Physicochemical and Digestion Properties of Potato Starch Were Modified by Complexing with Grape Seed Proanthocyanidins
Abstract
1. Introduction
2. Results
2.1. Binding Ability of GSP with Potato Starch
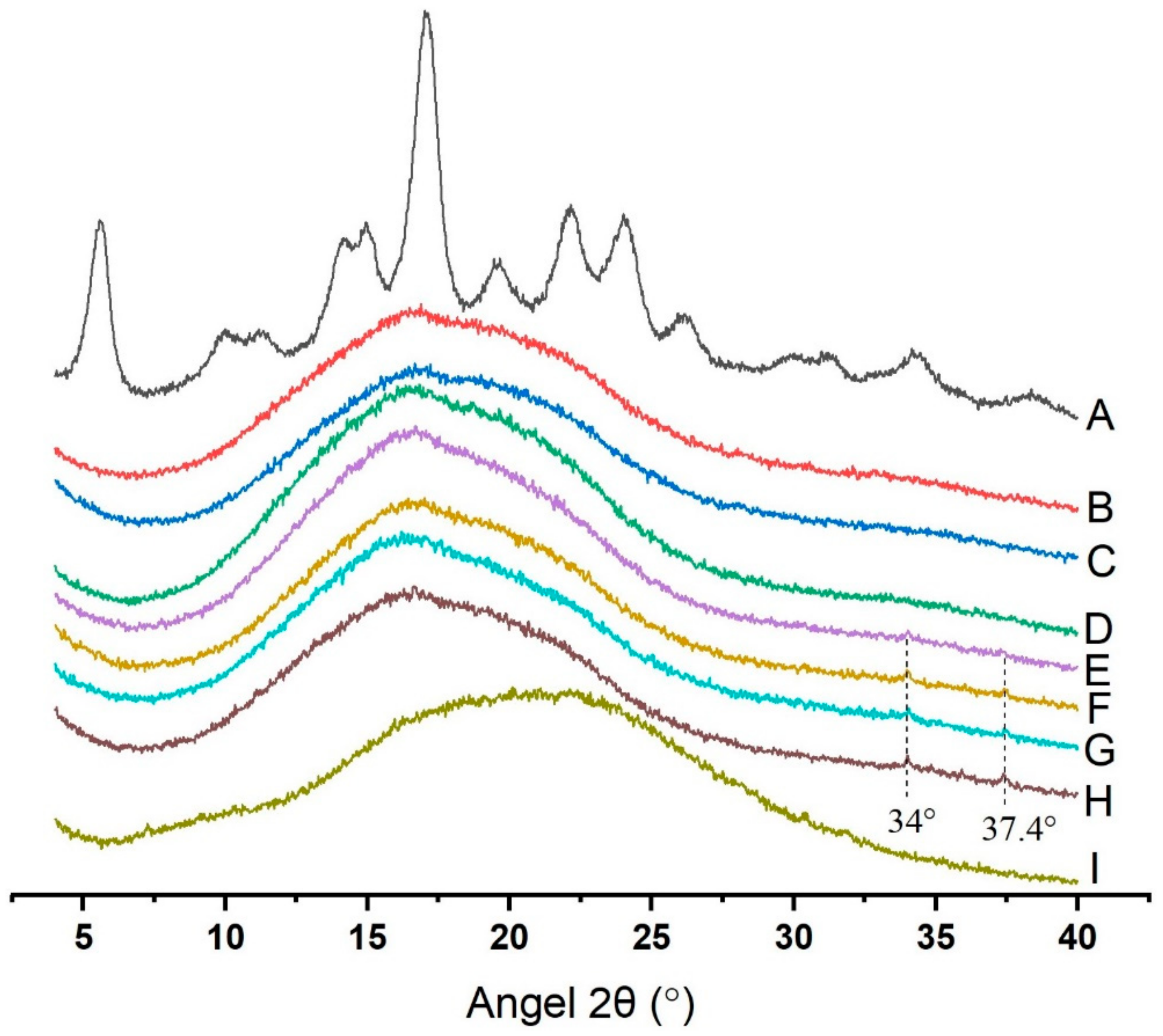
2.2. Semi-Crystalline Character of Potato Starch with GSP
2.3. Pasting Properties of GSP–Potato Starch Complexes
2.4. Thermal Properties of GSP–Potato Starch Complexes
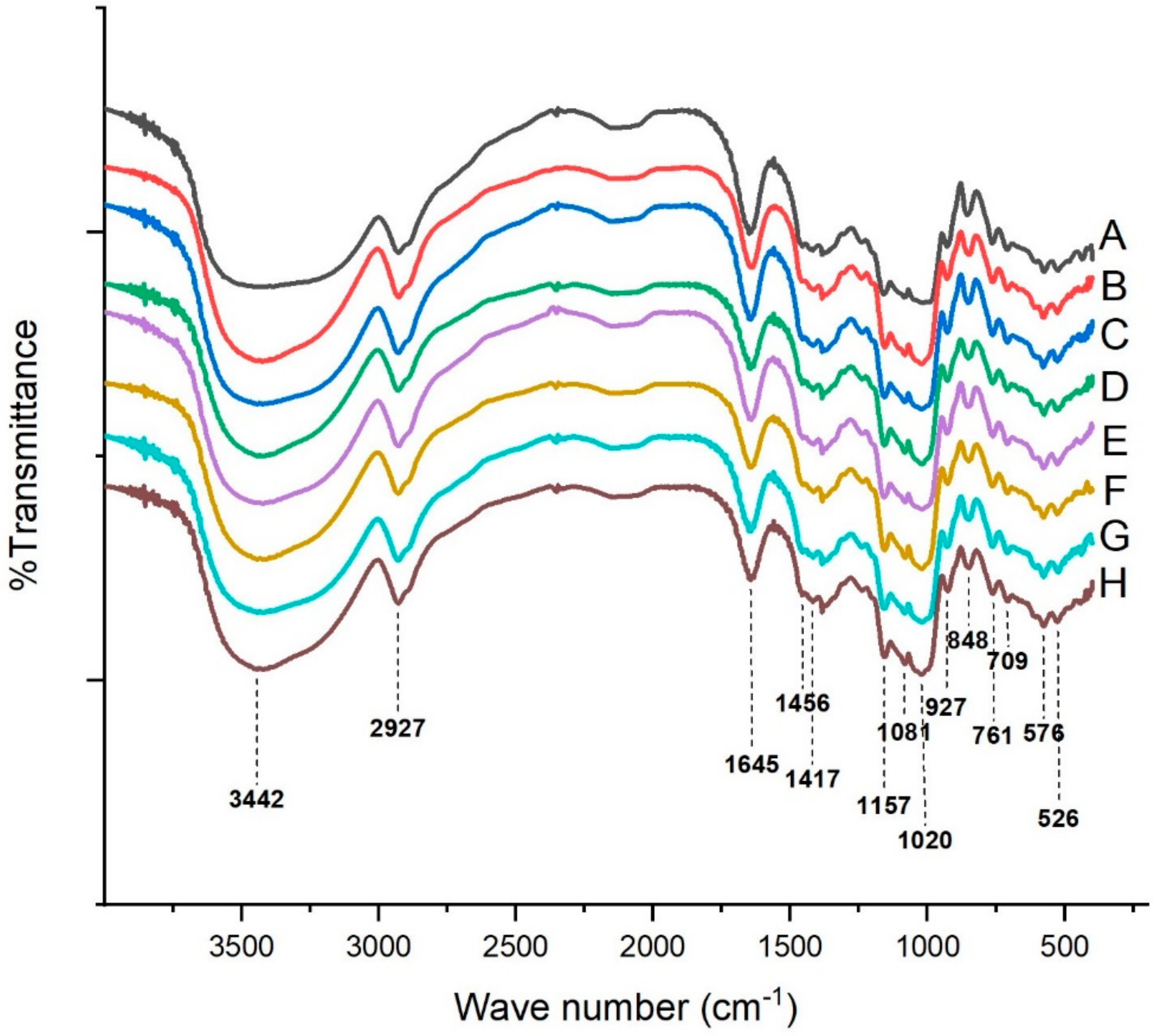
2.5. FT-IR Analysis
2.6. Texture Profile of the Gel
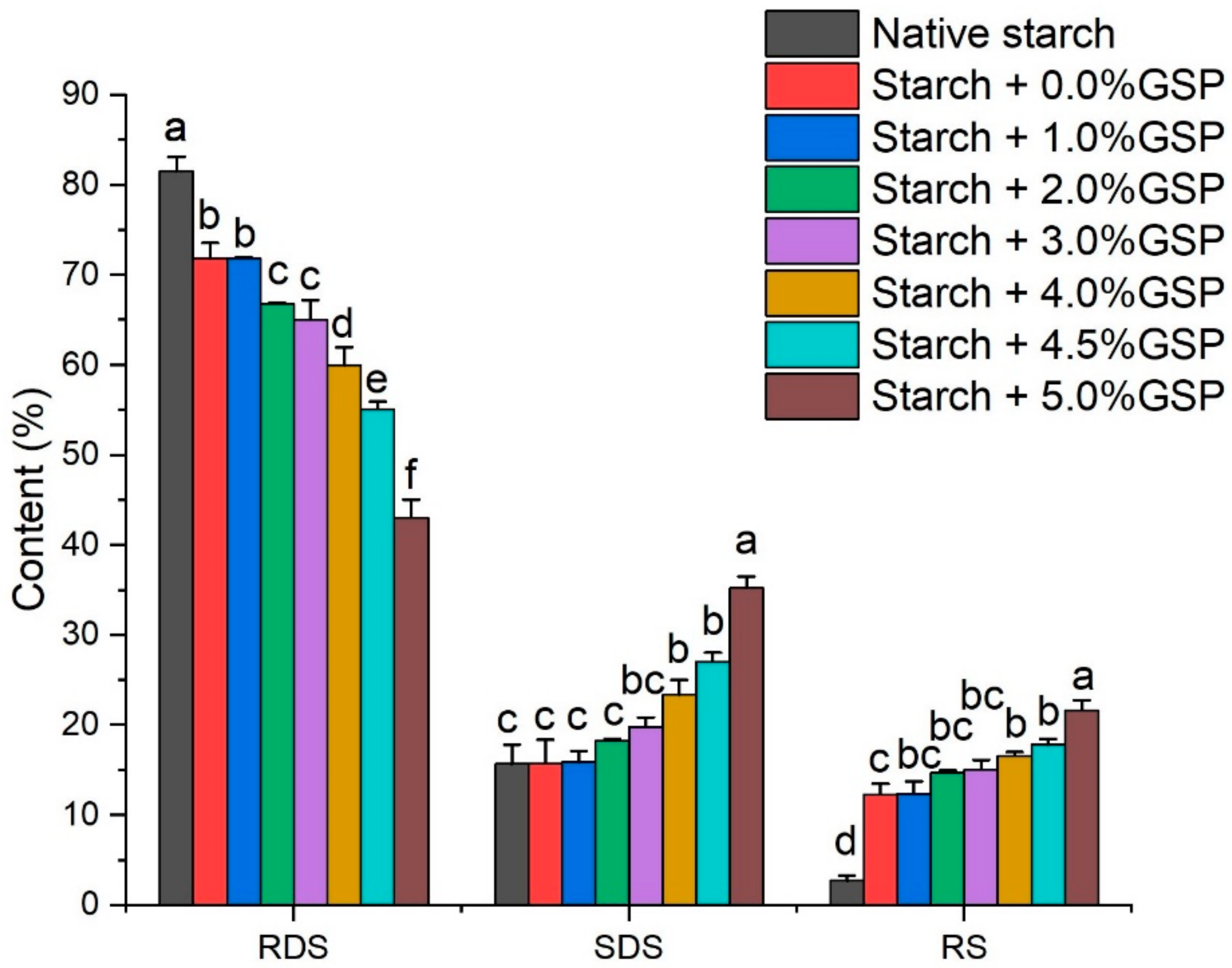
2.7. Digestibility of Potato Starch Complexed with GSP
3. Materials and Methods
3.1. Materials
3.2. Preparation of Grape Seed Proanthocyanidins-Potato Starch Complexes
3.3. Binding Ability of GSP with Potato Starch
3.4. X-ray Diffractometer Analysis
3.5. Pasting Properties Analysis
3.6. Thermal Properties Analysis
3.7. Fourier Transform Infrared Analysis
3.8. Gel Texture Analysis
3.9. In Vitro Digestion
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tian, J.; Chen, S.; Chen, J.; Liu, D.; Ye, X. Cooking Methods Altered the Microstructure and Digestibility of the Potato. Starch 2018, 70, 1700241. [Google Scholar] [CrossRef]
- Food and Agricultural Organization. Available online: http://www.fao.org/faostat/en/#data (accessed on 11 December 2019).
- Ek, K.L.; Brand-Miller, J.; Copeland, L. Glycemic effect of potatoes. Food Chem. 2012, 133, 1230–1240. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Oladele, A.K.; Duodu, K.G.; Emmambux, N.M. Pasting, flow, thermal and molecular properties of maize starch modified with crude phenolic extracts from grape pomace and sorghum bran under alkaline conditions. Food Chem. 2019, 297, 124879. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crop. Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, J.; Tian, J.; Chen, S.; Ye, X.; Hu, Y.; Chen, J. Inhibitory mechanism of novel allosteric inhibitor, Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins against α-glucosidase. J. Funct. Foods 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Amoako, D.B.; Awika, J.M. Resistant starch formation through intrahelical V-complexes between polymeric proanthocyanidins and amylose. Food Chem. 2019, 285, 326–333. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, M.; Lin, S.; Yang, X.; Fu, C.; Huang, D. Binding Interaction of Selected Proanthocyanidins Possessing Hypoglycemic Activity with Common Food Raw Materials. Food Sci. 2017, 38, 156–163. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2019, 1–15. [Google Scholar] [CrossRef]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins—A review of efficacy and mechanisms. LWT 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Yi, J.; Liang, J.; Liu, Y.; Zhang, L.M. Preparation, characterization, and properties of amylose-ibuprofen inclusion complexes. Starch 2013, 65, 593–602. [Google Scholar] [CrossRef]
- Van Hung, P.; Phat, N.H.; Phi, N.T.L. Physicochemical properties and antioxidant capacity of debranched starch–ferulic acid complexes. Starch 2013, 65, 382–389. [Google Scholar] [CrossRef]
- Star, A.; Steuerman, D.W.; Heath, J.R.; Stoddart, J.F. Starched Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 2508–2512. [Google Scholar] [CrossRef]
- Obiro, W.C.; Sinha Ray, S.; Emmambux, M.N. V-amylose Structural Characteristics, Methods of Preparation, Significance, and Potential Applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef]
- Tian, J.; Ogawa, Y.; Shi, J.; Chen, S.; Zhang, H.; Liu, D.; Ye, X. The microstructure of starchy food modulates its digestibility. Crit. Rev. Food Sci. 2019, 59, 3117–3128. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Feng, T.; Zeng, X.; Chen, L.; Li, L. Improvement in Nutritional Attributes of Rice Starch with Dodecyl Gallate Complexation: A Molecular Dynamic Simulation and in Vitro Study. J. Agric. Food Chem. 2018, 66, 9282–9290. [Google Scholar] [CrossRef]
- Tian, J.; Chen, S.; Zhang, H.; Fang, H.; Sun, Y.; Liu, D.; Linhart, R.J.; Ye, X. Existing cell wall fragments modify the thermal properties and hydrolysis of potato starch. Food Hydrocolloids 2018, 85, 229–232. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Peng, S.; Zhang, G. Effect of Green Tea Catechins on the Postprandial Glycemic Response to Starches Differing in Amylose Content. J. Agric. Food Chem. 2011, 59, 4582–4588. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Q.; Hu, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Physicochemical properties, structure and in vitro digestibility on complex of starch with lotus (Nelumbo nucifera Gaertn.) leaf flavonoids. Food Hydrocolloids 2018, 81, 191–199. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, S.; Wang, S. Annealing improves paste viscosity and stability of starch. Food Hydrocolloids 2017, 62, 203–211. [Google Scholar] [CrossRef]
- Stute, R. Hydrothermal Modification of Starches: The Difference between Annealing and Heat/Moisture -Treatment. Starch 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between Amylose and Tea Polyphenols Modulates the Postprandial Glycemic Response to High-Amylose Maize Starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, X.; Jin, Z.; Tian, Y.; Bai, Y.; Xie, Z. Retrogradation properties of rice starch gelatinized by heat and high hydrostatic pressure (HHP). J. Food Eng. 2011, 106, 262–266. [Google Scholar] [CrossRef]
- Cardoso, M.B.; Putaux, J.; Samios, D.; Da Silveira, N.P. Influence of alkali concentration on the deproteinization and/or gelatinization of rice starch. Carbohyd. Polym. 2007, 70, 160–165. [Google Scholar] [CrossRef]
- Zhou, X.; Baik, B.; Wang, R.; Lim, S. Retrogradation of waxy and normal corn starch gels by temperature cycling. J. Cereal Sci. 2010, 51, 57–65. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Physico-chemical characterization of starch ferulates of different degrees of substitution. Food Chem. 2007, 105, 579–589. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33. [Google Scholar] [CrossRef]
- Martínez, P.; Peña, F.; Bello-Pérez, L.A.; Núñez-Santiago, C.; Yee-Madeira, H.; Velezmoro, C. Physicochemical, functional and morphological characterization of starches isolated from three native potatoes of the Andean region. Food Chem. 2019, 2, 100030. [Google Scholar] [CrossRef]
- Dupuis, J.H.; Liu, Q. Potato Starch: A Review of Physicochemical, Functional and Nutritional Properties. Am. J. Potato Res. 2019, 96, 127–138. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape Seed and Tea Extracts and Catechin 3-Gallates Are Potent Inhibitors of alpha-Amylase and alpha-Glucosidase Activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Awika, J.; Rooney, L.W. Effect of molecular weight profile of sorghum proanthocyanidins on resistant starch formation. J. Sci. Food Agric. 2014, 94, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Kong, X.; Yang, W.; Yin, X.; Xu, E.; Chen, S.; Liu, D.; Ye, X. Physicochemical and digestibility characterisation of maize starch–caffeic acid complexes. LWT 2020, 121, 108857. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Tian, J.; Cai, Y.; Qin, W.; Matsushita, Y.; Ye, X.; Ogawa, Y. Parboiling reduced the crystallinity and in vitro digestibility of non-waxy short grain rice. Food Chem. 2018, 257, 23–28. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the Relationship Between Water-satured State and Crystallinity by the Diffraction Method for Moistened Potato Starch. Starch 1983, 35, 407–410. [Google Scholar] [CrossRef]
Sample Availability: Samples of the proanthocyanidins and all complexes are not available from the authors. |

| Samples | Binding Amount (mg/g) | Loading Efficiency (%) |
|---|---|---|
| Starch + 1.0% GSP | 7.36 ± 0.05 | 73.41 ± 0.63 |
| Starch + 2.0% GSP | 13.86 ± 0.06 | 69.19 ± 0.36 |
| Starch + 3.0% GSP | 20.57 ± 0.06 | 68.49 ± 0.13 |
| Starch + 4.0% GSP | 27.98 ± 0.04 | 69.91 ± 0.08 |
| Starch + 4.5% GSP | 32.20 ± 0.04 | 71.51. ± 0.05 |
| Starch + 5.0% GSP | 35.72 ± 0.02 | 71.40 ± 0.05 |
| Samples 1 | Final Viscosity (cP) | Pasting Temperature (°C) |
|---|---|---|
| Native starch | 1406.5 ± 24.75 f | 65.08 ± 0.04 f |
| Starch + 0.0% GSP | 4119.0 ± 49.50 a | 91.90 ± 0.64 e |
| Starch + 1.0% GSP | 3823.5 ± 105.36 b | 93.10 ± 0.57 c,d |
| Starch + 2.0% GSP | 3604.5 ± 71.42 b,c | 92.70 ± 0.71 d,e |
| Starch + 3.0% GSP | 3487.5 ± 84.15 c | 93.08 ± 0.53 c,d |
| Starch + 4.0% GSP | 3356.0 ± 155.56 c,d | 93.90 ± 0.57 b,c |
| Starch + 4.5% GSP | 3149.5 ± 183.14 d | 94.50 ± 0.28 a,b |
| Starch + 5.0% GSP | 2623.0 ± 93.34 e | 95.10 ± 0.03 a |
| Samples 1 | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|
| Native starch | 62.7 ± 0.7 a | 65.5 ± 0.3 a | 68.4 ± 1.0 a | 13.7 ± 0.3 a |
| Starch + 0.0% GSP | 52.0 ± 0.2 b | 58.6 ± 0.4 b | 64.6 ± 0.8 b | 0.9 ± 0.2 d |
| Starch + 1.0% GSP | 53.5 ± 0.9 b | 59.5 ± 0.7 b | 65.4 ± 1.5 b | 1.2 ± 0.6 b,c,d |
| Starch + 2.0% GSP | 53.6 ± 2.1 b | 60.1 ± 1.6 b | 65.2 ± 0.4 b | 0.9 ± 0.4 d |
| Starch + 3.0% GSP | 53.4 ± 2.8b | 59.8 ± 0.2 b | 65.2 ± 1.3 b | 1.1 ± 0.2 c,d |
| Starch + 4.0% GSP | 54.8 ± 0.8 b | 59.4 ± 0.3 b | 65.5 ± 0.3 b | 1.3 ± 0.1 b,c,d |
| Starch + 4.5% GSP | 53.0 ± 1.4 b | 59.3 ± 0.8 b | 66.0 ± 0.9 b | 1.5 ± 0.1 b |
| Starch + 5.0% GSP | 53.2 ± 0.8 b | 59.7 ± 1.1 b | 66.1 ± 1.0 b | 1.5 ± 0.2 b,c |
| Samples 1 | Hardness (g) | Springiness (mm) | Cohesiveness | Chewiness (g) | Resilience |
|---|---|---|---|---|---|
| Native starch | 1290.007 ± 37.875 b | 0.874 ± 0.031 a | 0.83 ± 0.014 a | 936.999 ± 65.191 a | 0.426 ± 0.013 b |
| Starch + 0.0% GSP | 1392.628 ± 42.612 a | 0.909 ± 0.023 a,b | 0.811 ± 0.02 a | 1029.505 ± 82.358 a | 0.532 ± 0.023 a |
| Starch + 1.0% GSP | 1153.262 ± 35.037 c | 0.929 ± 0.02 a,b | 0.677 ± 0.022 b | 725.317 ± 33.782 b | 0.47 ± 0.013 c |
| Starch + 2.0% GSP | 947.100 ± 31.757 d | 0.862 ± 0.022 b | 0.601 ± 0.029 c | 491.446 ± 45.062 c | 0.32 ± 0.017 d |
| Starch + 3.0% GSP | 852.324 ± 26.475 e | 0.724 ± 0.021 c | 0.569 ± 0.018 c | 350.977 ± 10.008 d | 0.277 ± 0.021 e |
| Starch + 4.0% GSP | 816.614 ± 31.379 e,f | 0.644 ± 0.023 d | 0.551 ± 0.03 c | 289.698 ± 24.558 d,e | 0.201 ± 0.011 f |
| Starch + 4.5% GSP | 747.402 ± 13.089 g,h | 0.614 ± 0.011 d,e | 0.425 ± 0.021 d | 194.882 ± 8.587 e,f | 0.205 ± 0.017 f |
| Starch + 5.0% GSP | 719.548 ± 4.184 h | 0.580 ± 0.027 e | 0.394 ± 0.015 d | 164.122 ± 3.529 f | 0.185 ± 0.016 f |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Tian, J.; Fang, H.; Zhang, H.; Kong, X.; Wu, D.; Zheng, J.; Liu, D.; Ye, X.; Chen, S. Physicochemical and Digestion Properties of Potato Starch Were Modified by Complexing with Grape Seed Proanthocyanidins. Molecules 2020, 25, 1123. https://doi.org/10.3390/molecules25051123
Zhang Z, Tian J, Fang H, Zhang H, Kong X, Wu D, Zheng J, Liu D, Ye X, Chen S. Physicochemical and Digestion Properties of Potato Starch Were Modified by Complexing with Grape Seed Proanthocyanidins. Molecules. 2020; 25(5):1123. https://doi.org/10.3390/molecules25051123
Chicago/Turabian StyleZhang, Zirui, Jinhu Tian, Haitian Fang, Huiling Zhang, Xiangli Kong, Dongmei Wu, Jiaqi Zheng, Donghong Liu, Xingqian Ye, and Shiguo Chen. 2020. "Physicochemical and Digestion Properties of Potato Starch Were Modified by Complexing with Grape Seed Proanthocyanidins" Molecules 25, no. 5: 1123. https://doi.org/10.3390/molecules25051123
APA StyleZhang, Z., Tian, J., Fang, H., Zhang, H., Kong, X., Wu, D., Zheng, J., Liu, D., Ye, X., & Chen, S. (2020). Physicochemical and Digestion Properties of Potato Starch Were Modified by Complexing with Grape Seed Proanthocyanidins. Molecules, 25(5), 1123. https://doi.org/10.3390/molecules25051123







