Hepatoprotective Effects of Standardized Extracts from an Ancient Italian Apple Variety (Mela Rosa dei Monti Sibillini) against Carbon Tetrachloride (CCl4)-Induced Hepatotoxicity in Rats
Abstract
1. Introduction
2. Results
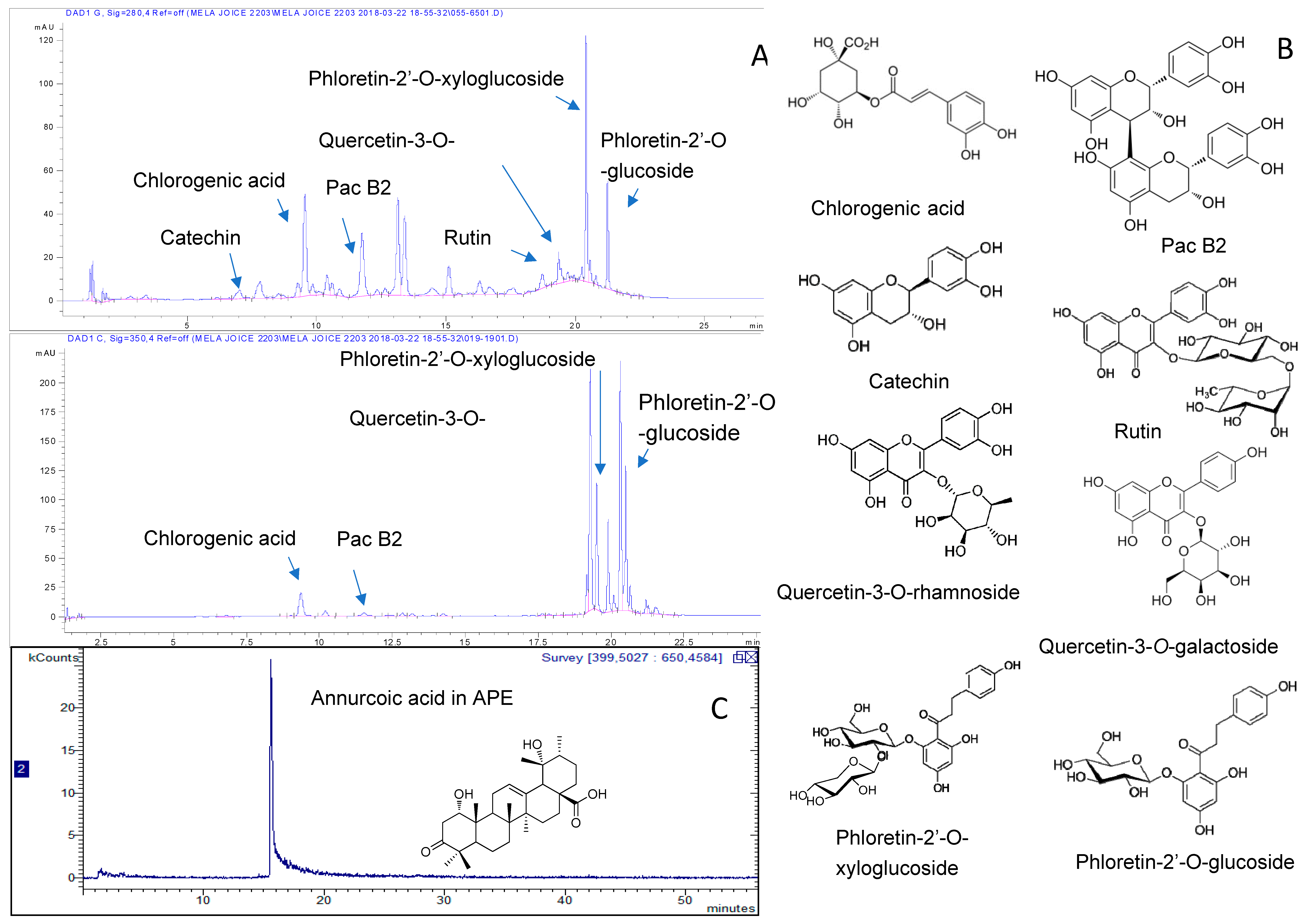
2.1. Chemical Composition of APE and APP
2.2. Effects of APP and APE on Serum Biochemical Parameters
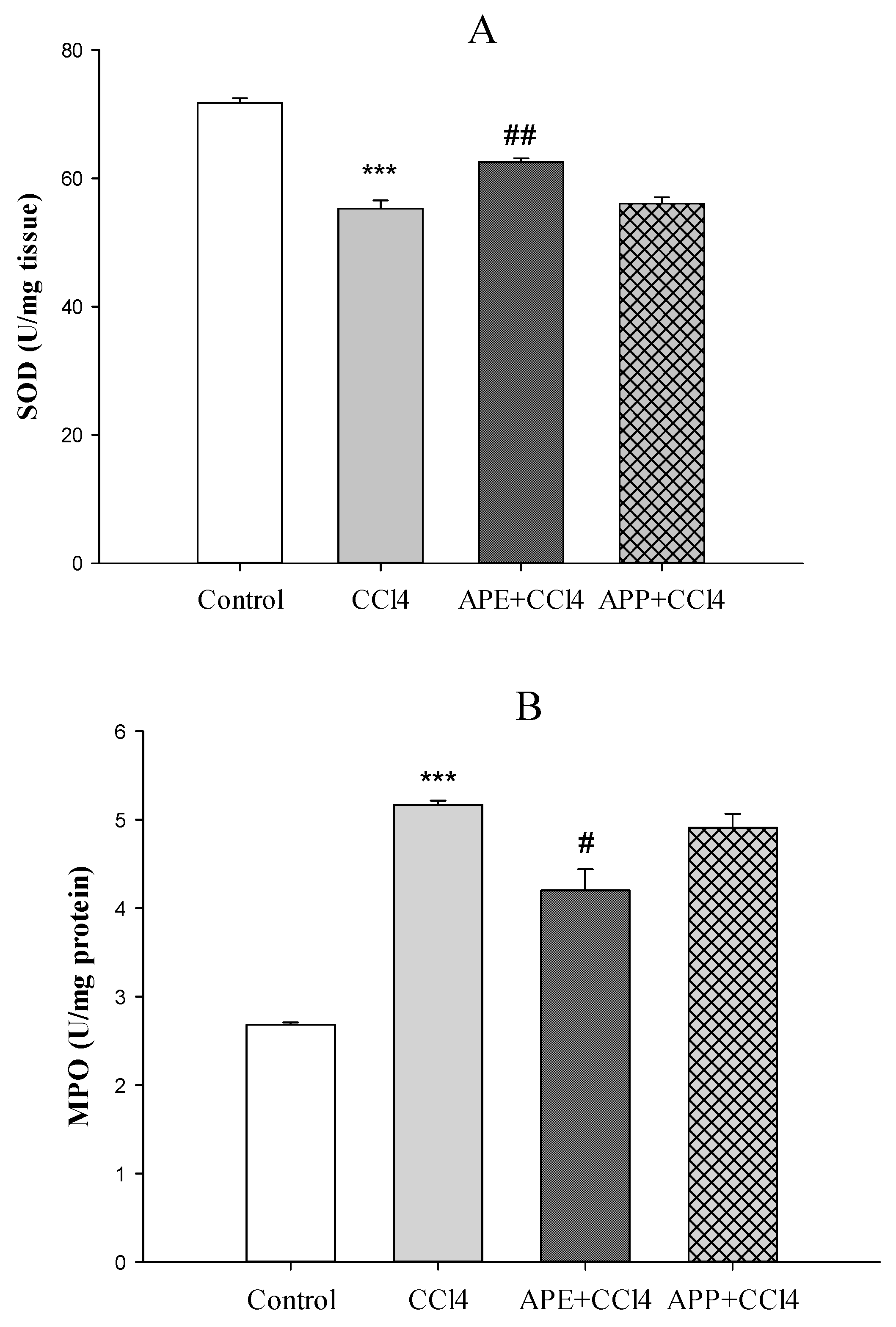
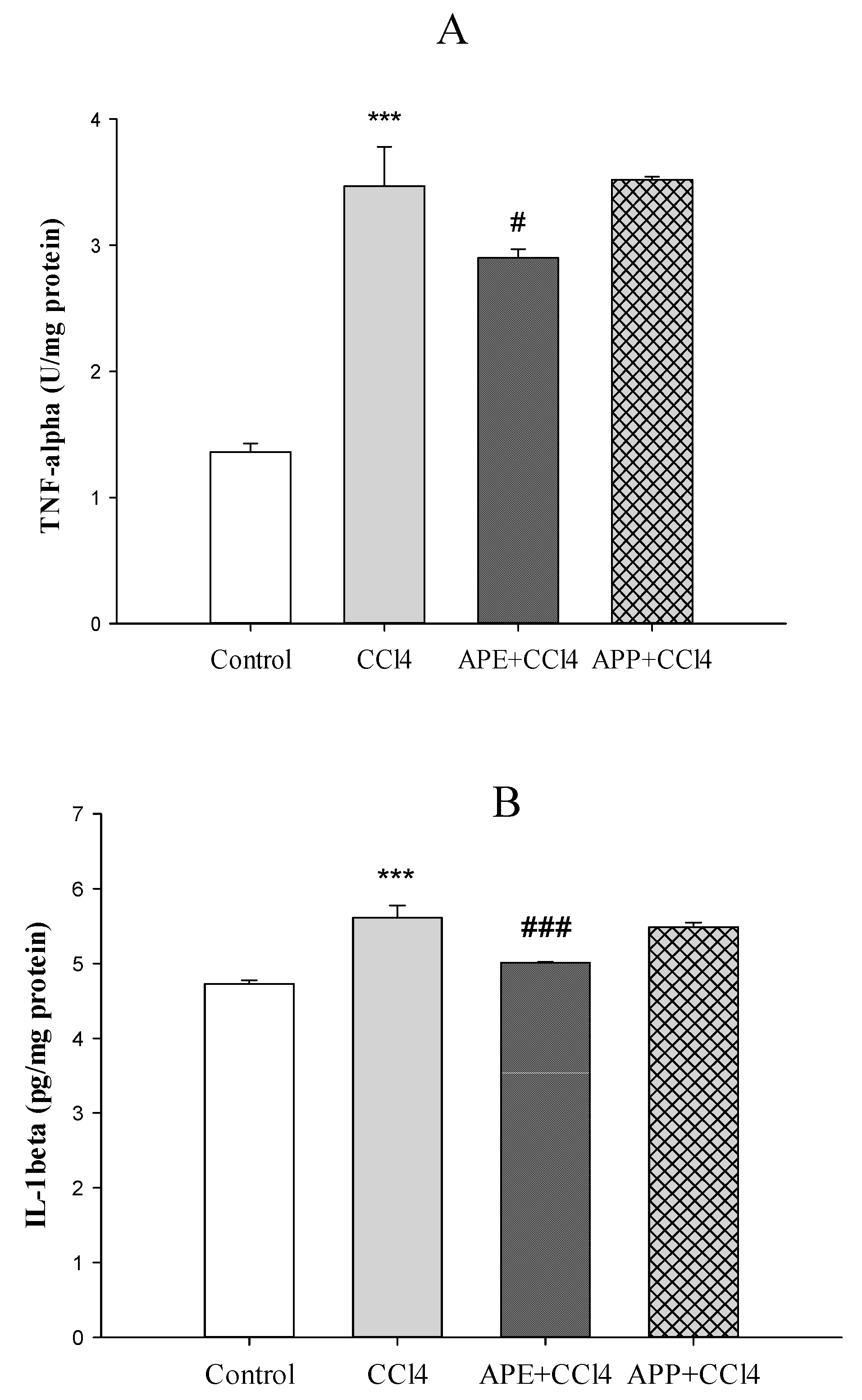
2.3. Effects of APP and APE on Liver Enzyme Assessments
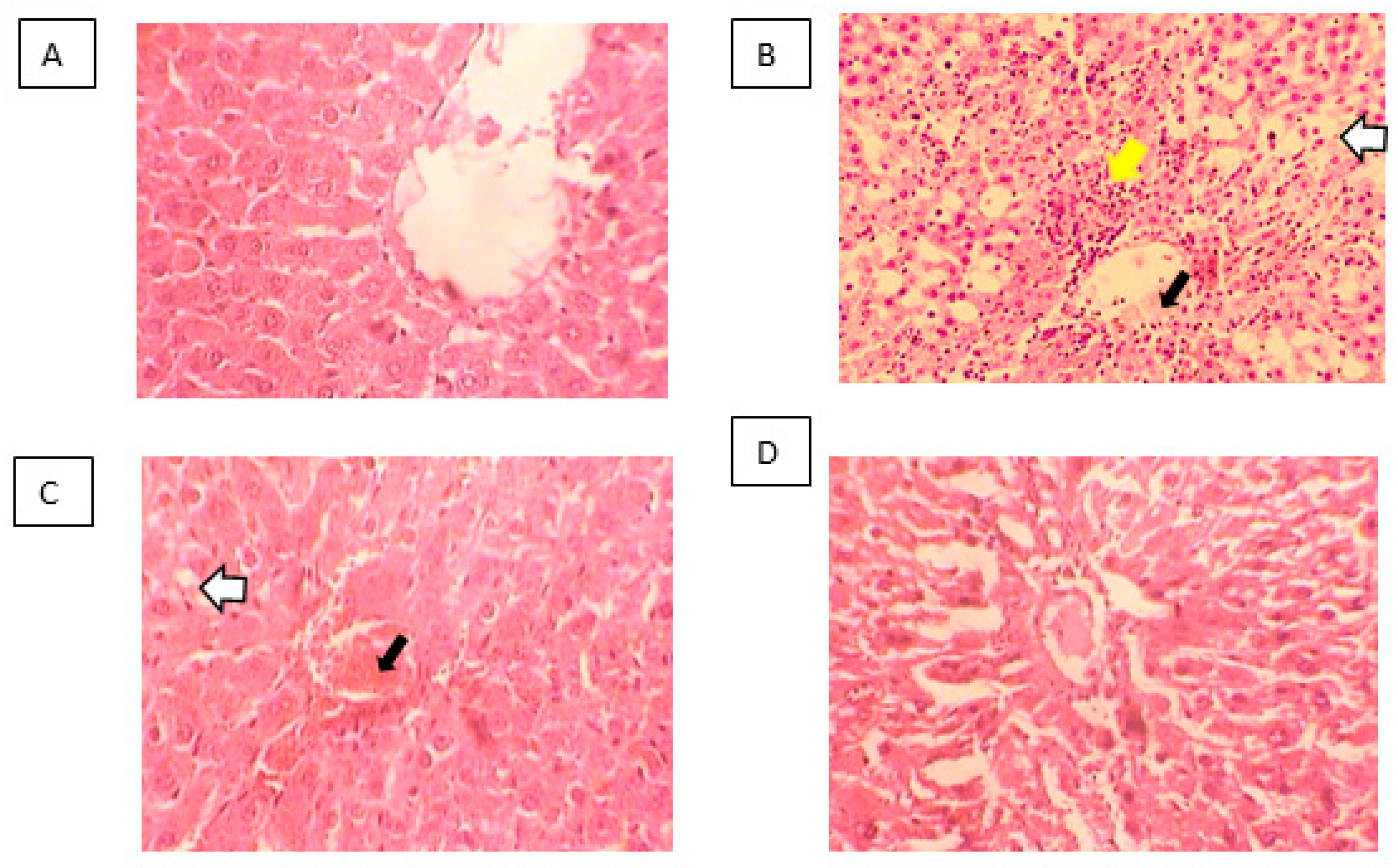
2.4. Histopathological Examination of the Liver
3. Discussion
4. Materials and Methods
4.1. Apple Sampling
4.2. Preparation of Hydroalcoholic Extracts
4.3. HPLC-DAD-MSn Analysis
4.4. Animals
4.5. Chemicals
4.6. Experimental Design
- Control group: received only normal saline every day
- CCl4 group: received CCl4 (25% CCl4-paraffin oil mixture, 2 mL/kg body weight, i.p (intraperitoneal); on the third day)
- APE group: received 30 mg/kg/day b.w (body weight). per os (oral administration) of APE and CCl4
- APP group: received 30 mg/kg/day b.w. per os of APP and CCl4
4.7. Biochemical Analysis
4.8. Evaluation of Antioxidant Markers
4.9. Histopathological Examination
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nkuimi Wandjou, J.G.; Sut, S.; Giuliani, C.; Fico, G.; Papa, F.; Ferraro, S.; Caprioli, G.; Maggi, F.; Dall’Acqua, S. Characterization of nutrients, polyphenols and volatile components of the ancient apple cultivar ‘Mela Rosa Dei Monti Sibillini’from Marche region, central Italy. Int. J. Food Sci. Nutr. 2019, 70, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Fathy, S.M.; Drees, E.A. Protective effects of Egyptian cloudy apple juice and apple peel extract on lipid peroxidation, antioxidant enzymes and inflammatory status in diabetic rat pancreas. BMC Complement. Med. Ther. 2016, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Bhaskar, A.; Kumar, A. Antihyperglycemic, antioxidant and hypolipidemic effect of Punica granatum L. flower extract in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, S1764–S1769. [Google Scholar] [CrossRef]
- Yao, W.Y.; Zhou, Y.F.; Qian, A.H.; Zhang, Y.P.; Qiao, M.M.; Zhai, Z.K.; Yuan, Y.Z.; Yang, S.L. Emodin has a protective effect in cases of severe acute pancreatitis via inhibition of nuclear factorkappaB activation resulting in antioxidation. Mol. Med. Rep. 2015, 11, 1416–1420. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Hemmati, S.; Shirooie, S.; Nabavi, S.M.; Talebzadeh Bonakdar, A.; Fayaznia, R.; Asgardoon, M.H.; Dehnavi, A.Z.; Ghafouri, M.; Nkuimi Wandjou, J.G.; et al. Protective effects of hydroalcoholic extracts from an ancient apple variety ’Mela Rosa dei Monti Sibillini’ against renal ischemia/reperfusion injury in rats. Food Funct. 2019, 10, 7544–7552. [Google Scholar] [CrossRef]
- Hong, I.H.; Lewis, K.; Iakova, P.; Jin, J.; Sullivan, E.; Jawanmardi, N.; Timchenko, L.; Timchenko, N. Age-associated change of C/EBP family proteins causes severe liver injury and acceleration of liver proliferation after CCl4 treatments. J. Biol. Chem. 2014, 289, 1106–1118. [Google Scholar] [CrossRef]
- Xu, G.; Han, X.; Yuan, G.; An, L.; Du, P. Screening for the protective effect target of deproteinized extract of calf blood and its mechanisms in mice with CCl4-induced acute liver injury. PLoS ONE 2017, 12, e0180899. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Shirooie, S.; Partoazar, A.; Nikoui, V.; Estakhri, M.R.A.; Bakhtiarian, A. Hepatoprotective effects of phosphatidylserine liposomes on carbon tetrachloride-induced hepatotoxicity in rats. J. Cell. Biochem. 2019, 120, 11853–11858. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.F.; Dai, M.G. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Bian, Y.; Wang, Z.; Chang, T.M. Hepatoprotective effects of Poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] on alcohol-damaged primary rat hepatocyte culture in vitro. Artif. Cells Nanomed. Biotechnol. 2017, 45, 46–50. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, K.Q.; Moura, F.A.; Dos Santos, J.M.; De Araújo, O.R.P.; de Farias Santos, J.C.; Goulart, M.O.F. Oxidative stress and inflammation in hepatic diseases: Therapeutic possibilities of N-acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Renal Inj. Prev. 2015, 4, 20–27. [Google Scholar]
- Lu, Y.; Chen, J.; Ren, D.; Yang, X.; Zhao, Y. Hepatoprotective effects of phloretin against CCl4-induced liver injury in mice. Food Agric. Immunol. 2017, 28, 211–222. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.Y.; Yu, L.M.; Liu, B.; Li, M.Y.; Zhu, R.Z. Phycocyanobilin accelerates liver regeneration and reduces mortality rate in carbon tetrachloride-induced liver injury mice. World J. Gastroenterol. 2015, 21, 5465–5472. [Google Scholar] [CrossRef]
- Miltonprabu, S.; Tomczyk, M.; Skalicka-Woźniak, K.; Rastrelli, L.; Daglia, M.; Nabavi, S.F.; Alavian, S.M.; Nabavi, S.M. Hepatoprotective effect of quercetin: From chemistry to medicine. Food Chem. Toxicol. 2017, 108, 365–374. [Google Scholar] [CrossRef]
- Vladimir-Knezevic, S.; Cvijanovic, O.; Blazekovic, B.; Kindl, M.; Stefan, M.B.; Domitrovic, R. Hepatoprotective effects of Micromeria croatica ethanolic extract against CCl4-induced liver injury in mice. BMC Complement. Altern. Med. 2015, 15, 233. [Google Scholar] [CrossRef]
- Fahmy, M.A.; Diab, K.A.; Abdel-Samie, N.S.; Omara, E.A.; Hassan, Z.M. Carbon tetrachloride induced hepato/renal toxicity in experimental mice: Antioxidant potential of Egyptian Salvia officinalis L essential oil. Environ. Sci. Pollut Res. 2018, 25, 27858–27876. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Q.L.; Song, Y.N.; Sun, Y.; Wei, B.; Li, X.Y.; Hu, Y.Y.; Liu, P.; Su, S.B. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J. Toxicol. Sci. 2016, 41, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, H.; Ito, H.; Ando, T.; Arioka, Y.; Kanbe, A.; Ando, K.; Ishikawa, T.; Saito, K.; Hara, A.; Moriwaki, H.; et al. The Deficiency of Indoleamine 2,3-Dioxygenase Aggravates the CCl4-Induced Liver Fibrosis in Mice. PLoS ONE 2016, 211, e0162183. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Imai, Y.; Matsura, T.; Kitagawa, A.; Yamada, K. Preventive effect of neutropenia on carbon tetrachloride-induced hepatotoxicity in rats. J. Appl. Toxicol. 2006, 26, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Al Mamun, M.A.; Faruk, M.; Ul Islam, M.T.; Rahman, M.M.; Alam, M.N.; Rahman, A.F.M.T.; Reza, H.M.; Alam, M.A. Astaxanthin Ameliorates Hepatic Damage and Oxidative Stress in Carbon Tetrachloride-administered Rats. Pharmacognosy Res. 2017, 9, S84–S91. [Google Scholar] [PubMed]
- Liu, Y.; Wen, P.H.; Zhang, X.X.; Dai, Y.; He, Q. Breviscapine ameliorates CCl4induced liver injury in mice through inhibiting inflammatory apoptotic response and ROS generation. Int. J. Mol. Med. 2018, 42, 755–768. [Google Scholar]
- Zhong, W.; Gao, L.; Zhou, Z.; Lin, H.; Chen, C.; Huang, P.; Huang, W.; Zhou, C.; Huang, S.; Nie, L.; et al. Indoleamine 2,3-dioxygenase 1 deficiency attenuates CCl4-induced fibrosis through Th17 cells down-regulation and tryptophan 2,3-dioxygenase compensation. Oncotarget 2017, 8, 40486–40500. [Google Scholar] [CrossRef]
- Singab, A.N.; Youssef, D.T.; Noaman, E.; Kotb, S. Hepatoprotective effect of flavonol glycosides rich fraction from Egyptian Vicia calcarata Desf. against CCl4-induced liver damage in rats. Arch. Pharm. Res. 2005, 28, 791–798. [Google Scholar] [CrossRef]
- Hu, L.; Li, L.; Xu, D.; Xia, X.; Pi, R.; Xu, D.; Song, E.; Song, Y. Protective effects of neohesperidin dihydrochalcone against carbon tetrachloride-induced oxidative damage in vivo and in vitro. Chem. Biol. Interact. 2014, 213, 51–59. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Bakr, A.G.; Osman, A.T. Protective effects of phloridzin against methotrexate-induced liver toxicity in rats. Biomed. Pharmacother. 2017, 95, 529–535. [Google Scholar] [CrossRef]
- Ma, J.Q.; Ding, J.; Zhang, L.; Liu, C.M. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ. Toxicol. Pharmacol. 2014, 37, 975–983. [Google Scholar] [CrossRef]
- Padua, T.A.; de Abreu, B.S.; Costa, T.E.; Nakamura, M.J.; Valente, L.M.; Henriques, M.; Siani, A.C.; Rosas, E.C. Anti-inflammatory effects of methyl ursolate obtained from a chemically derived crude extract of apple peels: Potential use in rheumatoid arthritis. Arch. Pharm. Res. 2014, 37, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.C.; Furtos, A.; Dudonne, S.; Montoudis, A.; Garofalo, C.; Desjardins, Y.; Delvin, E.; Levy, E. Apple peel polyphenols and their beneficial actions on oxidative stress and inflammation. PLoS ONE 2013, 8, e53725. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of apple polyphenols on inflammatory gene expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Poloniato, G.; Malagoli, M.; Dall’Acqua, S. Fragmentation of the main triterpene acids of apple by LC-APCI-MSn. J. Mass Spectr. 2018, 53, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Nkuimi Wandjou, J.G.; Mevi, S.; Sagratini, G.; Vittori, S.; Dall’Acqua, S.; Caprioli, G.; Lupidi, G.; Mombelli, G.; Arpini, S.; Allegrini, P.; et al. Antioxidant and Enzyme Inhibitory Properties of the Polyphenolic-Rich Extract from an Ancient Apple Variety of Central Italy (Mela Rosa dei Monti Sibillini). Plants 2020, 9, 9. [Google Scholar] [CrossRef]
Sample Availability: Samples of the apple extracts are available from the authors. |

| Examinations | Urea | Creatinine | AST | ALT | ALP |
|---|---|---|---|---|---|
| Control | 50.28 ± 1.85 | 0.63 ± 0.07 | 125.5 ± 26.8 | 48 ± 6.46 | 316.1 ± 24.45 |
| CCl4 | 53.10 ± 0.90 | 0.71 ± 0.10 | 633.0 ± 22.8 ### | 632.2 ± 53.59 ## | 599 ± 55.96 # |
| APE + CCl4 | 46.00 ± 7.00 | 0.75 ± 0.03 | 230.5 ± 33.5 ** | 181 ± 69 ** | 361.5 ± 41.5 * |
| APP + CCl4 | 37 ± 2.00 | 0.63 ± 0.07 | 151.5 ± 3.50 ** | 110.5 ± 3.50 ** | 375 ± 25 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi-Manesh, H.; Dehpour, A.R.; Ansari-Nasab, S.; Hemmati, S.; Sadeghi, M.A.; Shahraki, R.H.; Shirooie, S.; Nabavi, S.M.; Nkuimi Wandjou, J.G.; Sut, S.; et al. Hepatoprotective Effects of Standardized Extracts from an Ancient Italian Apple Variety (Mela Rosa dei Monti Sibillini) against Carbon Tetrachloride (CCl4)-Induced Hepatotoxicity in Rats. Molecules 2020, 25, 1816. https://doi.org/10.3390/molecules25081816
Yousefi-Manesh H, Dehpour AR, Ansari-Nasab S, Hemmati S, Sadeghi MA, Shahraki RH, Shirooie S, Nabavi SM, Nkuimi Wandjou JG, Sut S, et al. Hepatoprotective Effects of Standardized Extracts from an Ancient Italian Apple Variety (Mela Rosa dei Monti Sibillini) against Carbon Tetrachloride (CCl4)-Induced Hepatotoxicity in Rats. Molecules. 2020; 25(8):1816. https://doi.org/10.3390/molecules25081816
Chicago/Turabian StyleYousefi-Manesh, Hasan, Ahmad Reza Dehpour, Sedighe Ansari-Nasab, Sara Hemmati, Mohammad Amin Sadeghi, Reza Hashemi Shahraki, Samira Shirooie, Seyed Mohammad Nabavi, Joice G. Nkuimi Wandjou, Stefania Sut, and et al. 2020. "Hepatoprotective Effects of Standardized Extracts from an Ancient Italian Apple Variety (Mela Rosa dei Monti Sibillini) against Carbon Tetrachloride (CCl4)-Induced Hepatotoxicity in Rats" Molecules 25, no. 8: 1816. https://doi.org/10.3390/molecules25081816
APA StyleYousefi-Manesh, H., Dehpour, A. R., Ansari-Nasab, S., Hemmati, S., Sadeghi, M. A., Shahraki, R. H., Shirooie, S., Nabavi, S. M., Nkuimi Wandjou, J. G., Sut, S., Caprioli, G., Dall’Acqua, S., & Maggi, F. (2020). Hepatoprotective Effects of Standardized Extracts from an Ancient Italian Apple Variety (Mela Rosa dei Monti Sibillini) against Carbon Tetrachloride (CCl4)-Induced Hepatotoxicity in Rats. Molecules, 25(8), 1816. https://doi.org/10.3390/molecules25081816










