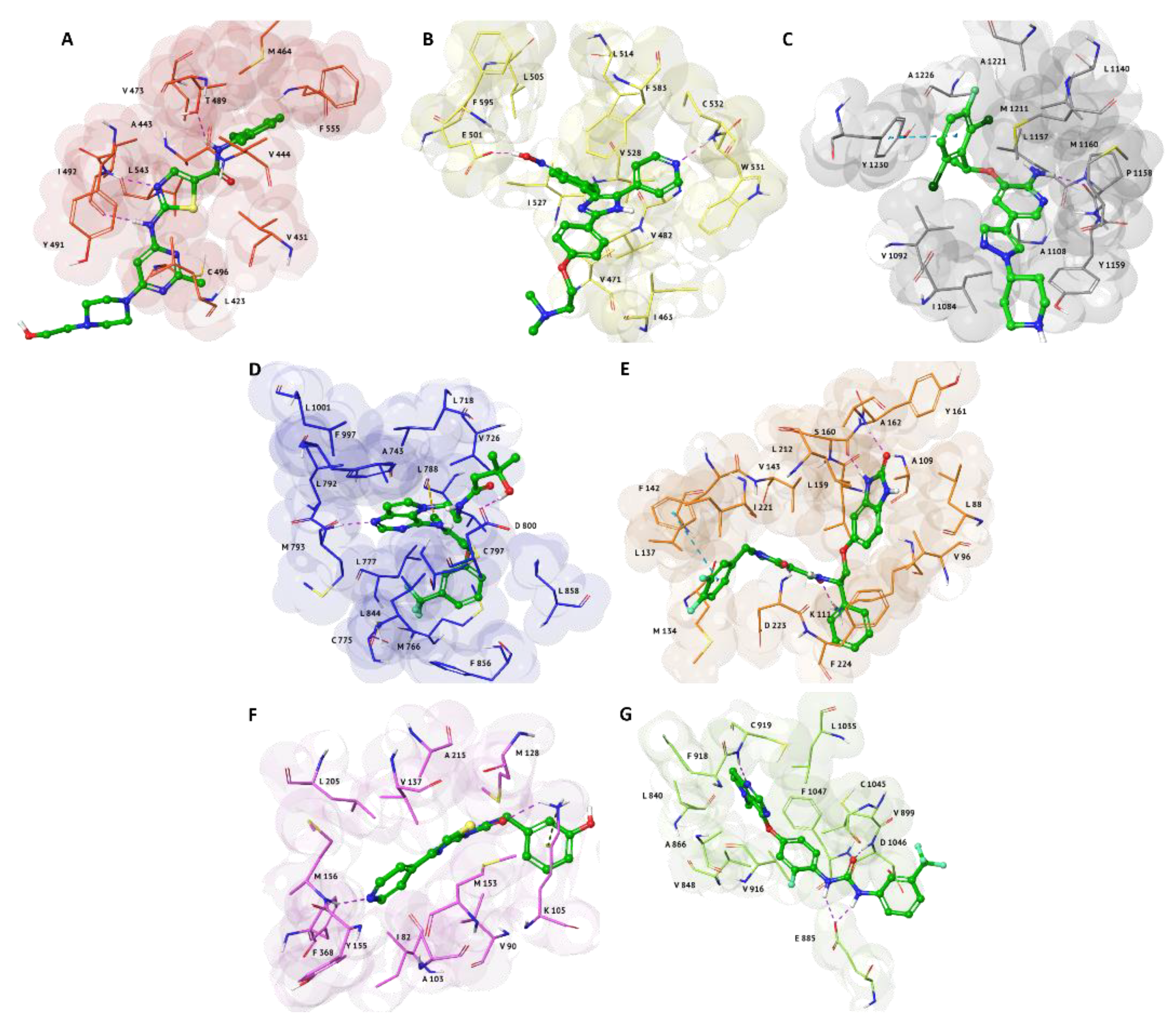
2.1. Structure-Based Virtual Screening (SBVS)
Cinnamyl Cinnamate and α-Terpinen-7-al
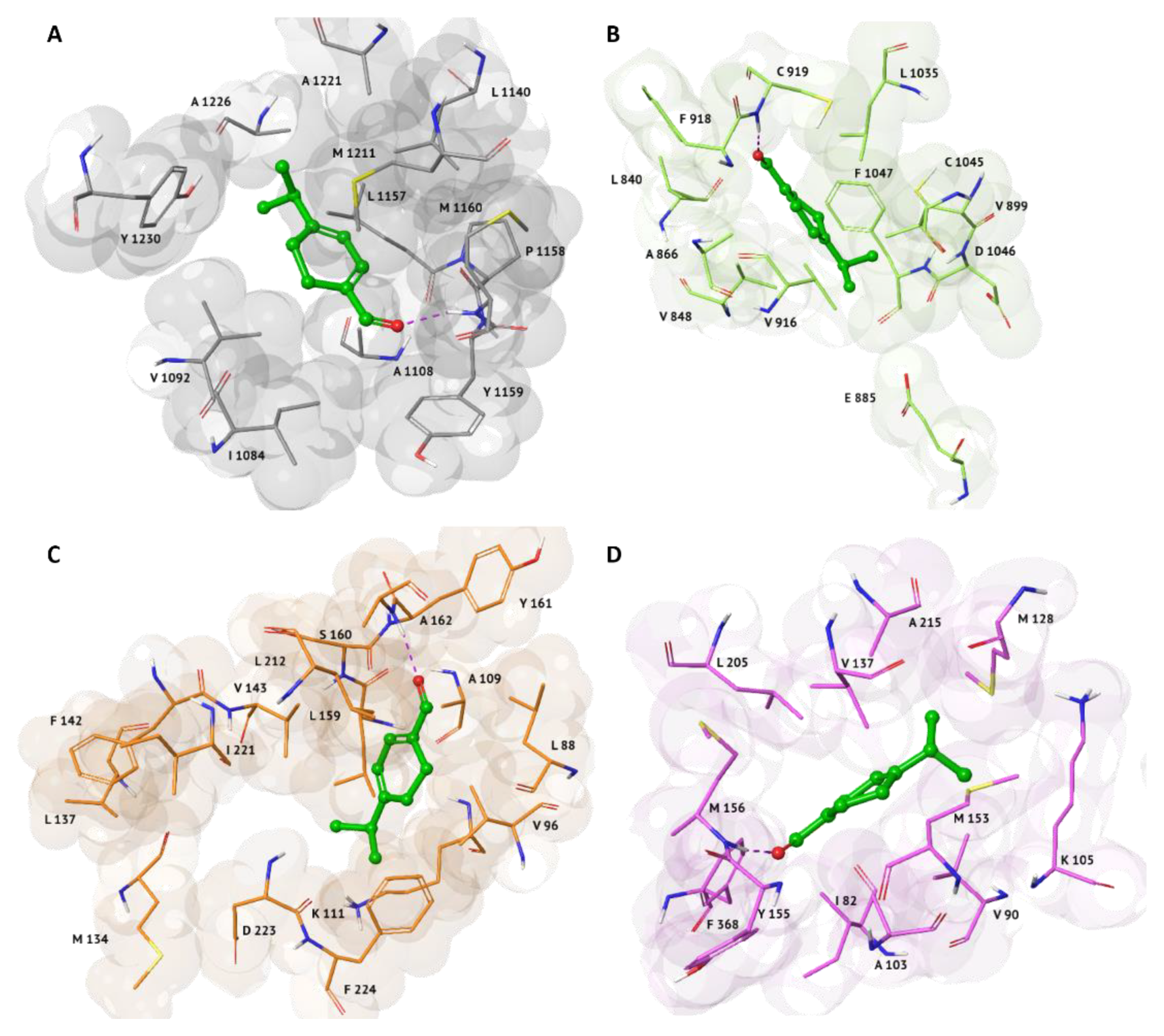
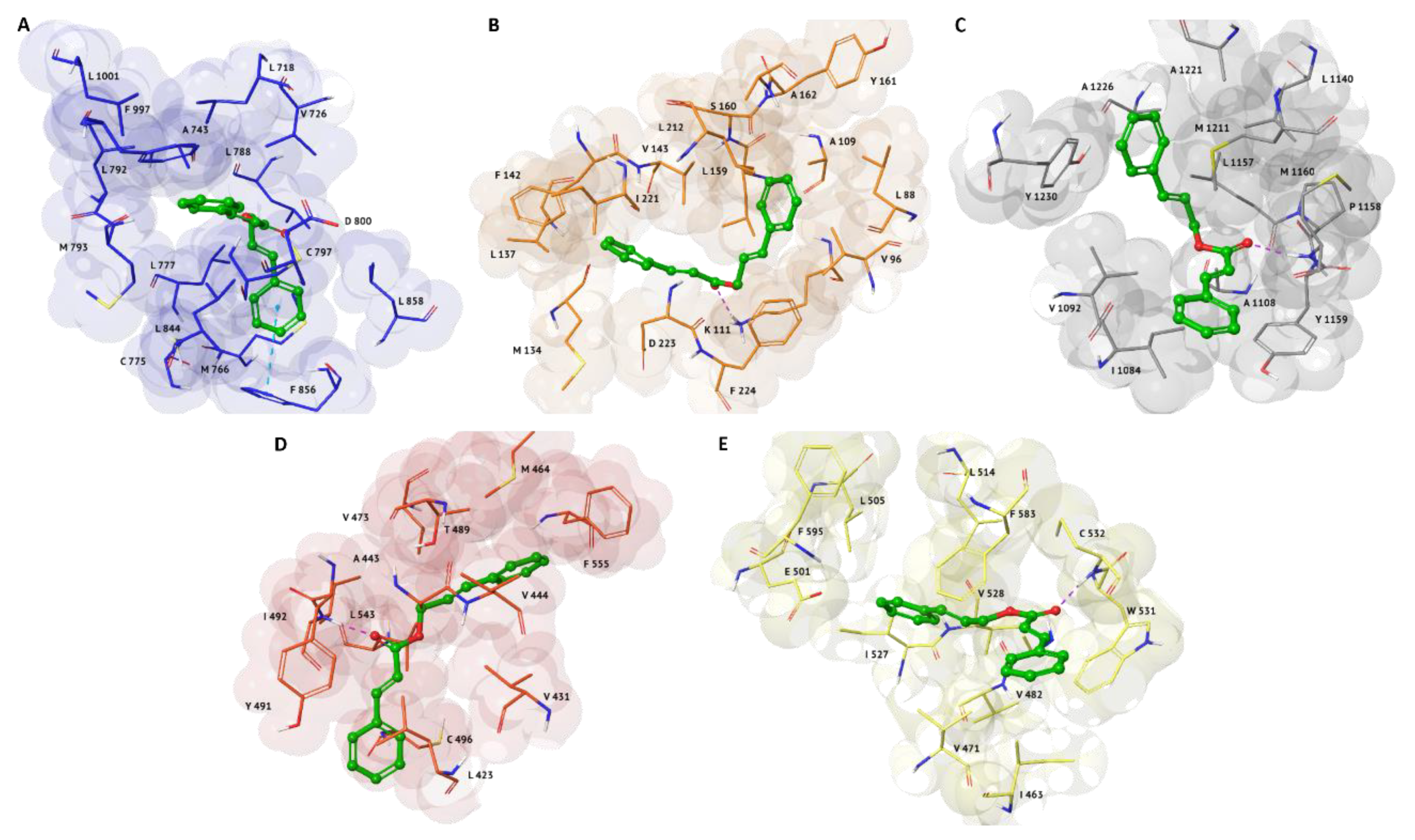
2.2. Induced Fit Docking
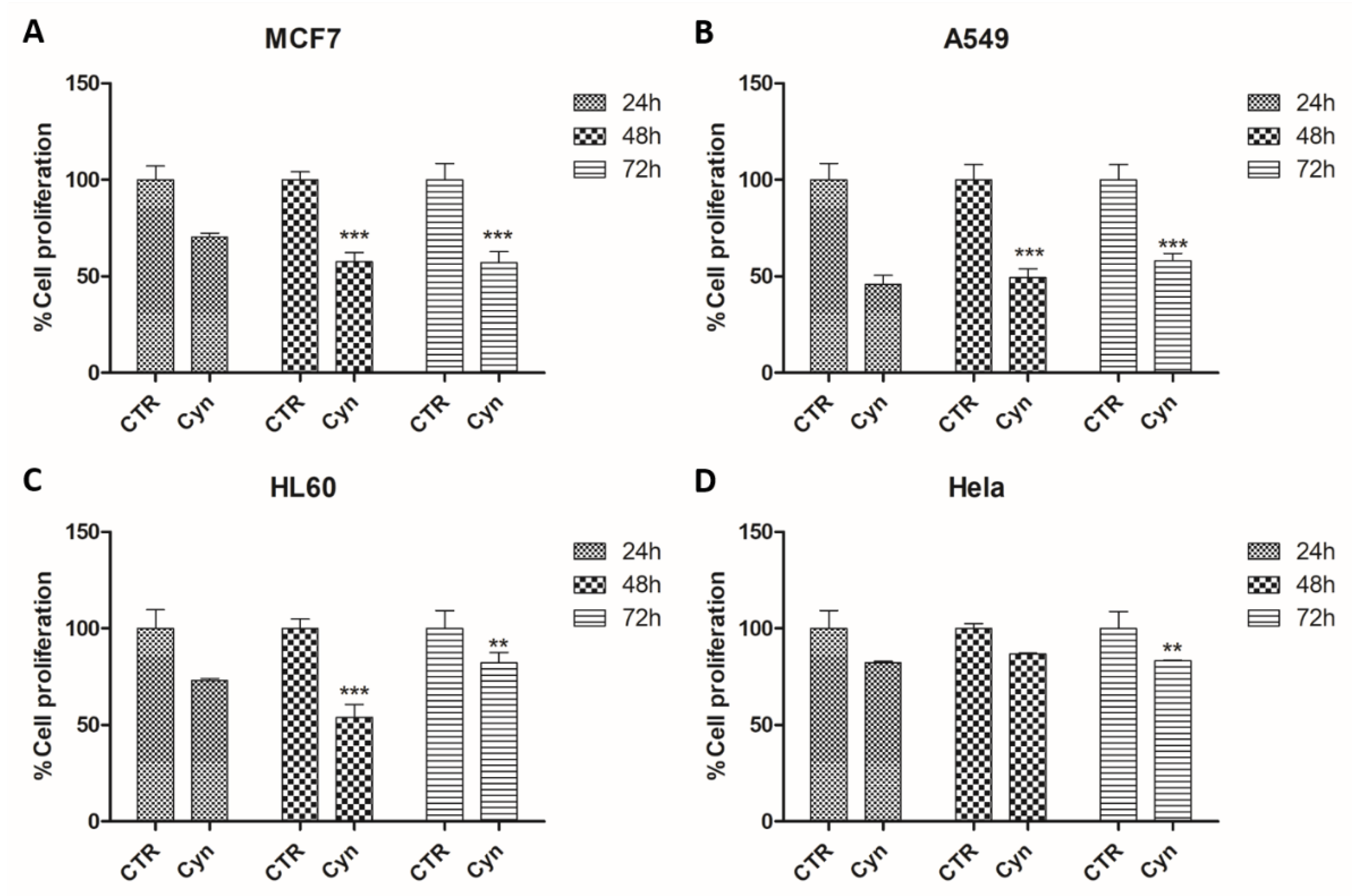
2.3. Cell Viability Assay
3. Conclusions
4. Materials and Methods
4.1. Ligands Database Preparation
4.2. Targets Preparation
4.3. Glide Docking
4.4. Induced Fit Docking Protocol
4.5. Cell Viability Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines (Basel) 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.; Ademiluyi, A. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. Protein kinases--the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Martin, J.; Anamika, K.; Srinivasan, N. Classification of protein kinases on the basis of both kinase and non-kinase regions. PLoS ONE 2010, 5, e12460. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid Based Complement. Alternat. Med. 2015, 2015, 397821. [Google Scholar] [CrossRef]
- Cho, S.M.; Lee, E.O.; Kim, S.H.; Lee, H.J. Essential oil of Pinus koraiensis inhibits cell proliferation and migration via inhibition of p21-activated kinase 1 pathway in HCT116 colorectal cancer cells. BMC Complement. Altern. Med. 2014, 14, 275. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Lee, S.S.; Yang, M.L.; Huang-Liu, R.; Lee, C.Y.; Li, Y.C.; Kuan, Y.H. Zerumbone reduced the inflammatory response of acute lung injury in endotoxin-treated mice via Akt-NFκB pathway. Chem. Biol. Interact. 2017, 271, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Souza, L.F.; Alloisio, S.; Cornara, L.; De Feo, V. Coriandrum sativum and Lavandula angustifolia Essential Oils: Chemical Composition and Activity on Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1999. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhu, Z.; Han, S.L.; Zhang, Z.L. Bergapten exerts inhibitory effects on diabetes-related osteoporosis via the regulation of the PI3K/AKT, JNK/MAPK and NF-κB signaling pathways in osteoprotegerin knockout mice. Int. J. Mol. Med. 2016, 38, 1661–1672. [Google Scholar] [CrossRef]
- Yang, X.Q.; Zheng, H.; Ye, Q.; Li, R.Y.; Chen, Y. Essential oil of Cinnamon exerts anti-cancer activity against head and neck squamous cell carcinoma via attenuating epidermal growth factor receptor-tyrosine kinase. J. BUON 2015, 20, 1518–1525. [Google Scholar]
- Wei, J.; Zhang, X.; Bi, Y.; Miao, R.; Zhang, Z.; Su, H. Anti-Inflammatory Effects of Cumin Essential Oil by Blocking JNK, ERK, and NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 474509. [Google Scholar] [CrossRef]
- Maruca, A.; Moraca, F.; Rocca, R.; Molisani, F.; Alcaro, F.; Gidaro, M.C.; Alcaro, S.; Costa, G.; Ortuso, F. Chemoinformatic Database Building and in Silico Hit-Identification of Potential Multi-Targeting Bioactive Compounds Extracted from Mushroom Species. Molecules 2017, 22, 1571. [Google Scholar] [CrossRef]
- Rocca, R.; Moraca, F.; Costa, G.; Nadai, M.; Scalabrin, M.; Talarico, C.; Distinto, S.; Maccioni, E.; Ortuso, F.; Artese, A.; et al. Identification of G-quadruplex DNA/RNA binders: Structure-based virtual screening and biophysical characterization. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 1329–1340. [Google Scholar] [CrossRef]
- Rocca, R.; Moraca, F.; Costa, G.; Talarico, C.; Ortuso, F.; Da Ros, S.; Nicoletto, G.; Sissi, C.; Alcaro, S.; Artese, A. Identification of Piperidinyl-amine Derivatives as Novel Dual Binders of Oncogene c-myc/c-Kit G-quadruplexes. ACS Med. Chem. Lett. 2018, 9, 848–853. [Google Scholar] [CrossRef]
- Costa, G.; Carta, F.; Ambrosio, F.A.; Artese, A.; Ortuso, F.; Moraca, F.; Rocca, R.; Romeo, I.; Lupia, A.; Maruca, A.; et al. A computer-assisted discovery of novel potential anti-obesity compounds as selective carbonic anhydrase VA inhibitors. Eur. J. Med. Chem. 2019, 181, 111565. [Google Scholar] [CrossRef]
- Catalano, R.; Rocca, R.; Juli, G.; Costa, G.; Maruca, A.; Artese, A.; Caracciolo, D.; Tagliaferri, P.; Alcaro, S.; Tassone, P.; et al. A drug repurposing screening reveals a novel epigenetic activity of hydroxychloroquine. Eur J. Med. Chem. 2019, 183, 111715. [Google Scholar] [CrossRef] [PubMed]
- Catalano, R.; Moraca, F.; Amato, J.; Cristofari, C.; Rigo, R.; Dalla Via, L.; Rocca, R.; Lupia, A.; Maruca, A.; Costa, G. Targeting multiple G-quadruplex–forming DNA sequences: Design, biophysical and biological evaluations of indolo-naphthyridine scaffold derivatives. Eur. J. Med. Chem. 2019, 182, 111627. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Della Volpe, S.; Ambrosio, F.A.; Costa, G.; Unver, M.Y.; Zucal, C.; Rossi, D.; Martino, E.; Provenzani, A.; Hirsch, A.K.H.; et al. Exploration of ligand binding modes towards the identification of compounds targeting HuR: A combined STD-NMR and Molecular Modelling approach. Sci. Rep. 2018, 8, 13780. [Google Scholar] [CrossRef] [PubMed]
- Catalogna, G.; Moraca, F.; D’Antona, L.; Dattilo, V.; Perrotti, G.; Lupia, A.; Costa, G.; Ortuso, F.; Iuliano, R.; Trapasso, F.; et al. Review about the multi-target profile of resveratrol and its implication in the SGK1 inhibition. Eur. J. Med. Chem. 2019, 183, 111675. [Google Scholar] [CrossRef]
- Rastelli, G.; Pinzi, L. Computational polypharmacology comes of age. Front. Pharmacol 2015, 6, 157. [Google Scholar] [CrossRef]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef]
- Anighoro, A.; Stumpfe, D.; Heikamp, K.; Beebe, K.; Neckers, L.M.; Bajorath, J.; Rastelli, G. Computational polypharmacology analysis of the heat shock protein 90 interactome. J. Chem. Inf. Model. 2015, 55, 676–686. [Google Scholar] [CrossRef]
- Reddy, A.S.; Tan, Z.; Zhang, S. Curation and analysis of multitargeting agents for polypharmacological modeling. J. Chem. Inf. Model. 2014, 54, 2536–2543. [Google Scholar] [CrossRef]
- Artese, A.; Alcaro, S.; Moraca, F.; Reina, R.; Ventura, M.; Costantino, G.; Beccari, A.R.; Ortuso, F. State-of-the-art and dissemination of computational tools for drug-design purposes: A survey among Italian academics and industrial institutions. Future Med. Chem. 2013, 5, 907–927. [Google Scholar] [CrossRef]
- Essential oil University (EOU). Available online: https://essentialoils.org/ (accessed on 15 July 2019).
- Buckle, J. Use of aromatherapy as a complementary treatment for chronic pain. Altern. Ther. Health Med. 1999, 5, 42–51. [Google Scholar]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukic, N.; Bozin, B.; Sokovic, M.; Simin, N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef]
- Sylvestre, M.; Legault, J.; Dufour, D.; Pichette, A. Chemical composition and anticancer activity of leaf essential oil of Myrica gale L. Phytomedicine 2005, 12, 299–304. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A. Antiproliferative effects of essential oils and their major constituents in human renal adenocarcinoma and amelanotic melanoma cells. Cell Prolif. 2008, 41, 1002–1012. [Google Scholar] [CrossRef]
- Heinrich, M.; Bremner, P. Ethnobotany and ethnopharmacy-their role for anti-cancer drug development. Curr. Drug Targets 2006, 7, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadi, M.; Riahi-Madvar, A.; Hadian, J.; Rezaee, F.; Rafiee, R.; Biniaz, M. Toxicity of essential oil of Satureja khuzistanica: In vitro cytotoxicity and anti-microbial activity. J. Immunotoxicol. 2014, 11, 50–55. [Google Scholar] [CrossRef]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef]
- Fogang, H.P.; Maggi, F.; Tapondjou, L.A.; Womeni, H.M.; Papa, F.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Lupidi, G.; et al. In vitro biological activities of seed essential oils from the Cameroonian spices Afrostyrax lepidophyllus MILDBR. and Scorodophloeus zenkeri HARMS rich in sulfur-containing compounds. Chem. Biodivers. 2014, 11, 161–169. [Google Scholar] [CrossRef]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
- Costa, G.; Gidaro, M.C.; Vullo, D.; Supuran, C.T.; Alcaro, S. Active Components of Essential Oils as Anti-Obesity Potential Drugs Investigated by in Silico Techniques. J. Agric. Food Chem. 2016, 64, 5295–5300. [Google Scholar] [CrossRef] [PubMed]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Bettaieb, I.; Bourgou, S.; Wannes, W.A.; Hamrouni, I.; Limam, F.; Marzouk, B. Essential oils, phenolics, and antioxidant activities of different parts of cumin (Cuminum cyminum L.). J. Agric. Food Chem. 2010, 58, 10410–10418. [Google Scholar] [CrossRef] [PubMed]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. The pharmacological activities of Cuminum cyminum-A review. IOSR J. Pharm. 2016, 6, 46–65. [Google Scholar]
- Lee, I.K.; Han, M.S.; Kim, D.W.; Yun, B.S. Phenylpropanoid acid esters from Korean propolis and their antioxidant activities. Bioorg Med. Chem. Lett. 2014, 24, 3503–3505. [Google Scholar] [CrossRef] [PubMed]
- El-Readi, M.Z.; Eid, H.H.; Ashour, M.L.; Eid, S.Y.; Labib, R.M.; Sporer, F.; Wink, M. Variations of the chemical composition and bioactivity of essential oils from leaves and stems of Liquidambar styraciflua (Altingiaceae). J. Pharm. Pharmacol. 2013, 65, 1653–1663. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Telikepalli, H.; Wang, P.B.; Kuo, S.; Shankel, D.M.; Stewart, G. Antimutagenicity of secondary metabolites from higher plants. Mutat. Res. 1992, 267, 229–241. [Google Scholar] [CrossRef]
- Corigliano, D.M.; Syed, R.; Messineo, S.; Lupia, A.; Patel, R.; Reddy, C.V.R.; Dubey, P.K.; Colica, C.; Amato, R.; De Sarro, G.; et al. Indole and 2,4-Thiazolidinedione conjugates as potential anticancer modulators. Peer J. 2018, 6, e5386. [Google Scholar] [CrossRef]
- Andrade, M.A.; Braga, M.A.; Cesar, P.H.S.; Trento, M.V.C.; Esposito, M.A.; Silva, L.F. Anticancer Properties of Essential Oils: An overview. Curr. Cancer Drug Targets 2018, 18, 957–966. [Google Scholar] [CrossRef]
- Maruca, A.; Ambrosio, F.A.; Lupia, A.; Romeo, I.; Rocca, R.; Moraca, F.; Talarico, C.; Bagetta, D.; Catalano, R.; Costa, G. Computer-based techniques for lead identification and optimization I: Basics. Phys. Sci. Rev. 2018, 4, 113–114. [Google Scholar] [CrossRef]
- Ortuso, F.; Bagetta, D.; Maruca, A.; Talarico, C.; Bolognesi, M.L.; Haider, N.; Borges, F.; Bryant, S.; Langer, T.; Senderowitz, H.; et al. The Mu.Ta.Lig. Chemotheca: A Community-Populated Molecular Database for Multi-Target Ligands Identification and Compound-Repurposing. Front. Chem. 2018, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Chemotheca. Available online: http://chemotheca.unicz.it/ (accessed on 15 July 2019).
- COST Action CA15135. Available online: http://www.mutalig.eu/ (accessed on 15 July 2019).
- Schrödinger Release 2018-4: LigPrep; Schrödinger, LLC: New York, NY, USA, 2018.
- ZINC15. Available online: http://zinc15.docking.org/ (accessed on 15 July 2019).
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- The Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB). Available online: https://www.rcsb.org/ (accessed on 15 July 2019).
- Schrödinger Release 2018-4: Protein Preparation Wizard. Epik, Schrödinger, LLC: New York, NY, USA, 2016; Impact, Schrödinger, LLC, New York, NY, USA, 2016; Prime, Schrödinger, LLC, New York, NY, USA, 2018.
- Schrödinger Release 2018-4: Glide; Schrödinger, LLC: New York, NY, USA, 2018.
Sample Availability: Samples of the compounds not are available from the authors. |
| Name CAS Number | Structure | Target | G-Score |
|---|---|---|---|
| Psoralen CAS 66-97-7 |  | VEGFR2 | −8.09 |
| c-Met | −8.46 | ||
| 1-H-indol-2-ol CAS 16990-73-1 |  | VEGFR2 | −8.00 |
| PDK1 | −8.64 | ||
| α-terpinen-7-al CAS 1197-15-5 |  | VEGFR2 | −8.38 |
| PDK1 | −8.15 | ||
| ROCK1 | −8.00 | ||
| c-Met | −8.16 | ||
| Hinokitiol CAS 499-44-5 |  | VEGFR2 | −8.42 |
| c-Met | −8.20 | ||
| β-Vetivone CAS 18444-79-6 |  | ROCK1 | −8.05 |
| c-Met | −8.26 | ||
| Precocene II CAS 644-06-4 |  | VEGFR2 | −8.02 |
| MEK2 | −8.08 | ||
| 1-H-benzochromene CAS 5153-92-4 |  | VEGFR2 | −8.18 |
| PI3K-γ | −8.95 | ||
| c-Met | −8.88 | ||
| Piperonylacetone CAS 55418-52-5 |  | VEGFR2 | −8.36 |
| ROCK1 | −8.33 | ||
| c-Met | −9.10 | ||
| Thymohydroquinone CAS 2217-60-9 |  | SGK1 | −8.57 |
| VEGFR2 | −8.70 | ||
| PDK1 | −8.66 | ||
| CDK2 | −8.00 | ||
| GSK3β | −8.13 | ||
| ERK2 | −8.18 | ||
| 3-Phenil Benzaldeide CAS 1204-60-0 |  | VEGFR2 | −9.47 |
| MEK2 | −8.41 | ||
| ROCK1 | −8.35 | ||
| B-Raf | −8.10 | ||
| c-Met | −8.07 | ||
| Cinnamyl cinnamate CAS 122-69-0 |  | EGFR | −8.12 |
| PDK1 | −8.19 | ||
| BMX | −8.06 | ||
| B-Raf | −8.02 | ||
| c-Met | −8.54 | ||
| Isoquinoline CAS 119-65-3 |  | VEGFR2 | −8.23 |
| PKA | −8.00 | ||
| ROCK1 | −8.04 | ||
| c-Met | −8.12 | ||
| Atronorin CAS 479-20-9 |  | SGK1 | −8.02 |
| EGFR | −9.69 | ||
| VEGFR2 | −10.97 | ||
| ABK | −8.17 | ||
| B-Raf | −8.01 | ||
| ERK1 | −9.06 | ||
| c-Met | −8.46 |
| Name CAS Number | Target | G-Score | IFD Score |
|---|---|---|---|
| α-terpinen-7-al CAS 1197-15-5 | VEGFR2 | −8.82 | −665.28 |
| PDK1 | −8.52 | −603.38 | |
| ROCK1 | −8.18 | −870.20 | |
| c-Met | −8.62 | −633.60 | |
| Cinnamyl cinnamate CAS 122-69-0 | EGFR | −8.31 | −654.13 |
| PDK1 | −9.46 | −605.92 | |
| BMX | −8.72 | −575.64 | |
| B-Raf | −8.64 | −563.84 | |
| c-Met | −9.50 | −635.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruca, A.; Lanzillotta, D.; Rocca, R.; Lupia, A.; Costa, G.; Catalano, R.; Moraca, F.; Gaudio, E.; Ortuso, F.; Artese, A.; et al. Multi-Targeting Bioactive Compounds Extracted from Essential Oils as Kinase Inhibitors. Molecules 2020, 25, 2174. https://doi.org/10.3390/molecules25092174
Maruca A, Lanzillotta D, Rocca R, Lupia A, Costa G, Catalano R, Moraca F, Gaudio E, Ortuso F, Artese A, et al. Multi-Targeting Bioactive Compounds Extracted from Essential Oils as Kinase Inhibitors. Molecules. 2020; 25(9):2174. https://doi.org/10.3390/molecules25092174
Chicago/Turabian StyleMaruca, Annalisa, Delia Lanzillotta, Roberta Rocca, Antonio Lupia, Giosuè Costa, Raffaella Catalano, Federica Moraca, Eugenio Gaudio, Francesco Ortuso, Anna Artese, and et al. 2020. "Multi-Targeting Bioactive Compounds Extracted from Essential Oils as Kinase Inhibitors" Molecules 25, no. 9: 2174. https://doi.org/10.3390/molecules25092174
APA StyleMaruca, A., Lanzillotta, D., Rocca, R., Lupia, A., Costa, G., Catalano, R., Moraca, F., Gaudio, E., Ortuso, F., Artese, A., Trapasso, F., & Alcaro, S. (2020). Multi-Targeting Bioactive Compounds Extracted from Essential Oils as Kinase Inhibitors. Molecules, 25(9), 2174. https://doi.org/10.3390/molecules25092174












