Fermented Wild Ginseng by Rhizopus oligosporus Improved l-Carnitine and Ginsenoside Contents
Abstract
1. Introduction
2. Results and Discussion
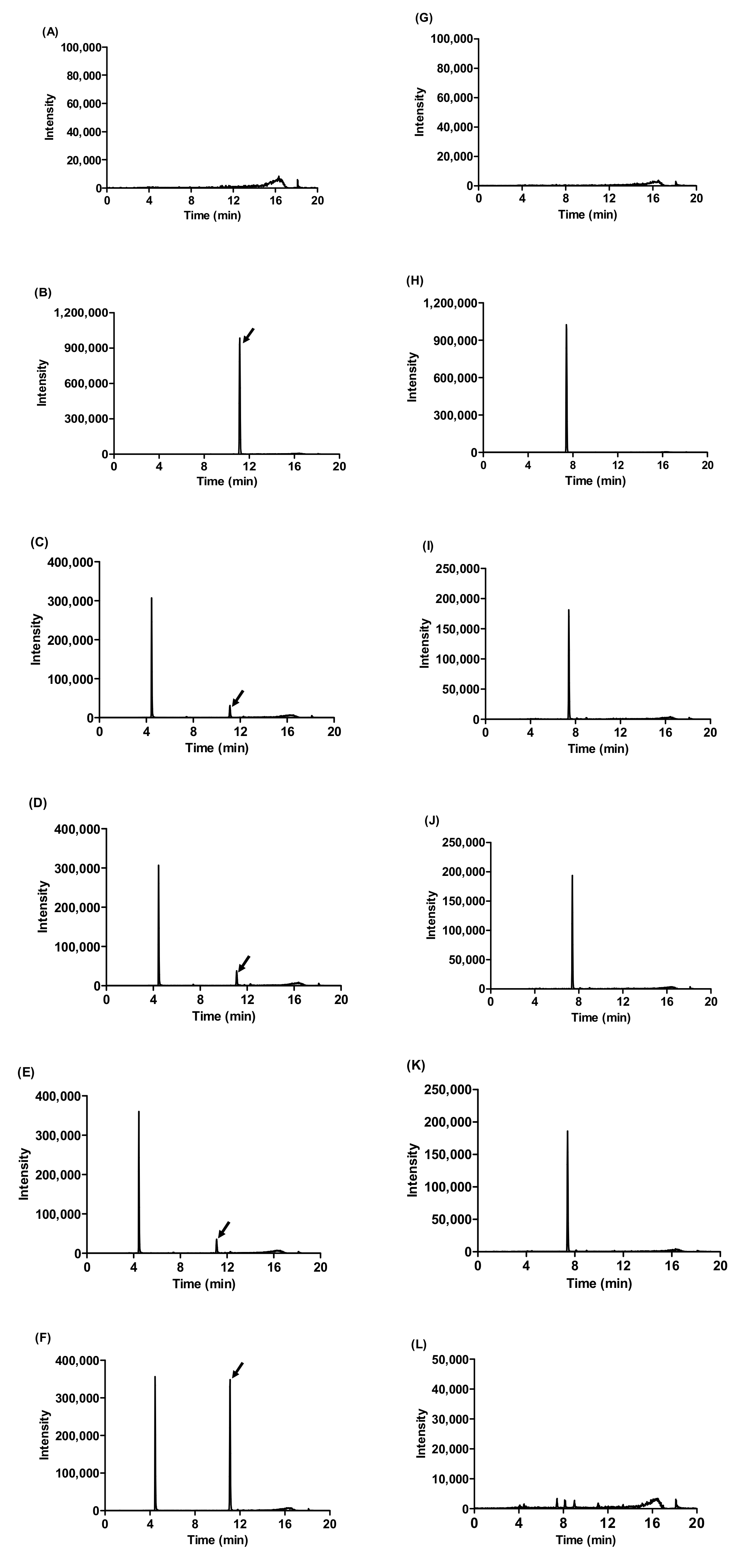
2.1. Changes in Ginsenoside Composition of Fermented Wild Ginseng
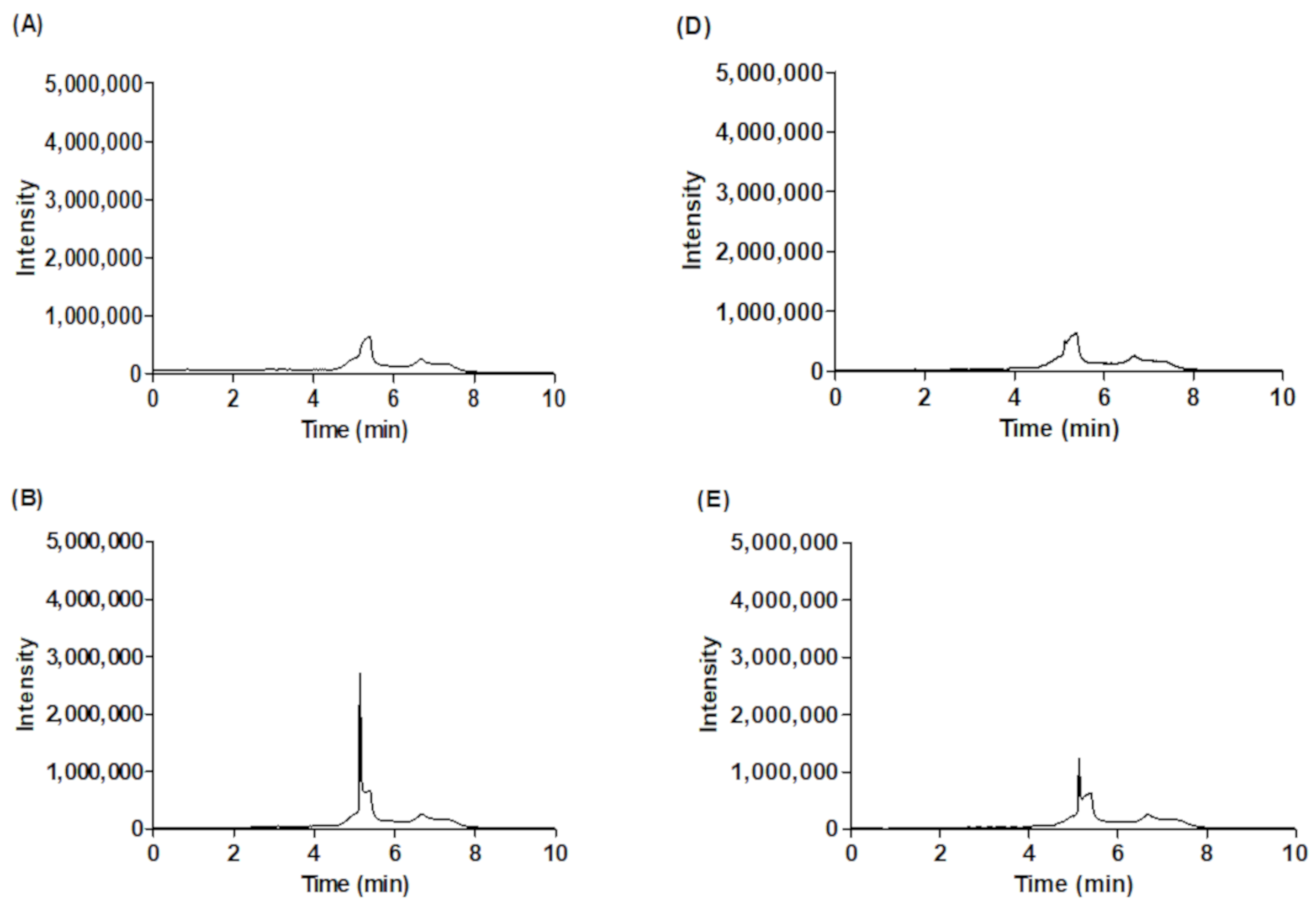
2.2. Amount of l-Carnitine in Fermented Wild Ginseng
2.3. Antioxidant Activity of Fermented Ginseng by R. oligosporus
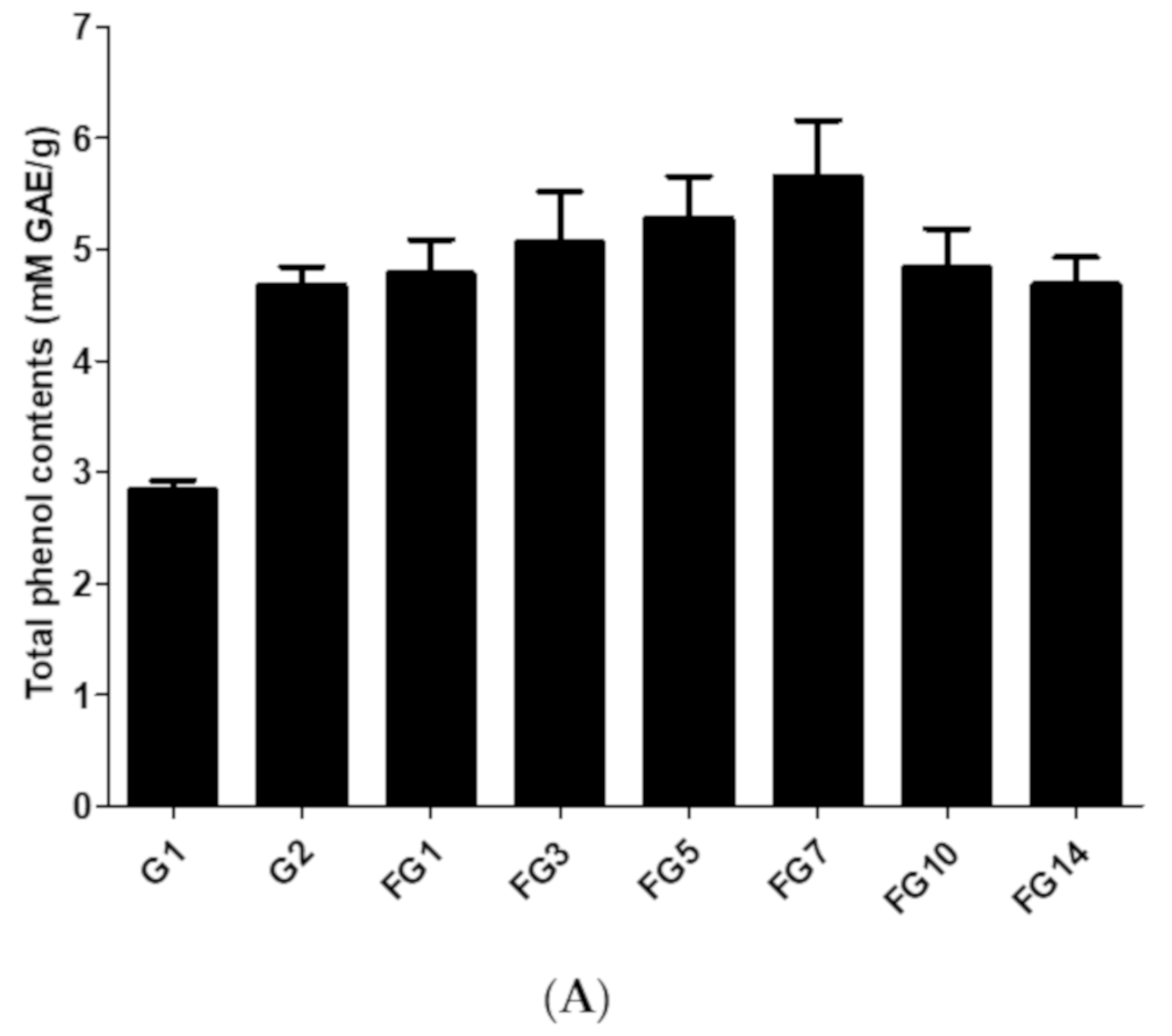
2.3.1. Total Phenolic Content
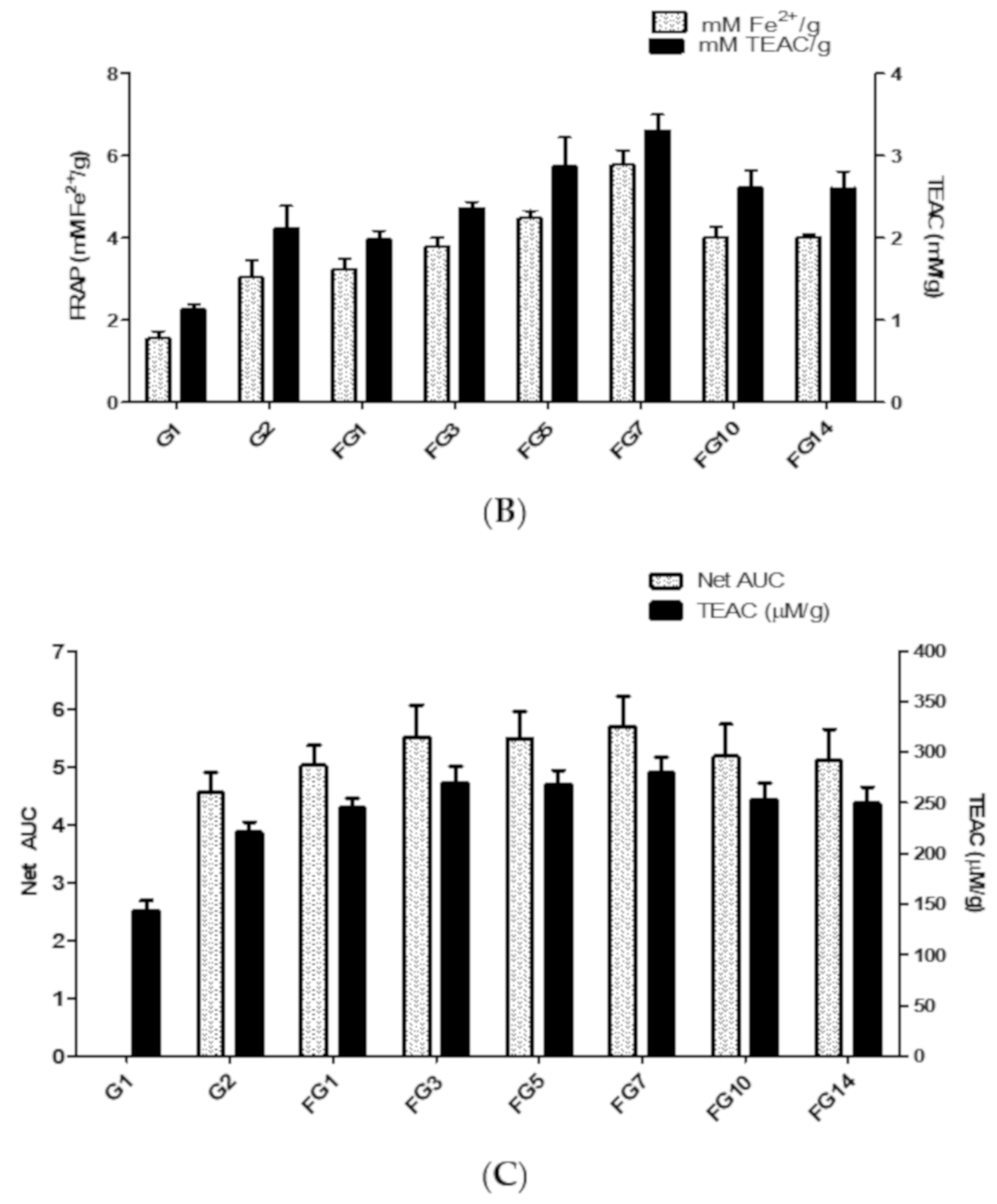
2.3.2. Ferric Reducing Antioxidant Power (FRAP)
2.3.3. Oxygen Radical Absorbance Capacity (ORAC)
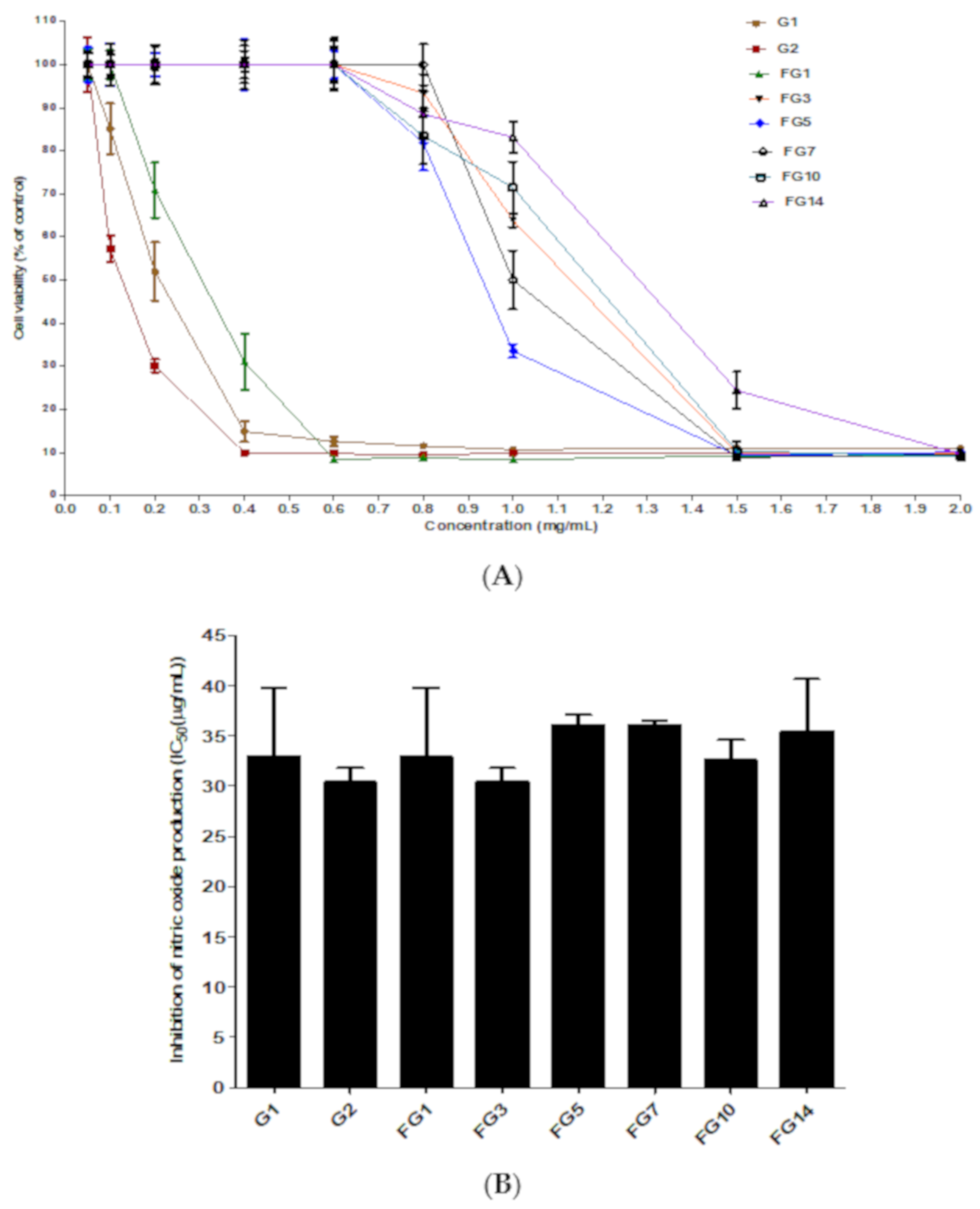
2.4. Inhibitory Effect of Fermented Ginseng against Nitric Oxide Production
3. Materials and Methods
3.1. Materials
3.2. Fermentation of Wild Ginseng by R. oligosporus
3.3. Sample Extraction
3.4. Analysis of Ginsenoside Contents
3.5. Analysis of l-Carnitine Content
3.6. Antioxidant Activity
3.6.1. Determination of Total Phenolic Contents
3.6.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.7. Cell Viability Test
3.8. Measurement of Nitric Oxide Production
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Soldati, F. Panax ginseng: Standardization and biological activity. Biologic. Active Nat. 2000, 209–232. [Google Scholar]
- Lui, J.H.-c.; Staba, E.J. The ginsenosides of various ginseng plants and selected products. J. Nat. 1980, 43, 340–346. [Google Scholar] [CrossRef]
- Jeong, H.; Lim, C.; Cha, B.; Choi, S.; Kwon, K. Component analysis of cultivated ginseng, cultivated wild ginseng, and wild ginseng and the change of ginsenoside components in the process of red ginseng. J. Pharmacopunct. 2010, 13, 63–77. [Google Scholar] [CrossRef][Green Version]
- Gillis, C.N. Panax ginseng pharmacology: A nitric oxide link? Biochem. Pharmacol. 1997, 54, 1–8. [Google Scholar] [CrossRef]
- Xie, J.T.; Mehendale, S.; Yuan, C.S. Ginseng and diabetes. Am. J. Chinese Med. 2005, 33, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Dey, L.; Xie, J.T.; Wang, A.; Wu, J.; Maleckar, S.A.; Yuan, C.S. Anti-hyperglycemic effects of ginseng: Comparison between root and berry. Phytomedicine 2003, 10, 600–605. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Liu, L.L.; Rausch, W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef]
- Liu, J.F.; Yan, X.D.; Li, L.; Zhu, Y.; Qin, K.F.; Zhou, L.F.; Sun, D.; Zhang, X.H.; Ye, R.D.; Zhao, G. Ginsennoside Rd attenuates cognitive dysfunction in a rat model of Alzheimer’s disease. Neurochem Res. 2012, 37, 2738–2747. [Google Scholar] [CrossRef]
- Shin, Y.M.; Jung, H.J.; Choi, W.Y.; Lim, C.J. Antioxidative, anti-inflammatory, and matrix metalloproteinase inhibitory activities of 20(S)-ginsenoside Rg3 in cultured mammalian cell lines. Mol. Biol. Rep. 2013, 40, 269–279. [Google Scholar] [CrossRef]
- Shin, K.C.; Oh, D.K. Characterization of a novel recombinant beta-glucosidase from Sphingopyxis alaskensis that specifically hydrolyzes the outer glucose at the C-3 position in protopanaxadiol-type ginsenosides. J. Biotechnol. 2014, 172, 30–37. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, Y.Q.; Liang, X.J. Current evaluation of the millennium phytomedicine- ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional chinese medicine. Curr. Med. Chem. 2009, 16, 2924–2942. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Xu, J.H.; Lu, W.Y.; Lin, G.Q. Enzymatic transformation of ginsenoside Rg(3) to Rh-2 using newly isolated Fusarium proliferatum ECU2042. J. Mol. Catal. B-Enzym. 2006, 38, 113–118. [Google Scholar] [CrossRef]
- Xu, Q.F.; Fang, X.L.; Chen, D.F. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J. Ethnopharmacol. 2003, 84, 187–192. [Google Scholar] [CrossRef]
- Hu, C.; Song, G.; Zhang, B.; Liu, Z.C.; Chen, R.; Zhang, H.; Hu, T.H. Intestinal metabolite compound K of panaxoside inhibits the growth of gastric carcinoma by augmenting apoptosis via Bid-mediated mitochondrial pathway. J. Cell Mol. Med. 2012, 16, 96–106. [Google Scholar] [CrossRef]
- Joh, E.H.; Lee, I.A.; Jung, I.H.; Kim, D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation--the key step of inflammation. Biochem. Pharmacol. 2011, 82, 278–286. [Google Scholar] [CrossRef]
- Park, S.E.; Na, C.S.; Yoo, S.A.; Seo, S.H.; Son, H.S. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J. Ginseng Res. 2017, 41, 36–42. [Google Scholar] [CrossRef]
- Quan, L.H.; Piao, J.Y.; Min, J.W.; Yang, D.U.; Lee, H.N.; Yang, D.C. Bioconversion of ginsenoside Rb1 into compound K by Leuconostoc citreum Lh1 isolated from kimchi. Braz. J. Microbiol. 2011, 42, 1227–1237. [Google Scholar] [CrossRef]
- Han, B.H.; Park, M.H.; Han, Y.N.; Woo, L.K.; Sankawa, U.; Yahara, S.; Tanaka, O. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982, 44, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Lee, S.K.; Kim, N.D.; Park, M.K.; Kim, C.K.; Park, J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. 2000, 63, 1702–1704. [Google Scholar] [CrossRef]
- Zhao, X.S.; Gao, L.; Wang, J.; Bi, H.T.; Gao, J.; Du, X.L.; Zhou, Y.F.; Tai, G.H. A novel ginsenoside Rb-1-hydrolyzing beta-d-glucosidase from Cladosporium fulvum. Process Biochem. 2009, 44, 612–618. [Google Scholar] [CrossRef]
- Chang, K.H.; Jo, M.N.; Kim, K.T.; Paik, H.D. Evaluation of glucosidases of Aspergillus niger strain comparing with other glucosidases in transformation of ginsenoside Rbl to ginsenosides Rg3. J. Ginseng Res. 2014, 38, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Jang, H.J.; Eom, S.J.; Choi, N.S.; Lee, N.K.; Paik, H.D. Fermentation of red ginseng extract by the probiotic Lactobacillus plantarum KCCM 11613P: Ginsenoside conversion and antioxidant effects. J. Ginseng Res. 2019, 43, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Ye, M.; Guo, H.; Zheng, J.; Guo, D. Microbial transformation of ginsenoside Rb1 by Rhizopus stolonifer and Curvularia lunata. Biotechnol Lett. 2003, 25, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Zhu, X.M.; Lee, K.T.; Zheng, Y.N.; Li, W.; Han, L.K.; Fang, Z.M.; Gu, L.J.; Sun, B.S.; Wang, C.Y.; et al. Optimization of ginsenosides hydrolyzing beta-glucosidase production from Aspergillus niger using response surface methodology. Biol. Pharm. Bull. 2008, 31, 1870–1874. [Google Scholar] [CrossRef]
- Handoyo, T.; Morita, N. Structural and functional properties of fermented soybean (tempeh) by using Rhizopus oligosporus. Int. J. Food Prop. 2006, 9, 347–355. [Google Scholar] [CrossRef]
- Park, N.; Nguyen, T.T.H.; Lee, G.H.; Jin, S.N.; Kwak, S.H.; Lee, T.K.; Choi, Y.H.; Kim, S.B.; Kimura, A.; Kim, D. Composition and biochemical properties of l-carnitine fortified Makgeolli brewed by using fermented buckwheat. Food Sci. Nutr. 2018, 6, 2293–2300. [Google Scholar] [CrossRef]
- Hur, J.; Nguyen, T.T.H.; Park, N.; Kim, J.; Kim, D. Characterization of quinoa (Chenopodium quinoa) fermented by Rhizopus oligosporus and its bioactive properties. AMB Express 2018, 8, 143. [Google Scholar] [CrossRef]
- Bremer, J. Carnitine—Metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef]
- Kalaiselvi, T.; Panneerselvam, C. Effect of L-carnitine on the status of lipid peroxidation and antioxidants in aging rats. J. Nutr. Biochem. 1998, 9, 575–581. [Google Scholar] [CrossRef]
- McCue, P.; Shetty, K. Role of carbohydrate-cleaving enzymes in phenolic antioxidant mobilization from whole soybean fermented with Rhizopus oligosporus. Food Biotechnol. 2003, 17, 27–37. [Google Scholar] [CrossRef]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. Int. J. Mol. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Lin, J.T.; Wu, J.Y.; Liu, W.H. Isoflavone conversion of black soybean by immobilized Rhizopus spp. Food Biotechnol. 2010, 24, 312–331. [Google Scholar] [CrossRef]
- Hwang, C.R.; Lee, S.H.; Jang, G.Y.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J. Ginseng Res. 2014, 38, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.H.; Son, J.W.; Kim, H.J.; Oh, D.K. Ginsenoside compound K production from ginseng root extract by a thermostable beta-glycosidase from Sulfolobus solfataricus. Biosci. Biotechnol. Biochem. 2009, 73, 316–321. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Nardini, M.; Ghiselli, A. Determination of free and bound phenolic acids in beer. Food Chem. 2004, 84, 137–143. [Google Scholar] [CrossRef]
- Park, N.; Lee, T.K.; Nguyen, T.T.H.; An, E.B.; Kim, N.M.; You, Y.H.; Park, T.S.; Kim, D. The effect of fermented buckwheat on producing l-carnitine- and gamma-aminobutyric acid (GABA)-enriched designer eggs. J. Sci. Food Agric. 2017, 97, 2891–2897. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.X.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.Y.; Kim, H.J.; Kim, D.H.; Lee, T.H.; Kang, M.S.; Park, W. Anti-inflammatory effects of Angelica sinensis (Oliv.) diels water extract on RAW 264.7 induced with lipopolysaccharide. Nutrients 2018, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Boonkaewwan, C.; Toskulkao, C.; Vongsakul, M. Anti-inflammatory and immunomodulatory activities of stevioside and its metabolite steviol on THP-1 cells. J. Agric Food Chem. 2006, 54, 785–789. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Park, J.H.; Choi, A.; Hwang, H.J.; Mah, J.H. Validation of an HPLC analytical method for determination of biogenic amines in agricultural products and mcellonitoring of biogenic amines in Korean fermented agricultural products. Toxicol. Res. 2015, 31, 299–305. [Google Scholar] [CrossRef]
- Jang, H.J.; Jung, J.; Yu, H.S.; Lee, N.K.; Paik, H.D. Evaluation of the quality of yogurt using ginseng extract powder and probiotic Lactobacillus plantarum NK181. Korean J. Food Sci. An. 2018, 38, 1160–1167. [Google Scholar] [CrossRef]
Sample Availability: Samples of wild ginseng and fermented wild ginseng are available from local market (Pyeongchang, Kangwondo, Korea) and the authors, respectively. |

| Control | Autoclaved-Control | Fermented Wild Ginseng | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 3 days | 5 days | 7 days | 10 days | 14 days | |||
| Extraction Yield (%) | 21.2 | 20.7 | 21.4 | 23.0 | 23.8 | 24.9 | 23.3 | 25.4 |
| Group | Chemicals | Ginsenoside Contents (mg/Kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control * | Autoclaved Control | Fermented Wild Ginseng | |||||||
| 1 day | 3 days | 5 days | 7 days | 10 days | 14 days | ||||
 Protopanaxadiol-type (PPD) | Rg3 | 70 a | 100 ± 10 e | 80 ± 10 e | 140 ± 10 c | 160 ± 10 d | 170 ± 10 de | 180 e | 230 ± 10 f |
| Rb1 | 4030 ± 540 c | 7890 ± 1240 d | 7840 ± 810 d | 4300 ± 210 c | 2380 ± 90 b | 3370 ±120 c | 410 ± 10 a | 400 ± 40 a | |
| Rb2 | 1410 ± 210 a | 2810 ± 410 c | 2770 ± 120 c | 2300 ± 160 b | 2850 ± 80 c | 2810 ± 200 c | 2660 ± 330 bc | 3730 ± 230 d | |
| Rb3 | 220 ± 40 a | 420 ± 20 cd | 310 ± 30 b | 380 ± 50 bc | 460 ± 50 d | 470 ± 50 d | 490 ± 60 d | 580 ± 20 e | |
| Rc | 2130 ± 130 a | 3800 ± 420 bc | 3660 ± 170 bc | 3220 ± 120 b | 4060 ± 540 c | 4150 ± 650 c | 3560 ± 320 bc | 5280 ± 500 d | |
| Rd | 1280 ± 60 a | 2020 ± 370 a | 2140 ± 100 a | 4870 ± 330 b | 6940 ± 910 cd | 6730 ± 300 c | 7760 ± 810 d | 11,300 ± 840 e | |
| Compound K | 20 a | 50 d | 40 b | 40 c | 50 d | 60 e | 70 f | 70 g | |
| F2 | 110 ± 10 a | 150 ± 20 b | 110 ± 10 ab | 190 ± 10 c | 240 ± 20 d | 260 ± 20 d | 240 ± 20 d | 310 ± 30 e | |
 Protopanaxatriol-type (PPT) | Rg1 | 6330 ± 340 a | 7060 ± 810 a | 8060 ± 470 bc | 6440 ±340 a | 7240 ± 670 ab | 7120 ± 280 ab | 6520 ± 340 a | 8950 ± 710 c |
| Rg2 | 850 ± 30 a | 1560 ± 50 bc | 1340 ± 60 b | 1320 ± 30 b | 1500 ± 90 c | 1850 ± 30 e | 1630 ± 40 e | 2050 ± 80 f | |
| Re | 9790 ± 340 a | 10,420 ± 1580 a | 12,500 ± 480 bc | 9900 ± 660 a | 11,330 ± 1380 ab | 10,740 ± 410 ab | 9750 ± 510 a | 13,730 ± 1560 c | |
| Rf | 6410 ± 220 a | 6870 ± 660 a | 7860 ± 640 bc | 6370 ± 390 a | 7110 ± 640 ab | 7120 ± 390 ab | 6460 ± 300 a | 8560 ± 570 c | |
| Rh1 | 60 ± 10 a | 310 d | 170 b | 200 ± 20 b | 260 ± 10 c | 400 ± 40 e | 340 ± 20 d | 410 ± 20 e | |
| F1 | 40 a | 110 c | 100 ± 10 bc | 90 ± 10 b | 100 ± 10 bc | 110 ± 10 c | 100 ± 10 bc | 140 d | |
| Control * | Autoclaved-Control | Fermented Wild Ginseng | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 3 days | 5 days | 7 days | 10 days | 14 days | |||
| l-carnitine (mg/kg) | ND * | ND * | 60 a | 230 b | 310 c | 440 d | 500 e | 630 f ± 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Nguyen, T.T.H.; Lim, T.Y.; Lim, J.; Park, B.; Lee, S.; Mok, I.-K.; Pal, K.; Lim, S.; Kim, D. Fermented Wild Ginseng by Rhizopus oligosporus Improved l-Carnitine and Ginsenoside Contents. Molecules 2020, 25, 2111. https://doi.org/10.3390/molecules25092111
Lee G, Nguyen TTH, Lim TY, Lim J, Park B, Lee S, Mok I-K, Pal K, Lim S, Kim D. Fermented Wild Ginseng by Rhizopus oligosporus Improved l-Carnitine and Ginsenoside Contents. Molecules. 2020; 25(9):2111. https://doi.org/10.3390/molecules25092111
Chicago/Turabian StyleLee, Ganghee, Thi Thanh Hanh Nguyen, Tae Yun Lim, Juho Lim, Byeongsu Park, Seonmin Lee, Il-Kyoon Mok, Kunal Pal, Sangyong Lim, and Doman Kim. 2020. "Fermented Wild Ginseng by Rhizopus oligosporus Improved l-Carnitine and Ginsenoside Contents" Molecules 25, no. 9: 2111. https://doi.org/10.3390/molecules25092111
APA StyleLee, G., Nguyen, T. T. H., Lim, T. Y., Lim, J., Park, B., Lee, S., Mok, I.-K., Pal, K., Lim, S., & Kim, D. (2020). Fermented Wild Ginseng by Rhizopus oligosporus Improved l-Carnitine and Ginsenoside Contents. Molecules, 25(9), 2111. https://doi.org/10.3390/molecules25092111








