Photocatalytic Cleavage of β-O-4 Ether Bonds in Lignin over Ni/TiO2
Abstract
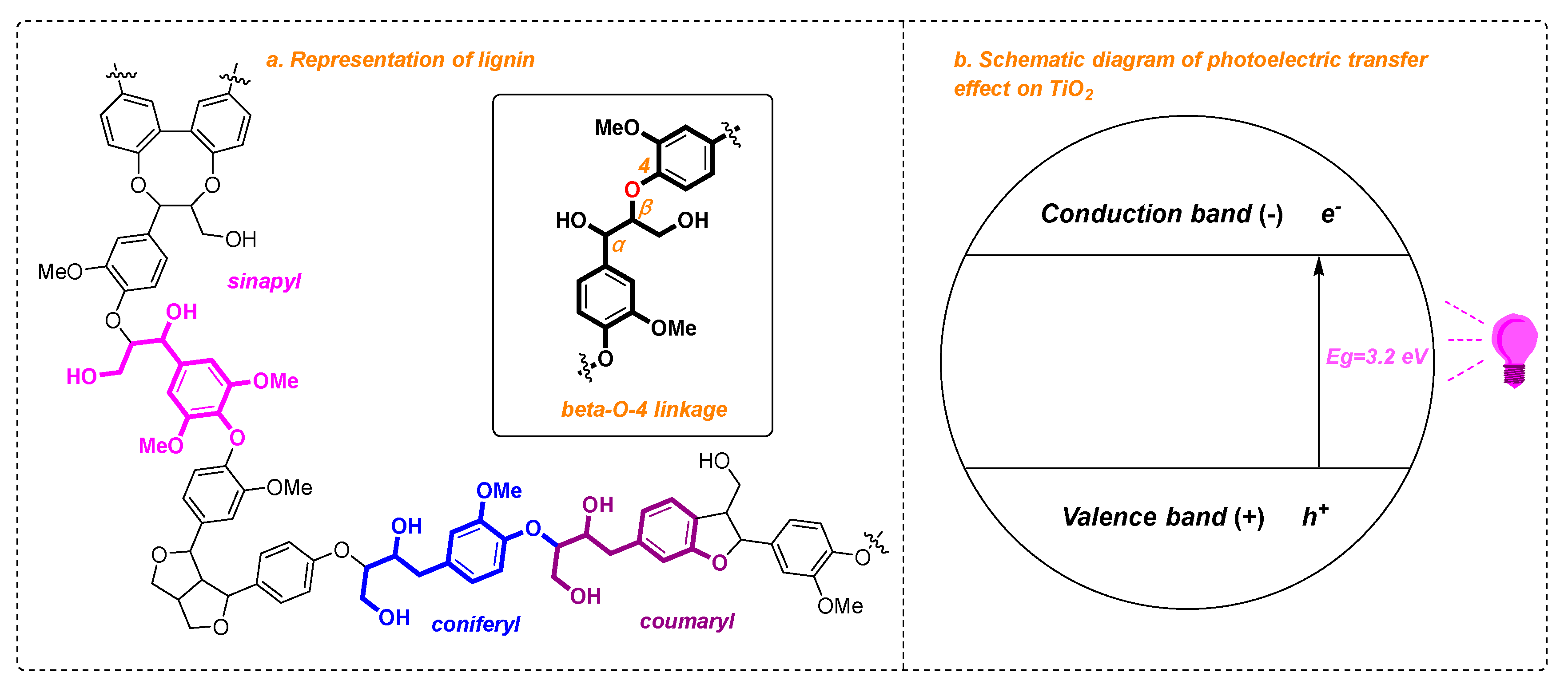
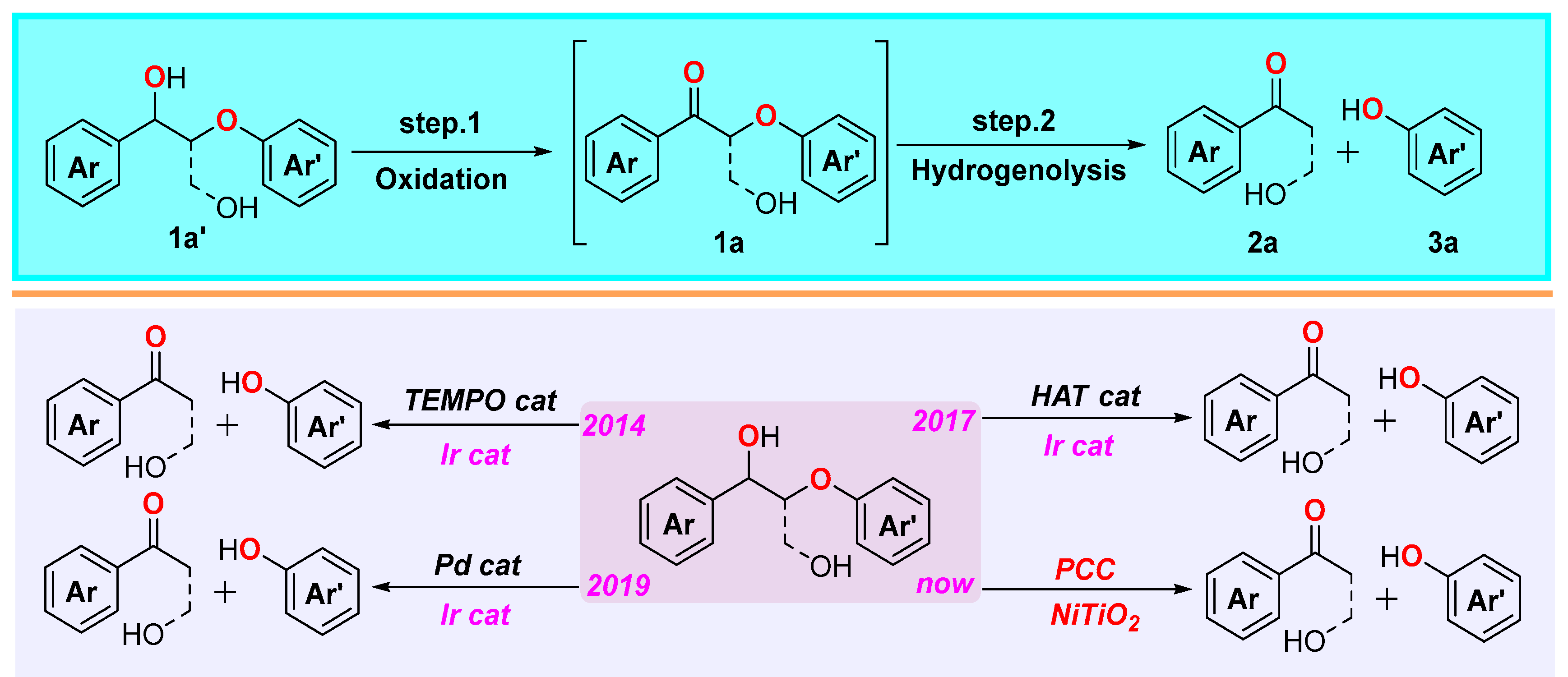
1. Introduction
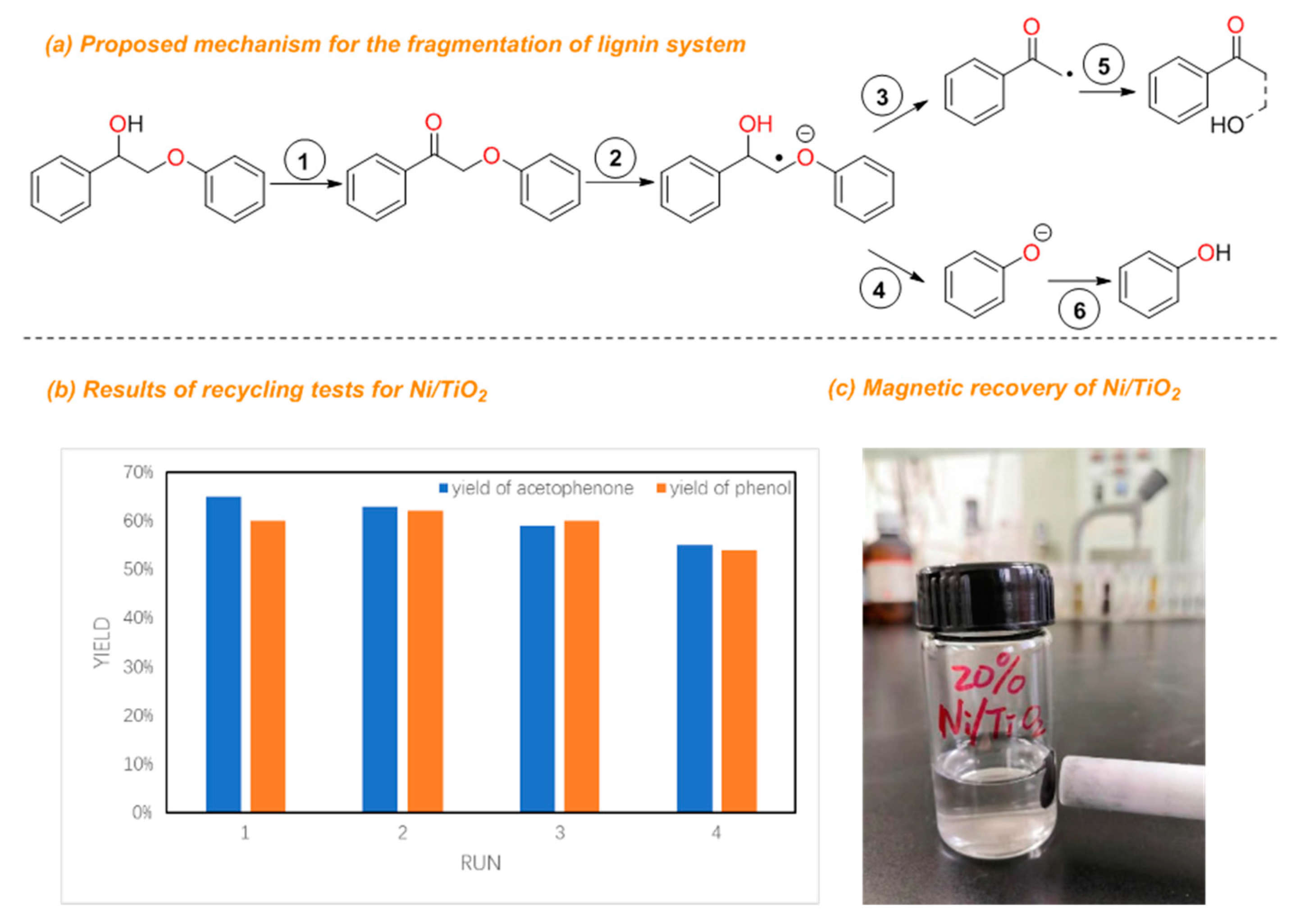
2. Result and Discussion
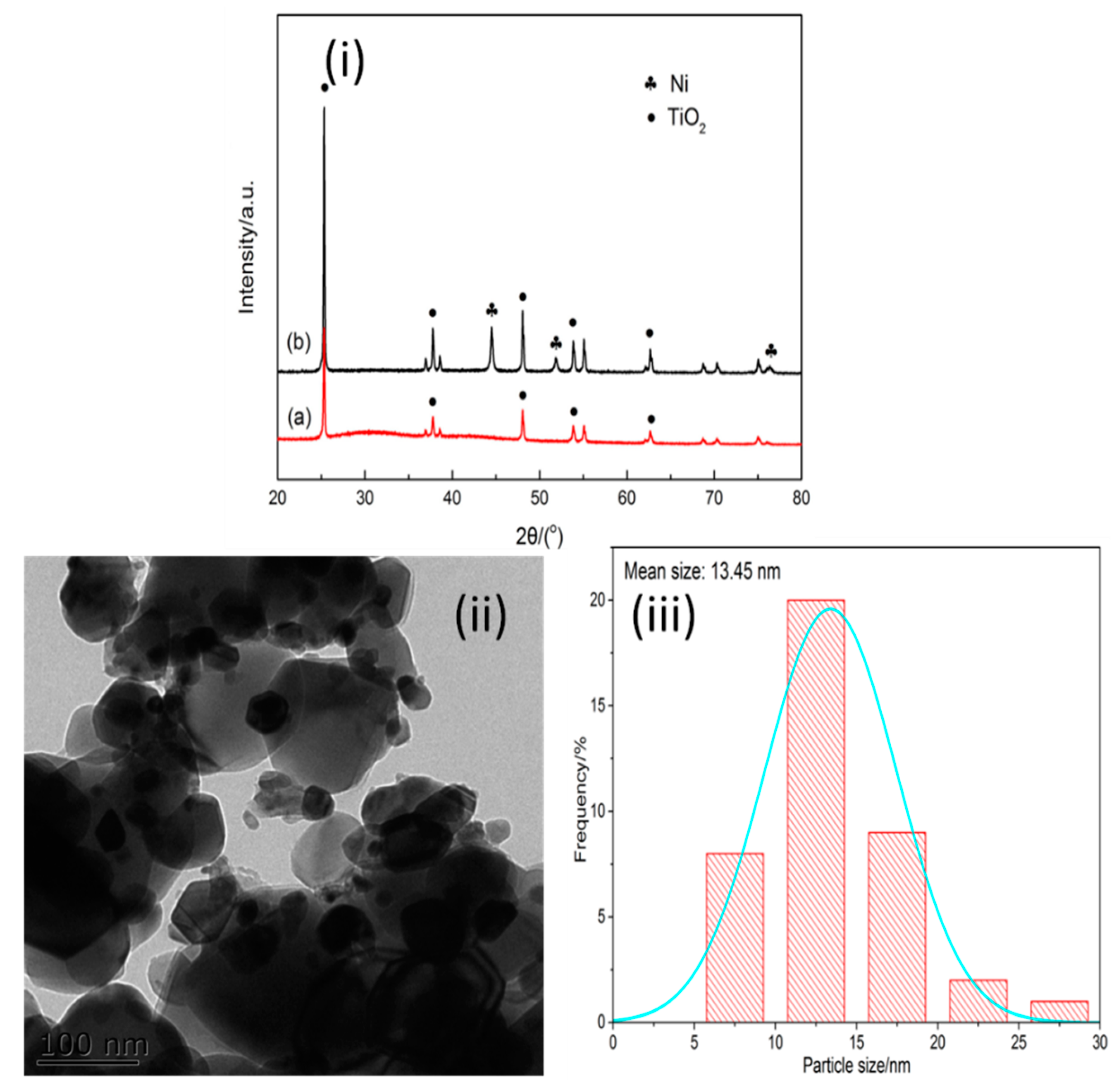
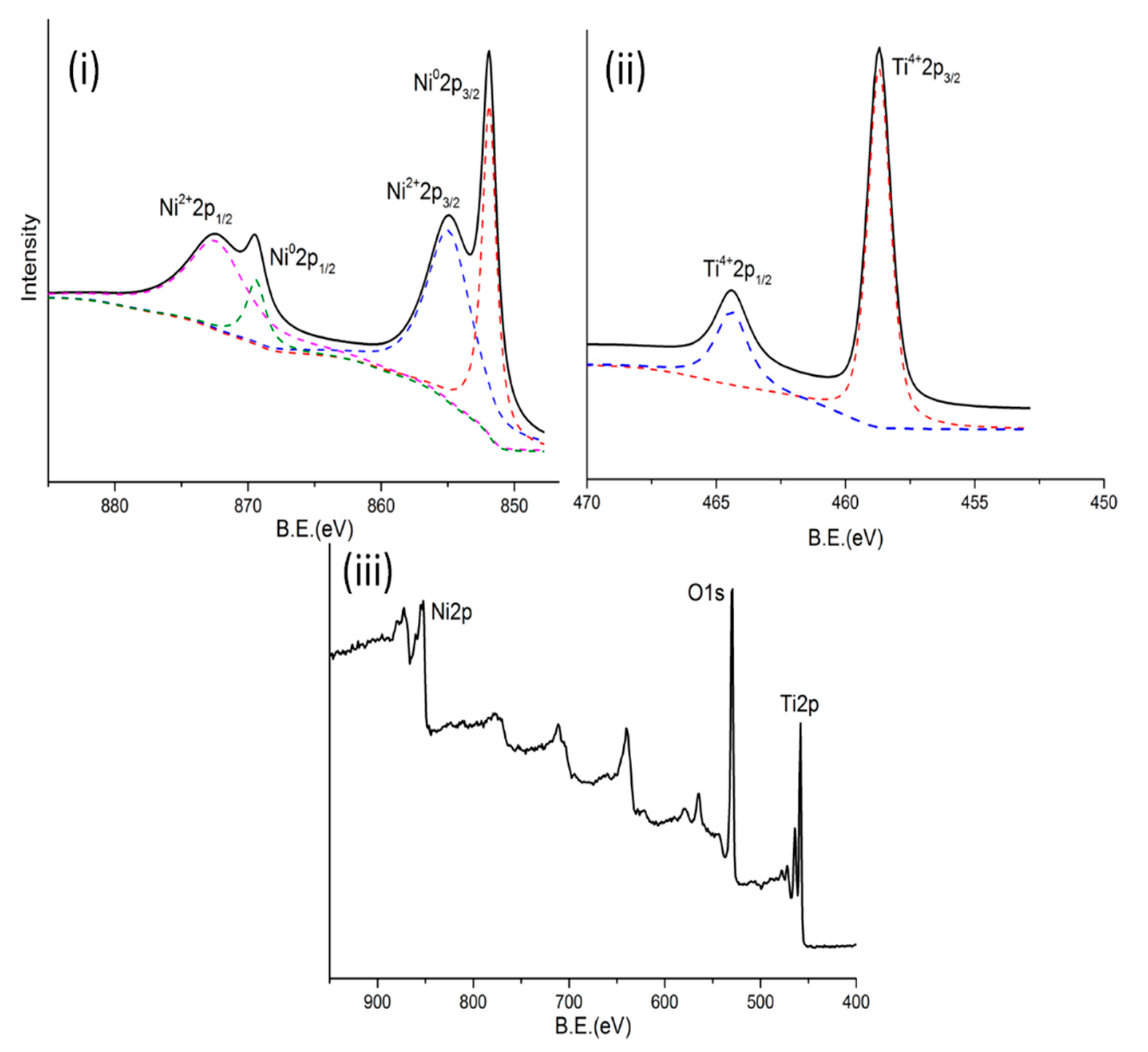
2.1. Catalyst Characterization
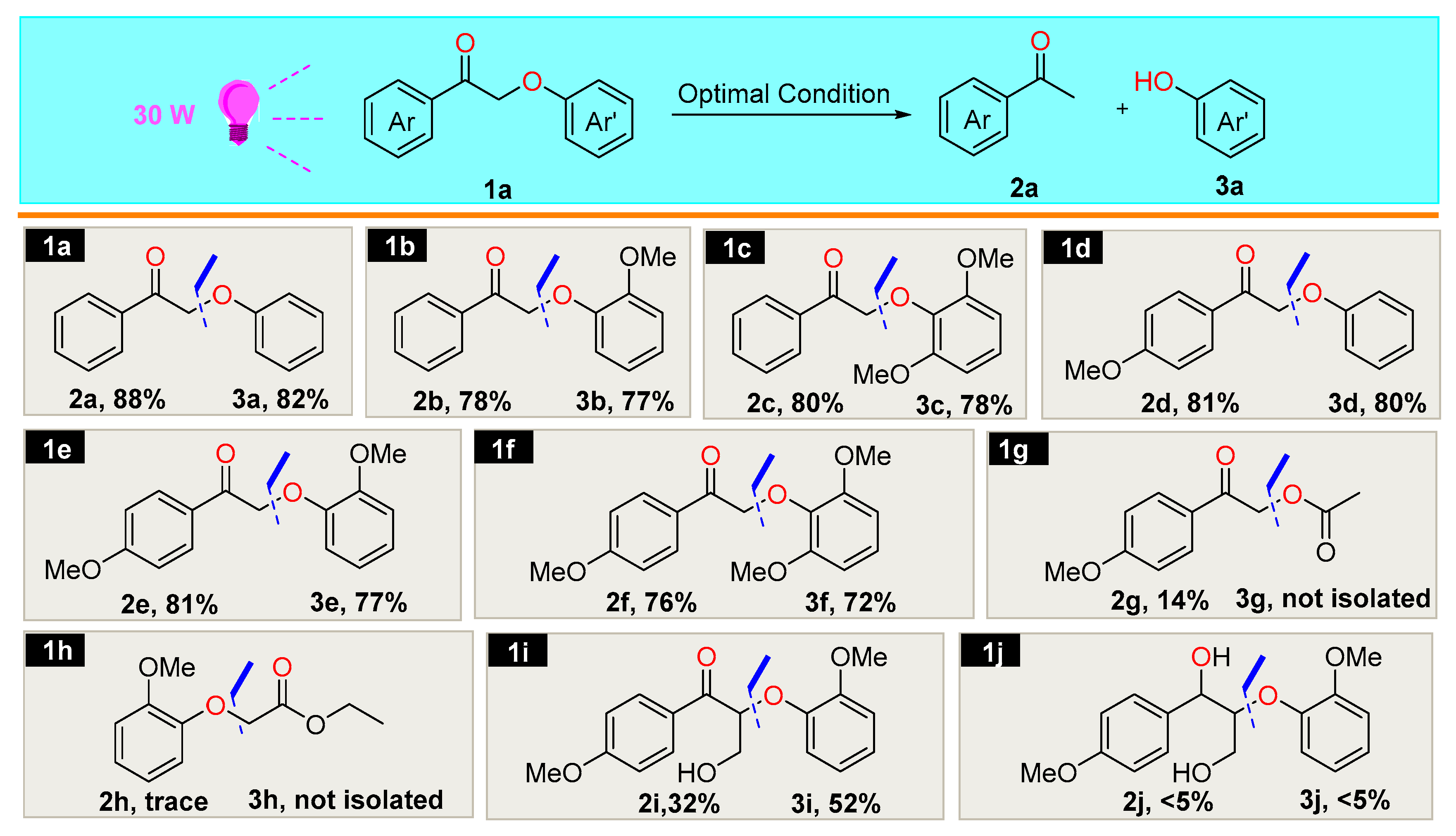
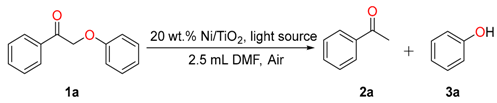
2.2. Optimization of the Reaction Condition
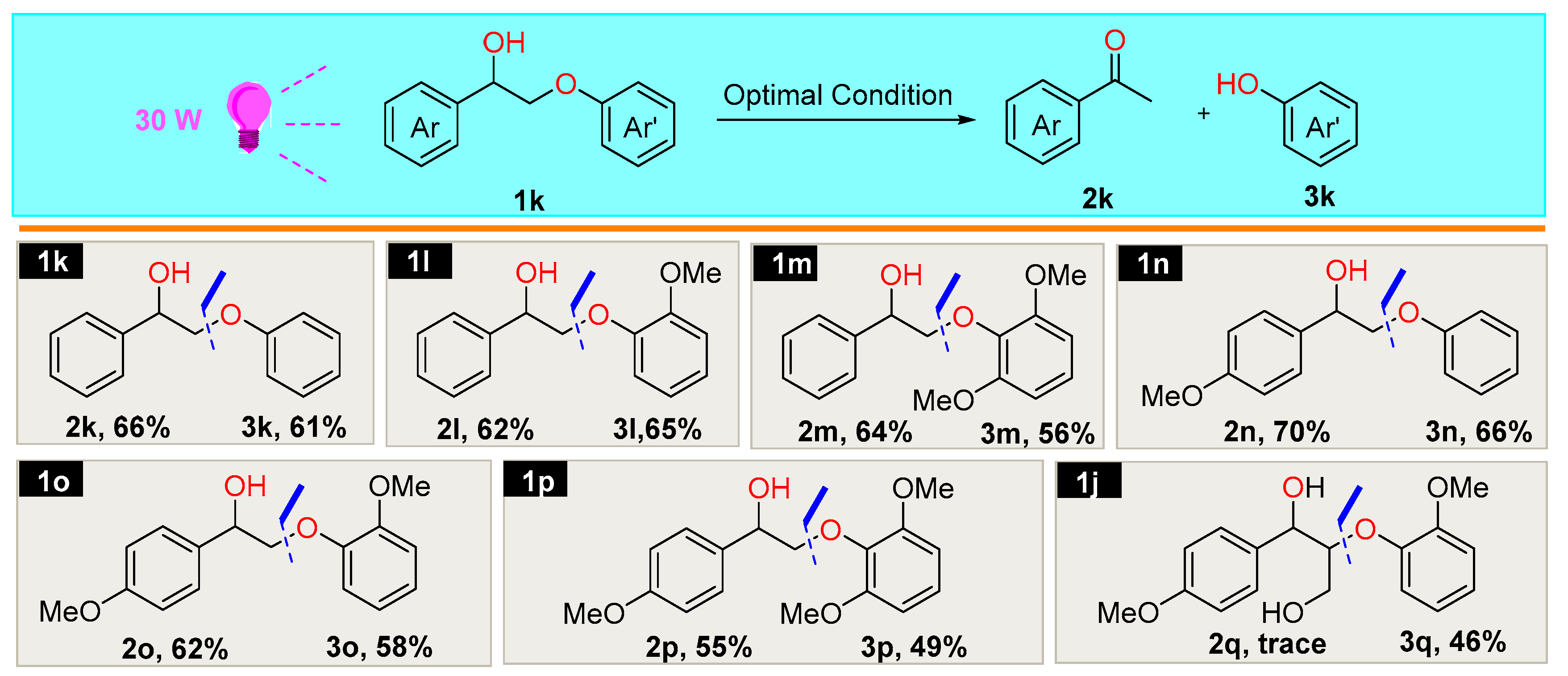
2.3. Scope of the Substrates
3. Experimental
3.1. Materials
3.2. General Procedure for Ni/TiO2 Catalyzed β-O-4 Model Compounds
3.3. Catalyst Preparation
3.4. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Binder, J.B.; Raines, R.T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Miao, W.; Tang, W.; Xue, D.; Xiao, J.; Zhang, T.; Wang, C. Rhodium-terpyridine catalyzed redox-neutral depolymerization of lignin in water. Green Chem. 2020, 22, 33–38. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Xiao, L.P.; Sun, R.C.; Song, G. Chemodivergent hydrogenolysis of eucalyptus lignin with Ni@ ZIF-8 catalyst. Green Chem. 2019, 21, 1498–1504. [Google Scholar] [CrossRef]
- Sergeev, A.G.; Hartwig, J.F. Selective, nickel-catalyzed hydrogenolysis of aryl ethers. Science 2011, 332, 439–443. [Google Scholar] [CrossRef]
- Sedai, B.; Diaz-Urrutia, C.; Baker, R.T.; Wu, R.; Silks, L.P.; Hanson, S.K. Aerobic oxidation of β-1 lignin model compounds with copper and oxovanadium catalysts. ACS Catal. 2013, 3, 3111–3122. [Google Scholar] [CrossRef]
- Liu, S.; Bai, L.; van Muyden, A.P.; Huang, Z.; Cui, X.; Fei, Z.; Li, X.; Hu, X.; Dyson, P.J. Oxidative cleavage of β-O-4 bonds in lignin model compounds with a single-atom Co catalyst. Green Chem. 2019, 21, 1974–1981. [Google Scholar] [CrossRef]
- Nichols, J.M.; Bishop, L.M.; Bergman, R.G.; Ellman, J.A. Catalytic C–O bond cleavage of 2-aryloxy-1-arylethanols and its application to the depolymerization of lignin-related polymers. J. Am. Chem. Soc. 2010, 132, 12554–12555. [Google Scholar] [CrossRef] [PubMed]
- Lahive, C.W.; Deuss, P.J.; Lancefield, C.S.; Sun, Z.; Cordes, D.B.; Young, C.M.; Tran, F.; Slawin, A.M.Z.; de Vires, J.G.; Kamer, P.C.; et al. Advanced model compounds for understanding acid-catalyzed lignin depolymerization: Identification of renewable aromatics and a lignin-derived solvent. J. Am. Chem. Soc. 2016, 138, 8900–8911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Zhang, Z.; Li, M.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. M-Gallate (M=Ni, Co) metal-organic framework-derived Ni/C and bimetallic Ni-Co/C catalysts for lignin conversion into monophenols. ACS Sustain. Chem. Eng. 2019, 7, 12955–12963. [Google Scholar] [CrossRef]
- Bernt, C.M.; Manesewan, H.; Chui, M.; Boscolo, M.; Ford, P.C. Temperature tuning the catalytic reactivity of Cu-doped porous metal oxides with lignin models. ACS Sustain. Chem. Eng. 2018, 6, 2510–2516. [Google Scholar] [CrossRef]
- Molinari, V.; Giordano, C.; Antonietti, M.; Esposito, D. Titanium nitride-nickel nanocomposite as heterogeneous catalyst for the hydrogenolysis of aryl ethers. J. Am. Chem. Soc. 2014, 136, 1758–1761. [Google Scholar] [CrossRef]
- Shuai, L.; Sitison, J.; Sadula, S.; Ding, J.; Thies, M.C.; Saha, B. Selective C-C bond cleavage of methylene-linked lignin models and kraft lignin. ACS Catal. 2018, 8, 6507–6512. [Google Scholar] [CrossRef]
- Hua, M.; Song, J.; Xie, C.; Wu, H.; Hu, Y.; Huang, X.; Han, B. Ru/hydroxyapatite as a dual-functional catalyst for efficient transfer hydrogenolytic cleavage of aromatic ether bonds without additional bases. Green Chem. 2019, 21, 5073–5079. [Google Scholar] [CrossRef]
- Kang, Y.; Lu, X.; Zhang, G.; Yao, X.; Xin, J.; Yang, S.; Yang, Y.; Xu, J.; Feng, M.; Zhang, S. Metal-free photochemical degradation of lignin-derived aryl ethers and lignin by autologous radicals through ionic liquid induction. ChemSusChem 2019, 12, 4005–4013. [Google Scholar] [CrossRef]
- Enright, M.J.; Gilbert-Bass, K.; Sarsito, H.; Cossairt, B.M. Photolytic C-O bond cleavage with quantum dots. Chem. Mater. 2019, 31, 2677–2682. [Google Scholar] [CrossRef]
- Han, G.; Yan, T.; Zhang, W.; Zhang, Y.C.; Lee, D.Y.; Cao, Z.; Sun, Y. Highly selective photocatalytic valorization of lignin model compounds using ultrathin metal/CdS. ACS Catal. 2019, 9, 11341–11349. [Google Scholar] [CrossRef]
- Nguyen, J.D.; Matsuura, B.S.; Stephenson, C.R. A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc. 2014, 136, 1218–1221. [Google Scholar] [CrossRef]
- Magallanes, G.; Kärkäs, M.D.; Bosque, I.; Lee, S.; Maldonado, S.; Stephenson, C.R. Selective C-O bond cleavage of lignin systems and polymers enabled by sequential palladium-catalyzed aerobic oxidation and visible-light photoredox catalysis. ACS Catal. 2019, 9, 2252–2260. [Google Scholar] [CrossRef]
- Dedeian, K.; Djurovich, P.I.; Garces, F.O.; Carlson, G.; Watts, R.J. A new synthetic route to the preparation of a series of strong photoreducing agents: Fac-tris-ortho-metalated complexes of iridium (III) with substituted 2-phenylpyridines. Inorg. Chem. 1991, 30, 1685–1687. [Google Scholar] [CrossRef]
- Slinker, J.D.; Gorodetsky, A.A.; Lowry, M.S.; Wang, J.; Parker, S.; Rohl, R.; Bernhard, S.; Malliaras, G.G. Efficient yellow electroluminescence from a single layer of a cyclometalated iridium complex. J. Am. Chem. Soc. 2004, 126, 2763–2767. [Google Scholar] [CrossRef] [PubMed]
- Kärkäs, M.D.; Bosque, I.; Matsuura, B.S.; Stephenson, C.R. Photocatalytic oxidation of lignin model systems by merging visible-light photoredox and palladium catalysis. Org. Lett. 2016, 18, 5166–5169. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, A. Introduction: Titanium dioxide (TiO2) nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, Z.; Zhou, C.; Ren, Z.; Yang, X. Single molecule photocatalysis on TiO2 surfaces: Focus review. Chem. Rev. 2019, 119, 11020–11041. [Google Scholar] [CrossRef]
- Chen, W.T.; Chan, A.; Sun-Waterhouse, D.; Llorca, J.; Idriss, H.; Waterhouse, G.I. Performance comparison of Ni/TiO2 and Au/TiO2 photocatalysts for H2 production in different alcohol-water mixtures. J. Catal. 2018, 367, 27–42. [Google Scholar] [CrossRef]
- Bansal, P.; Verma, A. In-situ dual effect studies using novel Fe-TiO2 composite for the pilot-plant degradation of pentoxifylline. Chem. Eng. J. 2018, 332, 682–694. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Remesal, E.R.; Ramírez, P.J.; Orozco, I.; Liu, Z.; Graciani, J.; Senanayake, S.D.; Sanz, J.F. Water-gas shift reaction on K/Cu (111) and Cu/K/TiO2 (110) surfaces: Alkali promotion of water dissociation and production of H2. ACS Catal. 2019, 9, 10751–10760. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, S.; Dong, M.; Wang, J.; Fan, W. Ru nanoparticles deposited on ultrathin TiO2 nanosheets as highly active catalyst for levulinic acid hydrogenation to γ-valerolactone. App. Catal. B 2019, 259, 118076. [Google Scholar] [CrossRef]
- Chen, S.; Abdel-Mageed, A.M.; Li, D.; Bansmann, J.; Cisneros, S.; Biskupek, J.; Huang, W.; Behm, R.J. Morphology-engineered highly active and stable Ru/TiO2 catalysts for selective CO methanation. Angew. Chem. Int. Ed. 2019, 58, 10732–10736. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Kolobov, N.S.; Bukhtiyarov, A.V.; Gerasimov, E.Y.; Gubanov, A.I.; Kozlov, D.V. Deposition of Pd nanoparticles on TiO2 using a Pd(acac)2 precursor for photocatalytic oxidation of CO under UV-LED irradiation. App. Catal. B 2018, 235, 214–224. [Google Scholar] [CrossRef]
- Gong, J.; Imbault, A.; Farnood, R. The promoting role of bismuth for the enhanced photocatalytic oxidation of lignin on Pt-TiO2 under solar light illumination. App. Catal. B 2017, 204, 296–303. [Google Scholar] [CrossRef]
- Srisasiwimon, N.; Chuangchote, S.; Laosiripojana, N.; Sagawa, T. TiO2/lignin-based carbon composited photocatalysts for enhanced photocatalytic conversion of lignin to high value chemicals. ACS Sustain. Chem. Eng. 2018, 6, 13968–13976. [Google Scholar] [CrossRef]
- Li, S.H.; Liu, S.; Colmenares, J.C.; Xu, Y.J. A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chem. 2016, 18, 594–607. [Google Scholar] [CrossRef]
- Lee, K.C.; Chen, Y.L.; Wang, C.C.; Huang, J.H.; Cho, E.C. Refluxed esterification of fullerene-conjugated P25 TiO2 promotes free radical scavenging capacity and facilitates antiaging potentials in human cells. ACS Appl. Mater. Interfaces 2018, 11, 311–319. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, X.; Huang, C.; Chen, X.; Dai, W.; Fu, X. CO gas sensitivity and its oxidation over TiO2 modified by PANI under UV irradiation at room temperature. App. Catal. B 2017, 219, 379–390. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Lu, S.; Qiu, X.; Qian, Y.; Li, P. Encapsulating TiO2 in lignin-Based colloidal spheres for high sunscreen performance and weak photocatalytic activity. ACS Sustain. Chem. Eng. 2019, 7, 6234–6242. [Google Scholar] [CrossRef]
- Li, C.; Ma, Z.; Zhang, L.; Qian, R. Preparation of Ni/TiO2 nanoparticles and their catalytic performance on the thermal decomposition of ammonium perchlorate. Chin. J. Chem. 2009, 27, 1863–1867. [Google Scholar] [CrossRef]
- Ye, J.; He, J.; Wang, S.; Zhou, X.; Zhang, Y.; Liu, G.; Yang, Y. Nickel-loaded black TiO2 with inverse opal structure for photocatalytic reduction of CO2 under visible light. Sep. Purif. Technol. 2019, 220, 8–15. [Google Scholar] [CrossRef]
- Dvoranova, D.; Brezova, V.; Mazúr, M.; Malati, M.A. Investigations of metal-doped titanium dioxide photocatalysts. App. Catal. B 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Kim, S.; Chmely, S.C.; Nimlos, M.R.; Bomble, Y.J.; Foust, T.D.; Paton, R.S.; Beckham, G.T. Computational study of bond dissociation enthalpies for a large range of native and modified lignins. J. Phys. Chem. Lett. 2011, 2, 2846–2852. [Google Scholar] [CrossRef]
- Bosque, I.; Magallanes, G.; Rigoulet, M.; Karkas, M.D.; Stephenson, C.R. Redox catalysis facilitates lignin depolymerization. ACS Cent. Sci. 2017, 3, 621–628. [Google Scholar] [CrossRef]
- Zhang, J.W.; Lu, G.P.; Cai, C. Self-hydrogen transfer hydrogenolysis of β-O-4 linkages in lignin catalyzed by MIL-100 (Fe) supported Pd-Ni BMNPs. Green Chem. 2017, 19, 4538–4543. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Entry | Catalysts | Solvent | T. (°C)/t. (h) b | Con. (%) b | Yield (%) b | |
|---|---|---|---|---|---|---|
| 2a | 3a | |||||
| 1 | TiO2 | DMF | r.t./12 | 56 | 40 | 42 |
| 2 | 10 wt% Ni/TiO2 | DMF | r.t./12 | 76 | 66 | 64 |
| 3 | 20 wt% Ni/TiO2 | DMF | 180/12 | 100 | 82 | 80 |
| 4 | 30 wt% Ni/TiO2 | DMF | r.t./12 | 100 | 83 | 82 |
| Entry | Light Source | Solvent | T. (°C)/t. (h) b | Con. (%) b | Yield (%) b | |
|---|---|---|---|---|---|---|
| 2a | 3a | |||||
| 1 | darkness | DMF | r.t./12 | 0 | 0 | 0 |
| 2 | sunlight | DMF | r.t./12 | 0 | 0 | 0 |
| 3 | sunlight | DMF | 180/12 | 28 | 11 | 8 |
| 4 | 30W, UV | DMF | r.t./12 | 100 | 82 | 80 |
| 5 | 30W, blue LED | DMF | r.t./12 | 0 | 0 | 0 |
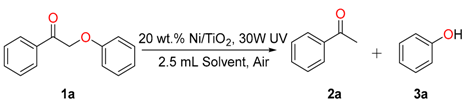
| Entry | Catalyst | Solvent | T. (oC)/t. (h) b | Con. (%) b | Yield (%) b | |
|---|---|---|---|---|---|---|
| 2a | 3a | |||||
| 1 | Ni/TiO2 | n-hexane | r.t./12 | 26 | 15 | 8 |
| 2 | Ni/TiO2 | cyclohexane | r.t./12 | 20 | 12 | 10 |
| 3 | Ni/TiO2 | iPrOH | r.t./12 | 100 | 88 | 82 |
| 4 | Ni/TiO2 | methanol | r.t./12 | 100 | 83 | 81 |
| 5 | Ni/TiO2 | DMF | r.t./12 | 100 | 82 | 80 |
| 6 | Ni/TiO2 | acetonitrile | r.t./12 | 60 | 32 | 30 |
| 7 | Ni/TiO2 | acetone | r.t./12 | 69 | 38 | 32 |
| 8 | Ni/TiO2 | H2O | r.t./12 | 15 | 6 | 5 |
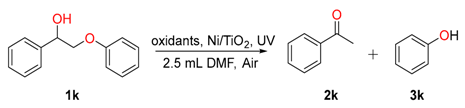
| Entry | Oxidant | Solvent | T. (°C)/t. (h) b | Con. (%) b | Yield (%) b | |
|---|---|---|---|---|---|---|
| 2k | 3k | |||||
| 1 | [4-Acetamido-TEMPO]BF4 | iPrOH | r.t./12 | 0 | 0 | 0 |
| 2 | H2O2 | iPrOH | r.t./12 | 0 | 0 | 0 |
| 3 | PCC | iPrOH | r.t./12 | 0 | 0 | 0 |
| 4 | PCC | iPrOH | r.t./12 | 100 | 0 | 0 |
| 5 c | PCC | iPrOH | r.t./12 | 100 | 66 | 61 |
| 6 d | PCC | iPrOH:DCM=1;1 | r.t./12 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liu, P.; Xia, H.; Zhou, M.; Zhao, J.; Sharma, B.K.; Jiang, J. Photocatalytic Cleavage of β-O-4 Ether Bonds in Lignin over Ni/TiO2. Molecules 2020, 25, 2109. https://doi.org/10.3390/molecules25092109
Chen C, Liu P, Xia H, Zhou M, Zhao J, Sharma BK, Jiang J. Photocatalytic Cleavage of β-O-4 Ether Bonds in Lignin over Ni/TiO2. Molecules. 2020; 25(9):2109. https://doi.org/10.3390/molecules25092109
Chicago/Turabian StyleChen, Changzhou, Peng Liu, Haihong Xia, Minghao Zhou, Jiaping Zhao, Brajendra K. Sharma, and Jianchun Jiang. 2020. "Photocatalytic Cleavage of β-O-4 Ether Bonds in Lignin over Ni/TiO2" Molecules 25, no. 9: 2109. https://doi.org/10.3390/molecules25092109
APA StyleChen, C., Liu, P., Xia, H., Zhou, M., Zhao, J., Sharma, B. K., & Jiang, J. (2020). Photocatalytic Cleavage of β-O-4 Ether Bonds in Lignin over Ni/TiO2. Molecules, 25(9), 2109. https://doi.org/10.3390/molecules25092109






