Simplified Procedure for General Synthesis of Monosubstituted Piperazines—From a Batch Reaction Vessel to a Flow (Microwave) Reactor
Abstract
1. Introduction
2. Results and Discussion
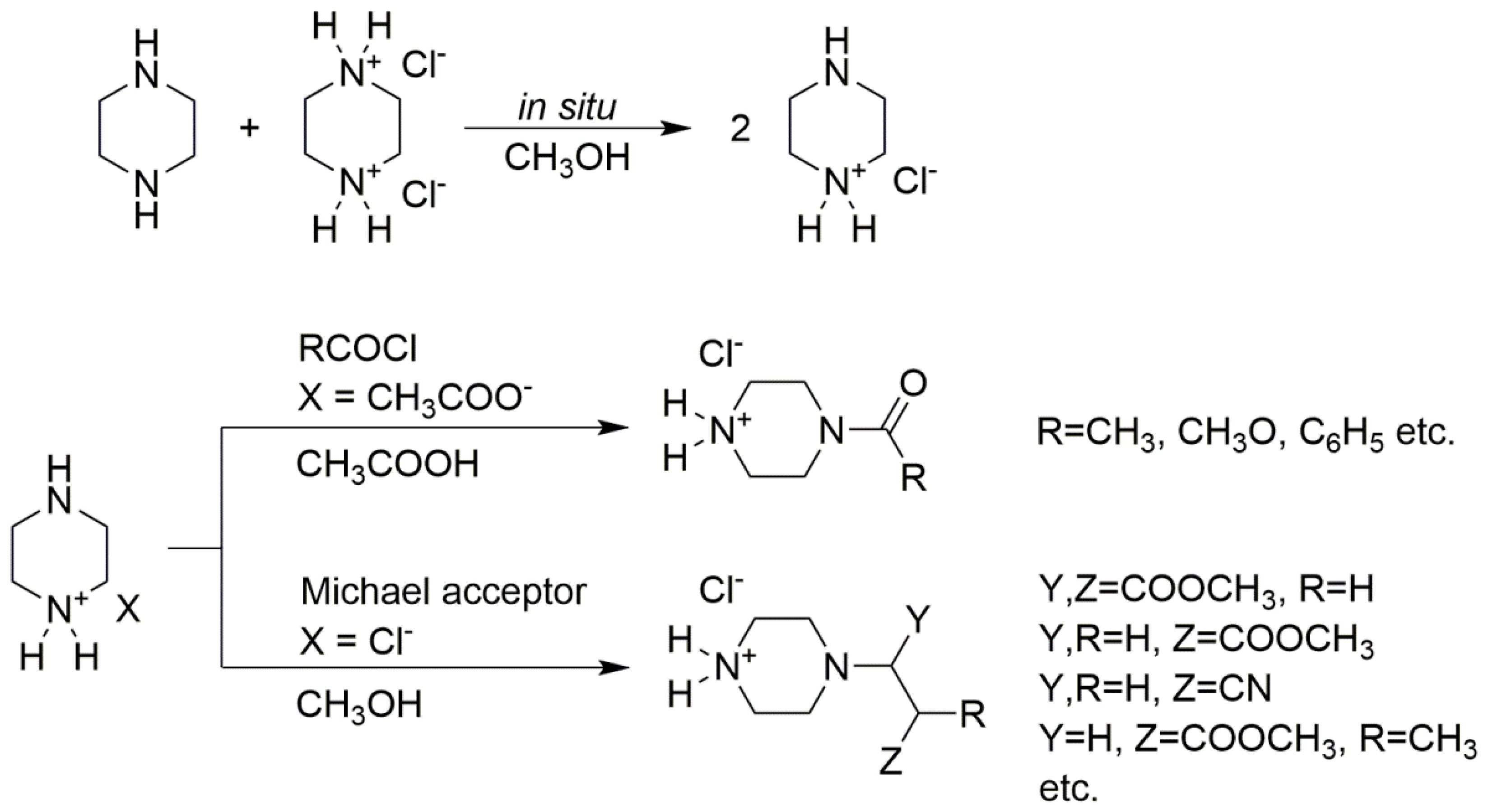
2.1. One-step Synthesis of Monosubstituted Piperazine Derivatives
2.2. Microwave Assisted Synthesis of Monosubstituted Piperazine Derivatives
2.3. Comparison of Synthetic Techniques
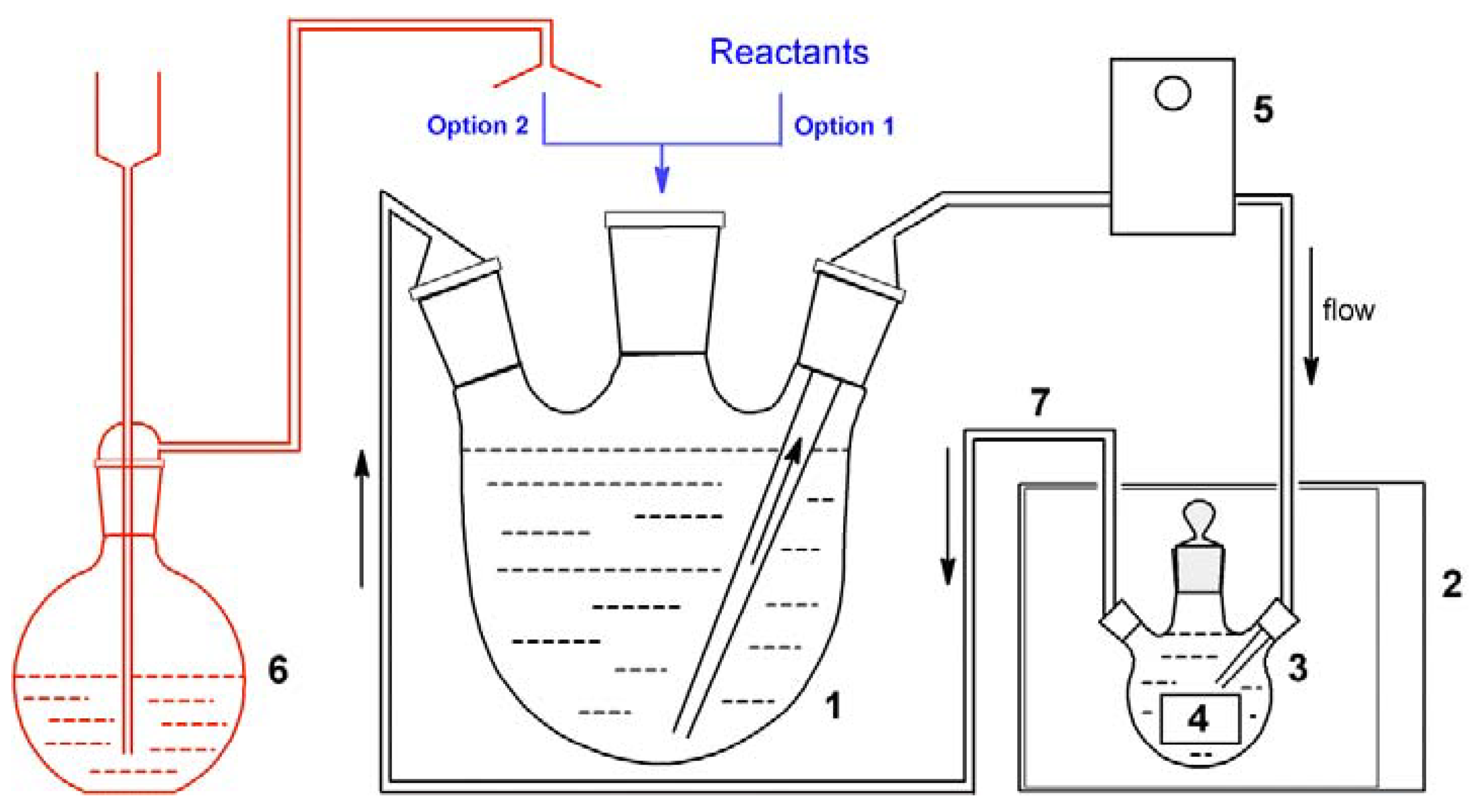
2.4. A Flow Reactor with a Microwave Unit and/or Catalytic Bed
3. Materials and Methods
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Al-Ghorbani, M.; Bushra, A.B.; Zabiulla, S.; Mamatha, S.V.; Khanum, S.A. Piperazine and Morpholine: Synthetic Preview and Pharmaceutical Applications. J. Chem. Pharm. Res. 2015, 7, 281–301. [Google Scholar] [CrossRef]
- Singh, K.; Siddiqui, H.H.; Shakya, P.; Bagga, P.; Kumar, A.; Khalid, M.; Arif, M.; Alok, S. Piperazine–A Biologically Active Scaffold. Int. J. Pharm. Sci. Res. 2015, 6, 4145–4158. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Chen, P.; Zhang, W.; Wu, C.; Sun, C.; Luo, W.; Zheng, L.; Liu, Z.; Liang, G. Development of 2-amino-4-phenylthiazole analogues to disrupt myeloid differentiation factor 88 and prevent inflammatory responses in acute lung injury. Eur. J. Med. Chem. 2019, 161, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, K.; Karcz, T.; Mogilski, S.; Siwek, A.; Kuder, K.J.; Latacz, G.; Kubacka, M.; Hagenow, S.; Lubelska, A.; Olejarz, A.; et al. Synthesis and biological activity of novel tert-butyl and tert-pentylphenoxyalkyl piperazine derivatives as histamine H3R ligands. Eur. J. Med. Chem. 2018, 152, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Satoskar, K.; Bhandarkar, B. Pharmacology and Pharmacotherapeutics, 5th ed.; Bombay Popular Prakashan Pvt. Ltd.: Bombay, India, 1985. [Google Scholar]
- Lu, S.; Zhang, Y.; Liu, J.; Zhao, C.; Liu, W.; Xi, R. Preparation of anti-Pefloxacin Antibody and Development of an Indirect Competitive Enzyme-Linked Immunosorbent Assay for Detection of Pefloxacin Residue in Chicken Liver. J. Agric. Food Chem. 2006, 54, 6995–7000. [Google Scholar] [CrossRef] [PubMed]
- Madrid, P.B.; Polgar, W.E.; Toll, L.; Tanga, M.J. Synthesis and antitubercular activity of phenothiazines with reduced binding to dopamine and serotonin receptors. Bioorg. Med. Chem. Lett. 2007, 17, 3014–3017. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Tang, H.; McKittrick, B.A.; Burnett, D.A.; Zang, H.; Smith-Torhan, A.; Fawzi, A.; Lachowicz, J. Modification of the clozapine structure by parallel synthesis. Bioorg. Med. Chem. Lett. 2006, 16, 4548–4553. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Brugel, T.A.; Balestra, M.; Brown, D.G.; Brush, K.A.; Hightower, C.; Hinkley, L.; Hoesch, V.; Kang, J.; Koether, G.M.; et al. 4-Aryl piperazine and piperidine amides as novel mGluR5 positive allosteric modulators. Bioorg. Med. Chem. Lett. 2010, 20, 7381–7384. [Google Scholar] [CrossRef]
- Reich, M.; Schunk, S.; Jostock, R.; Hees, S.; Germann, T.; Engels, M.F.-M. Substituted Disulfonamide Compounds. U.S. Patent 2010/0152158 A1, 17 June 2010. [Google Scholar]
- Liu, Y.; Zhou, E.; Yu, K.; Zhu, J.; Zhang, Y.; Xie, X.; Li, J.; Jiang, H. Discovery of a novel CCR5 antagonist lead compound through fragment assembly. Molecules 2008, 13, 2426. [Google Scholar] [CrossRef]
- Liu, T.; Weng, Z.; Dong, X.; Chen, L.; Ma, L.; Cen, S.; Zhou, N.; Hu, Y. Design, Synthesis and Biological Evaluation of Novel Piperazine Derivatives as CCR5 Antagonists. PLoS ONE 2013, 8, e53636. [Google Scholar] [CrossRef]
- Manetti, D.; Bartolini, A.; Borea, P.A.; Bellucci, C.; Dei, S.; Ghelardini, C.; Gualtieri, F.; Romanelli, M.N.; Scapecchi, S.; Teodori, E.; et al. Hybridized and isosteric analogues of N1-acetyl-N4-dimethyl-piperazinium iodide (ADMP) and N1-phenyl-N4-dimethyl-piperazinium iodide (DMPP) with central nicotinic action. Bioorg. Med. Chem. 1999, 7, 457–465. [Google Scholar] [CrossRef]
- Scott, J.S.; Degorce, S.L.; Anjum, R.; Culshaw, J.; Davies, R.D.M.; Davies, N.L.; Dillman, K.S.; Dowling, J.E.; Drew, L.; Ferguson, A.D.; et al. Discovery and Optimization of Pyrrolopyrimidine Inhibitors of Interleukin-1 Receptor Associated Kinase 4 (IRAK4) for the Treatment of Mutant MYD88L265P Diffuse Large B-Cell Lymphoma. J. Med. Chem. 2017, 60, 10071–10091. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Nakamura, T.; Nakamura, K.; Kurimoto, A.; Isobe, Y.; Ogita, H.; Millichip, I.; McInally, T.; Bonnert, R. Novel Adenine Compound. European Patent 1939198A1, 2 July 2008. [Google Scholar]
- Moore, T.S.; Boyle, M.; Thorn, V.M. N-substituted derivatives of piperazine and ethylenediamine. Part I. The preparation of N-monosubstituted derivatives. J. Chem. Soc. 1929, 1929, 39–51. [Google Scholar] [CrossRef]
- Baltzly, R.; Buck, J.S.; Lorz, E.; Schön, W. The preparation of N-mono-substituted and unsymmetrically disubstituted piperazines. J. Am. Chem. Soc. 1944, 66, 263–266. [Google Scholar] [CrossRef]
- Caille, S.; Allgeier, A.M.; Bernard, C.; Correll, T.L.; Cosbie, A.; Crockett, R.D.; Cui, S.; Faul, M.M.; Hansen, K.B.; Huggins, S.; et al. Development of a Factory Process for Omecamtiv Mecarbil, a Novel Cardiac Myosin Activator. Org. Process. Res. Dev. 2019, 23, 1558–1567. [Google Scholar] [CrossRef]
- Capuano, B.; Crosby, I.T.; Lloyd, E.J.; Taylor, D.A. Synthesis and Preliminary Pharmacological Evaluation of 4’-Arylmethyl Analogues of Clozapine. I. The Effect of Aromatic Substituents. Austral. J. Chem. 2002, 55, 565–576. [Google Scholar] [CrossRef]
- Zhou, A.; Wu, H.; Pan, J.; Wang, X.; Li, J.; Wu, Z.; Hui, A. Synthesis and Evaluation of Paeonol Derivatives as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease. Molecules 2015, 20, 1304–1318. [Google Scholar] [CrossRef]
- Devlin, J.P.; McNeil, D.W.; Keirns, J.J.; Barsumian, E.L. Bis(piperazinyl or homopiperazinyl)alkanes. U.S. Patent 4,725,597, 16 February 1988. [Google Scholar]
- Klaveness, J.; Brudeli, B.; Levy, F.O. 5-HTX Modulators. World Patent WO 2007/007072 A1, 18 January 2007. [Google Scholar]
- Panek, D.; Więckowska, A.; Wichur, T.; Bajda, M.; Godyń, J.; Jończyk, J.; Mika, K.; Janockova, J.; Soukup, O.; Knez, D.; et al. Design, synthesis and biological evaluation of new phthalimide and saccharin derivatives with alicyclic amines targeting cholinesterases, beta-secretase and amyloid beta aggregation. Eur. J. Med. Chem. 2017, 125, 676–695. [Google Scholar] [CrossRef]
- Zhang, C.; Tan, C.; Zu, X.; Zhai, X.; Liu, F.; Chu, B.; Ma, X.; Chen, Y.; Gong, P.; Jiang, Y. Exploration of (S)-3-aminopyrrolidine as a potentially interesting scaffold for discovery of novel Abl and PI3K dual inhibitors. Eur. J. Med. Chem. 2011, 46, 1404–1414. [Google Scholar] [CrossRef]
- Ferla, S.; Manganaro, R.; Benato, S.; Paulissen, J.; Neyts, J.; Jochmans, D.; Brancale, A.; Bassetto, M. Rational modifications, synthesis and biological evaluation of new potential antivirals for RSV designed to target the M2-1 protein. Bioorg. Med. Chem. 2020, 28, 115401. [Google Scholar] [CrossRef] [PubMed]
- Cunico, W.; Gomes, C.R.B.; Moreth, M.; Manhanini, D.P.; Figueiredo, I.H.; Penido, C.; Henriques, M.G.M.O.; Varotti, F.P.; Krettli, A.U. Synthesis and antimalarial activity of hydroxyethylpiperazine derivatives. Eur. J. Med. Chem. 2009, 44, 1363–1368. [Google Scholar] [CrossRef]
- Mehanna, A.S.; Jin, Y.K. Design, synthesis, and biological testing of thiosalicylamides as a novel class of calcium channel blockers. Bioorg. Med. Chem. 2005, 13, 4323–4331. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, A.S.; Kim, J.T. Calcium Channel Blockers. U.S. Patent 2002/0115655 A1, 22 August 2002. [Google Scholar]
- Peterson, Q.P.; Hsu, D.C.; Goode, D.R.; Novotny, C.J.; Totten, R.K.; Hergenrother, P.J. Procaspase-3 Activation as an Anti-Cancer Strategy: Structure−Activity Relationship of Procaspase-Activating Compound 1 (PAC-1) and Its Cellular Co-Localization with Caspase-3. J. Med. Chem. 2009, 52, 5721–5731. [Google Scholar] [CrossRef] [PubMed]
- Hergenrother, P.J.; Peterson, Q.P.; Hsu, D.C.; West, D.C.; Fan, T.M.; Novotny, C.J. Design, Synthesis and Evaluation of Procaspase Activating Compounds as Personalized Anti-Cancer Drugs. World Patent WO 2010/091382 A1, 12 August 2010. [Google Scholar]
- Li, J.; Huang, L.; Dong, W.; Ge, X.; Shi, C. Aralkyl-Alcohol Piperazine Derivatives and Their Uses as Antidepressant. U.S. Patent 2005/0267121 A1, 1 December 2005. [Google Scholar]
- Wang, J.; Xia, F.; Jin, W.-B.; Guan, J.-Y.; Zhao, H. Efficient synthesis and antioxidant activities of N-heterocyclyl substituted Coenzyme Q analogues. Bioorg. Chem. 2016, 68, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Biannic, B.; Bozell, J.J.; Elder, T. Steric effects in the design of Co-Schiff base complexes for the catalytic oxidation of lignin models to para-benzoquinones. Green Chem. 2014, 16, 3635–3642. [Google Scholar] [CrossRef]
- Wuts, P.G.M.; Greene, T.W. Greene’s Protective Groups in Organic Synthesis, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Morgan, B.P.; Muci, A.; Lu, P.-P.; Qian, X.; Tochimoto, T.; Smith, W.W.; Garard, M.; Kraynack, E.; Collibee, S.; Suehiro, I.; et al. Discovery of Omecamtiv Mecarbil the First, Selective, Small Molecule Activator of Cardiac Myosin. ACS Med. Chem. Lett. 2010, 1, 472–477. [Google Scholar] [CrossRef]
- Zulli, A.L.; Aimone, L.D.; Mathiasen, J.R.; Gruner, J.A.; Raddatz, R.; Bacon, E.R.; Hudkins, R.L. Substituted phenoxypropyl-(R)-2-methylpyrrolidine aminomethyl ketones as histamine-3 receptor inverse agonists. Bioorg. Med. Chem. Lett. 2012, 22, 2807–2810. [Google Scholar] [CrossRef]
- Sutton, J.C.; Bolton, S.A.; Hartl, K.S.; Huang, M.-H.; Jacobs, G.; Meng, W.; Ogletree, M.L.; Pi, Z.; Schumacher, W.A.; Seiler, S.M.; et al. Synthesis and SAR of 4-Carboxy-2-azetidinone Mechanism-Based Tryptase Inhibitors. Bioorg. Med. Chem. Lett. 2002, 12, 3229–3233. [Google Scholar] [CrossRef]
- Kantam, M.L.; Neeraja, V.; Kavita, B.; Neelima, B.; Chaudhuri, M.K.; Hussain, S. Cu(acac)2 Immobilized in Ionic Liquids: A Recoverable and Reusable Catalytic System for Aza-Michael Reactions. Adv. Synth. Catal. 2005, 347, 763–766. [Google Scholar] [CrossRef]
- Verma, A.K.; Attri, P.; Chopra, V.; Tiwari, R.K.; Chandra, R. Triethylammonium acetate (TEAA): A recyclable inexpensive ionic liquid promotes the chemoselective aza- and thia-Michael reactions. Mon. Chem. 2008, 139, 1041–1047. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, R.; Chaudhary, P.; Saxena, A.; Shankar, R.; Mozumdar, S.; Chandra, R. Cu-nanoparticles: A chemoselective catalyst for the aza-Michael reactions of N-alkyl- and N-arylpiperazines with acrylonitrile. Tetrahedron Lett. 2005, 46, 5229–5232. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kumar, N.S. Cellulose-Supported Copper (0) Catalyst for Aza-Michael Addition. Synlett 2006, 14, 2246–2250. [Google Scholar] [CrossRef]
- Varala, R.; Sreelatha, N.; Adapa, S.R. Ceric Ammonium Nitrate Catalyzed aza-Michael Addition of Aliphatic Amines to α,β-Unsaturated Carbonyl Compounds and Nitriles in Water. Synlett 2006, 10, 1549–1553. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Pandit, S.S. Highly rapid and direct synthesis of monoacylated piperazine derivatives from carboxylic acids under mild conditions. Tetrahedron Lett. 2003, 44, 3855–3858. [Google Scholar] [CrossRef]
- Paul, S.; Nanda, P.; Gupta, R.; Loupy, A. Ac2O–Py/basic alumina as a versatile reagent for acetylations in solvent-free conditions under microwave irradiation. Tetrahedron Lett. 2002, 43, 4261–4265. [Google Scholar] [CrossRef]
- Verma, S.K.; Acharya, B.N.; Kaushik, M.P. Imidazole-Catalyzed Monoacylation of Symmetrical Diamines. Org. Lett. 2010, 12, 4232–4235. [Google Scholar] [CrossRef]
- Pazdera, P.; Zberovská, B.; Němečková, D. Method of Piperazine Direct mono-N-substitution. Czech Patent 304,520, 30 April 2014. [Google Scholar]
- Pazdera, P.; Zberovská, B.; Herová, D. Method of Direct mono-N-substitution of Piperazine. Czech Patent 305,317, 17 June 2015. [Google Scholar]
- Pazdera, P.; Zberovská, B.; Herová, D. Process of Direct Piperazine mono-N-substitution. Czech Patent 305,854, 2 March 2016. [Google Scholar]
- Herová, D.; Pazdera, P. Efficient solid support catalyzed mono-aza-Michael addition reactions of piperazine. Mon. Chem. 2015, 146, 653–661. [Google Scholar] [CrossRef]
- Němečková, D.; Pazdera, P. A Simplified Protocol for Routine Chemoselective Syntheses of Piperazines Substituted In the 1-Position by an Electron Withdrawing Group. Curr. Org. Synth. 2015, 12, 173–179. [Google Scholar] [CrossRef]
- Estel, L.; Poux, M.; Benamara, N.; Polaert, I. Continuous flow-microwave reactor: Where are we? Chem. Eng. Process. 2017, 113, 56–64. [Google Scholar] [CrossRef]
- Moseley, J.D.; Lenden, P.; Lockwood, M.; Ruda, K.; Sherlock, J.-P.; Thomson, A.D.; Gilday, J.P. A Comparison of Commercial Microwave Reactors for Scale-Up within Process Chemistry. Org. Process. Res. Dev. 2008, 12, 30–40. [Google Scholar] [CrossRef]
- Biotage AB, Uppsala, Sweden. Available online: www.biotage.com (accessed on 4 May 2020).
- Anton Paar, GmbH, Graz, Austria. Available online: www.anton-paar.com (accessed on 4 May 2020).
- Milestone Srl, Sorisole (BG), Italy. Available online: www.milestonesrl.com (accessed on 4 May 2020).
- Nishioka, M.; Miyakawa, M.; Daino, Y.; Kataoka, H.; Koda, H.; Sato, K.; Suzuki, T.M. Single-Mode Microwave Reactor Used for Continuous Flow Reactions under Elevated Pressure. Ind. Eng. Chem. Res. 2013, 52, 4683–4687. [Google Scholar] [CrossRef]
- Macioszczyk, J.; Rac-Rumijowska, O.; Słobodzian, P.; Teterycz, H.; Malecha, K. Microfluidical Microwave Reactor for Synthesis of Gold Nanoparticles. Micromachines 2017, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Pazdera, P.; Zberovská, B.; Herová, D.; Datinská, V.; Šimbera, J. Catalyst for Chemical Syntheses Based on Metal Complex and Process for Preparing Thereof. Czech Patent 305 277, 3 June 2015. [Google Scholar]
- Pazdera, P.; Němečková, D.; Havránková, E.; Šimbera, J.; Ševčík, R. A Flow Reactor with a Microwave Source and a Catalytic Bed. Czech Patent 32 201, 16 October 2018. [Google Scholar]
- Pazdera, P.; Ševčík, R. Continuous Reactor with Ultrasonic Source. Czech Patent 24 590, 19 November 2012. [Google Scholar]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 5th ed.; Elsevier Science: London, UK, 2003. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

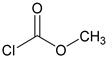
| Reactant (R) | Molar Ratio 1 | Catal. | Proc. 2 | Time (hr) | Product | Aver. Yield 3 (%) |
|---|---|---|---|---|---|---|
 | -: 1.3 | Cu(I) | A | 24 | 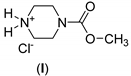 | 70 |
| - | - | B 4 | - | - | ||
| -: 1.1 | Ce(III) | C 4 | 0.58 | 61 | ||
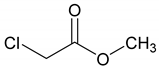 | 1: 1.1 | - | A | 19 | 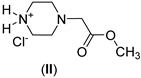 | 57 |
| 1: 1.2 | Ce(III) | A | 8 | 61 | ||
| 1: 1.2 | Cu(II) | B | 6 | 54 | ||
| 1: 1.2 | Cu(II) | C | 2.17 | 64 | ||
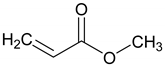 | 1: 2.2 | - | A | 16 | 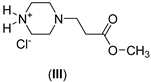 | 62 |
| 1: 2.6 | Ce(III) | A | 7 | 60 | ||
| 1: 2 | B | 4 | 50 | |||
| 1.2: 2.7 | C | 0.17 | 61 | |||
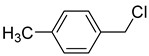 | 0.2: 1.2 | Cu(II) | A | 8 | 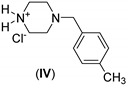 | 71 |
| 0.2: 1.1 | B | 1.83 | 72 | |||
| - | C | - | - | |||
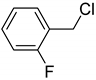 | 1: 1.1 | Cu(II) | A | 13 |  | 84 |
| 0.5: 1.1 | B | 2.5 | 67 | |||
| - | C | - | - | |||
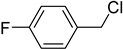 | 0.5: 1.1 | Cu(II) | A | 14 | 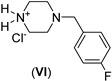 | 88 |
| 0.5: 1.1 | B | 1 | 69 | |||
| - | C | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Němečková, D.; Havránková, E.; Šimbera, J.; Ševčík, R.; Pazdera, P. Simplified Procedure for General Synthesis of Monosubstituted Piperazines—From a Batch Reaction Vessel to a Flow (Microwave) Reactor. Molecules 2020, 25, 2168. https://doi.org/10.3390/molecules25092168
Němečková D, Havránková E, Šimbera J, Ševčík R, Pazdera P. Simplified Procedure for General Synthesis of Monosubstituted Piperazines—From a Batch Reaction Vessel to a Flow (Microwave) Reactor. Molecules. 2020; 25(9):2168. https://doi.org/10.3390/molecules25092168
Chicago/Turabian StyleNěmečková, Dana, Eva Havránková, Jan Šimbera, Richard Ševčík, and Pavel Pazdera. 2020. "Simplified Procedure for General Synthesis of Monosubstituted Piperazines—From a Batch Reaction Vessel to a Flow (Microwave) Reactor" Molecules 25, no. 9: 2168. https://doi.org/10.3390/molecules25092168
APA StyleNěmečková, D., Havránková, E., Šimbera, J., Ševčík, R., & Pazdera, P. (2020). Simplified Procedure for General Synthesis of Monosubstituted Piperazines—From a Batch Reaction Vessel to a Flow (Microwave) Reactor. Molecules, 25(9), 2168. https://doi.org/10.3390/molecules25092168





