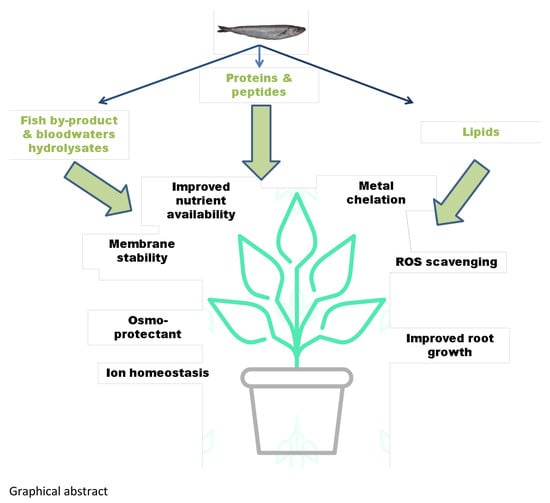
Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA
Abstract
1. Introduction
2. Protein Hydrolysates
2.1. Fish Hydrolysates as Plant Biostimulants
2.2. Individual Amino Acids
3. Humic Substances
4. Seaweed Extracts
5. Microorganisms
6. Production, Composition and Quality Control of Commercial Biostimulants
7. Plant Biostimulants’ Mode of Action
8. Regulation of Plant Biostimulants in Europe and USA
9. Conclusions
Funding
Conflicts of Interest
References
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, Y.; Jia, F.; Jin, H. Characterization of casein hydrolysates derived from enzymatic hydrolysis. Chem. Cent. J. 2013, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.; Mata, P. Evaluation of a Biostimulant (Pepton) Based in Enzymatic Hydrolyzed Animal Protein in Comparison to Seaweed Extracts on Root Development, Vegetative Growth, Flowering, and Yield of Gold Cherry Tomatoes Grown under Low Stress Ambient Field Conditions. Front. Plant Sci. 2018, 8, 2261. [Google Scholar] [CrossRef]
- Bradshaw, T.L.; Berkett, L.P.; Griffith, M.C.; Kingsley-Richards, S.L.; Moran, R.E.; Darby, H.M.; Garcia, M.E.; Parsons, R.L. Assessment of kelp extract biostimulants on tree growth, yield, and fruit quality in a certified organic apple orchard. Acta Hortic. 2013, 1001, 191–198. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Leskovar, D.I.; Colla, G.; Rouphael, Y. Watermelon and melon fruit quality: The genotypic and agro-environmental factors implicated. Sci. Hortic. 2018, 234, 393–408. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Tejada, M.; Rodríguez-Morgado, B.; Gómez, I.; Franco-Andreu, L.; Benítez, C.; Parrado, J. Use of biofertilizers obtained from sewage sludges on maize yield. Eur. J. Agron. 2016, 78, 13–19. [Google Scholar] [CrossRef]
- Mladenova, Y.I.; Maini, P.; Mallegni, C.; Goltsev, V.; Vladova, R.; Vinarova, K.; Rotcheva, S. Siapton—An amino-acid-based biostimulant reducing osmostress metabolic changes in maize. Agro Food Ind. Hi-Tech. 1998, 9, 18–22. [Google Scholar]
- Paradiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Mahro, B.; Timm, M. Potential of biowaste from the food industry as a biomass resource. Eng. Life Sci. 2007, 7, 457–468. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ramakrishnan, V.V.; Brooks, M.S.; Budge, S.M.; Dave, D. Fish processing wastes as a potential source of proteins, amino acids and oils: A critical review. J. Microb. Biochem. Technol. 2013, 5, 107–129. [Google Scholar]
- Wasswa, J.; Tang, J.; Gu, X.H.; Yuan, X.Q. Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2007, 104, 1698–1704. [Google Scholar] [CrossRef]
- Yin, H.; Pu, J.; Wan, Y.; Xiang, B.; Bechtel, P.J.; Sathivel, S. Rheological and functional properties of catfish skin protein hydrolysates. J. Food Sci. 2010, 75, E11–E17. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of Gelatin as a Biostimulant Seed Treatment to Improve Plant Performance. Front. Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 2004, 69, 615–622. [Google Scholar] [CrossRef]
- Sathivel, S.; Bechtel, P.J.; Babbitt, J.; Smiley, S.; Crapo, C.; Reppond, K.D.; Prinyawiwatkul, W. Biochemical and functional properties of herring (Clupea harengus) byproduct hydrolysates. J. Food Sci. 2003, 68, 2196–2200. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Nazeer, R.A.; Deeptha, R.; Jaiganesh, R.; Sampathkumar, N.S.; Naqash, S.Y. Radical scavenging activity of seela (sphyraena barracuda) and ribbon fish (lepturacanthus savala) backbone protein hydrolysates. Int. J. Pept. Res. Ther. 2011, 17, 209–216. [Google Scholar] [CrossRef]
- Liaset, B.; Lied, E.; Espe, M. Enzymatic hydrolysis of by-products from the fish-filleting industry; chemical characterisation and nutritional evaluation. J. Sci. Food Agric. 2000, 80, 581–589. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.; Zhao, X.; Zhang, Z.; Li, P. Optimization of enzymatic hydrolysis of Alaska pollock frame for preparing protein hydrolysates with low-bitterness. LWT Food Sci. Technol. 2011, 44, 421–428. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Rao, G.N.; Rao, D.G.; Jyothirmayi, T. Protein hydrolysates from meriga (Cirrhinus mrigala) egg and evaluation of their functional properties. Food Chem. 2010, 120, 652–657. [Google Scholar] [CrossRef]
- Ahn, C.B.; Lee, K.H.; Je, J.Y. Enzymatic production of bioactive protein hydrolysates from tuna liver: Effects of enzymes and molecular weight on bioactivity. Int. J. Food Sci. Technol. 2010, 44, 407–413. [Google Scholar] [CrossRef]
- Sierra Lopera, L.M.; Sepúlveda Rincón, C.T.; Vásquez Mazo, P.; Figueroa Moreno, O.A.; Zapata Montoya, J.E. Byproducts of aquaculture processes: Development and prospective uses. Rev. Vitae 2018, 25, 128–140. [Google Scholar] [CrossRef]
- Shavandi, A.; Hu, Z.; Teh, S.S.; Zhao, J.; Carne, A.; Bekhit, A.; Bekhit, A.E.D.A. Antioxidant and functional properties of protein hydrolysates obtained from squid pen chitosan extraction effluent. Food Chem. 2017, 227, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V. Enzymes from Seafood Processing Waste and Their Applications in Seafood Processing. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 47–69. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–29. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Geirsdottir, M.; Sigurgisladottir, S.; Hamaguchi, P.Y.; Thorkelsson, G.; Johannsson, R.; Kristinsson, H.G.; Kristjansson, M.M. Enzymatic Hydrolysis of Blue Whiting (Micromesistius poutassou); Functional and Bioactive Properties. J. Food Sci. 2011, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Salampessy, J.; Reddy, N.; Kailasapathy, K.; Phillips, M. Functional and potential therapeutic ACE-inhibitory peptides derived from bromelain hydrolysis of trevally proteins. J. Funct. Foods 2015, 14, 716–725. [Google Scholar] [CrossRef]
- Dong, Y.L.; Sheng, G.Y.; Fu, J.M.; Wen, K.W. Chemical characterization and anti-anaemia activity of fish protein hydrolysate from Saurida elongata. J. Sci. Food Agric. 2005, 85, 2033–2039. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Drench application of fish-derived protein hydrolysates affects lettuce growth, chlorophyll content, and gas exchange. Horttechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Bhaskar, N.; Benila, T.; Radha, C.; Lalitha, R.G. Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour. Technol. 2008, 99, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kechaou, E.S.; Dumay, J.; Donnay-Moreno, C.; Jaouen, P.; Gouygou, J.P.; Bergé, J.P.; Amar, R. Ben Enzymatic hydrolysis of cuttlefish (Sepia officinalis) and sardine (Sardina pilchardus) viscera using commercial proteases: Effects on lipid distribution and amino acid composition. J. Biosci. Bioeng. 2009, 107, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Lisiecka, J.; Knaflewski, M.; Spizewski, T.; Fra̧szczak, B.; KaŁuzewicz, A.; Krzesiński, W. The effect of animal protein hydrolysate on quantity and quality of strawberry daughter plants cv. “Elsanta”. Acta Sci. Pol. Hortorum Cultus 2011, 10, 31–40. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Corte, L.; Dell’Abate, M.T.; Magini, A.; Migliore, M.; Felici, B.; Roscini, L.; Sardella, R.; Tancini, B.; Emiliani, C.; Cardinali, G.; et al. Assessment of safety and efficiency of nitrogen organic fertilizers from animal-based protein hydrolysates-a laboratory multidisciplinary approach. J. Sci. Food Agric. 2014, 94, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Naroozlo, Y.A.; Souri, K.M.; Mojtaba, D. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164. [Google Scholar] [CrossRef]
- Tejada, M.; Rodríguez-Morgado, B.; Paneque, P.; Parrado, J. Effects of foliar fertilization of a biostimulant obtained from chicken feathers on maize yield. Eur. J. Agron. 2018, 96, 54–59. [Google Scholar] [CrossRef]
- Mohammadipour, N.; Souri, M.K. Effects of different levels of glycine in the nutrient solution on the growth, nutrient composition, and antioxidant activity of coriander (Coriandrum sativum L.). Acta Agrobot. 2019, 72, 13–16. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Hadadzadeh, H.; Jafari, M. Synthesis of Iron-Amino Acid Chelates and Evaluation of Their Efficacy as Iron Source and Growth Stimulator for Tomato in Nutrient Solution Culture. J. Plant Growth Regul. 2012, 2012, 498. [Google Scholar] [CrossRef]
- Visconti, F.; de Paz, J.M.; Bonet, L.; Jordà, M.; Quiñones, A.; Intrigliolo, D.S. Effects of a commercial calcium protein hydrolysate on the salt tolerance of Diospyros kaki L. cv. “Rojo Brillante” grafted on Diospyros lotus L. Sci. Hortic. 2015, 185, 129–138. [Google Scholar] [CrossRef]
- Cerdán, M.; Sánchez-Sánchez, A.; Oliver, M.; Juárez, M.; Sánchez-Andreu, J.J. Effect of foliar and root applications of amino acids on iron uptake by tomato plants. Acta Hortic. 2009, 830, 481–488. [Google Scholar] [CrossRef]
- Cerdán, M.; Sánchez-Sánchez, A.; Jordá, J.D.; Juárez, M.; Sánchez-Andreu, J. Effect of commercial amino acids on iron nutrition of tomato plants grown under lime-induced iron deficiency. J. Plant Nutr. Soil Sci. 2013, 176, 1–8. [Google Scholar] [CrossRef]
- Morales-Payan, P.; Stall, W. Passion Fruit (Passiflora Edulis) Transplant Production Is Affected By Selected Biostimulants. Proc. Florida State Hortic. Soc. 2004, 117, 224–227. [Google Scholar]
- Zheng, K.; Liang, M.; Yao, H.; Wang, J.; Chang, Q. Effect of size-fractionated fish protein hydrolysate on growth and feed utilization of turbot (Scophthalmus maximus L.). Aquac. Res. 2013, 44, 895–902. [Google Scholar] [CrossRef]
- Liang, M.; Wang, J.; Chang, Q.; Mai, K. Effects of different levels of fish protein hydrolysate in the diet on the nonspecific immunity of Japanese sea bass, Lateolabrax japonicus (Cuvieret Valenciennes, 1828). Aquac. Res. 2006, 37, 102–106. [Google Scholar] [CrossRef]
- Bernet, F.; Montel, V.; Noël, B.; Dupouy, J.P. Diazepam-like effects of a fish protein hydrolysate (Gabolysat PC60) on stress responsiveness of the rat pituitary-adrenal system and sympathoadrenal activity. Psychopharmacology 2000, 149, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Nedjar-Arroume, N.; Hedhili, K.; Chataigné, G.; Balti, R.; Nasri, M.; Dhulster, P.; Bougatef, A. Antibacterial peptides from barbel muscle protein hydrolysates: Activity against some pathogenic bacteria. LWT Food Sci. Technol. 2014, 55, 183–188. [Google Scholar] [CrossRef]
- Wang, J.; Wei, R.; Song, R. Novel Antibacterial Peptides Isolated from the Maillard Reaction Products of Half-Fin Anchovy (Setipinna taty) Hydrolysates/Glucose and Their Mode of Action in Escherichia coli. Mar. Drugs 2019, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Jenkelunas, P.J.; Li-Chan, E.C.Y. Production and assessment of Pacific hake (Merluccius productus) hydrolysates as cryoprotectants for frozen fish mince. Food Chem. 2018, 239, 535–543. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Guadix, A.; Guadix, E.M.; Jacobsen, C. Physical and oxidative stability of fish oil-in-water emulsions stabilized with fish protein hydrolysates. Food Chem. 2016, 203, 124–135. [Google Scholar] [CrossRef]
- Shahidi, F.; Onodenalore, A.C. Water dispersions of myofibrillar proteins from capelin (Mallotus villosus). Food Chem. 1995, 53, 51–54. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J. Agric. Food Chem. 2000, 48, 657–666. [Google Scholar] [CrossRef]
- Hatate, H.; Numata, Y.; Kochi, M. Synergistic Effect of Sardine Myofibril Protein Hydrolyzates with Antioxidants. Nippon Suisan. GAKKAISHI 1990, 56, 1011. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Antioxidant and Antibacterial Effect of Protein Hydrolysis of Yellowfin Tuna Waste on Flesh Quality Parameters of Minced Silver Carp. J. Genet. Resour. 2017, 3, 103–112. [Google Scholar]
- Je, J.-Y.; Lee, K.-H.; Lee, M.H.; Ahn, C.-B. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res. Int. 2009, 42, 1266–1272. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicrob. Proteins 2019, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Ween, O.; Stangeland, J.K.; Fylling, T.S.; Aas, G.H. Nutritional and functional properties of fishmeal produced from fresh by-products of cod (Gadus morhua L.) and saithe (Pollachius virens). Heliyon 2017, 3, e00343. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Skene, K.R.; Formanek, P. Non-protein amino acids: Plant, soil and ecosystem interactions. Plant Soil 2011, 342, 31–48. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxidants Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Koukounaras, A.; Tsouvaltzis, P.; Siomos, A.S. Effect of root and foliar application of amino acids on the growth and yield of greenhouse tomato in different fertilization levels. J. Food Agric. Environ. 2013, 11, 644–648. [Google Scholar]
- Walch-Liu, P.; Liu, L.H.; Remans, T.; Tester, M.; Forde, B.G. Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 1045–1057. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Flores, T.; Todd, C.D.; Tovar-Mendez, A.; Dhanoa, P.K.; Correa-Aragunde, N.; Hoyos, M.E.; Brownfield, D.M.; Mullen, R.T.; Lamattina, L.; Polacco, J.C. Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol. 2008, 147, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- McCarthy, M.D.; Bronk, D.A. Analytical Methods for the Study of Nitrogen. In Nitrogen in the Marine Environment; Academic Press: Cambridge, MA, USA, 2008; pp. 1219–1275. ISBN 9780123725226. [Google Scholar]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; Angelico, R.; Cho, H.G.; Francioso, O.; Ertani, A.; Nardi, S. Spontaneous aggregation of humic acid observed with AFM at different pH. Chemosphere 2015, 138, 821–828. [Google Scholar] [CrossRef]
- Canellas, L.P.; Balmori, D.M.; Médici, L.O.; Aguiar, N.O.; Campostrini, E.; Rosa, R.C.C.; Façanha, A.R.; Olivares, F.L. A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.). Plant Soil 2013, 366, 119–132. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Ramadan, W.A. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci. Hortic. 2015, 184, 101–105. [Google Scholar] [CrossRef]
- Michelloti Bettoni, M.; Mógor, Á.F.; Kogerastki, J.F.; Pauletti, V. Onion (Allium cepa L.) seedling growth using humic substances. Idesia 2016, 34, 57–62. [Google Scholar] [CrossRef]
- Hartz, T.K.; Bottoms, T.G. Humic substances generally ineffective in improving vegetable crop nutrient uptake or productivity. HortScience 2010, 45, 906–910. [Google Scholar] [CrossRef]
- Blunden, G.; Cripps, A.L.; Gordon, S.M.; Mason, T.G.; Turnei, C.H. The Characterisation and Quantitative Estimation of Betaines in Commercial Seaweed Extracts. Bot. Mar. 1986, 29, 155–160. [Google Scholar] [CrossRef]
- Gajc-Wolska, J.; Spiewski, T.; Grabowska, A. The Effect of Seaweed Extracts on the Yield and Quality Parameters of Broccoli (Brassica oleracea var. cymosa L.) in Open Field Production. Acta Hortic. 2012, 1009, 83–89. [Google Scholar] [CrossRef]
- Carvalho, M.E.; Castro, P.R.; Novembre, A.D.; Chamma, H.M.C. Seaweed Extract Improves the Vigor and Provides the Rapid Emergence of Dry Bean Seeds. Agric. Envirn. Sci. 2013, 13, 1104–1107. [Google Scholar]
- Abdel-Mawgoud, A.M.R.; Tantaway, A.S.; Hafez, M.M.; Habib, H.A.M. Seaweed Extract Improves Growth, Yield and Quality of Different Watermelon Hybrids. Res. J. Agric. Biol. Sci. 2010, 6, 161–168. [Google Scholar]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A Commercial Extract of Brown Macroalga (Ascophyllum nodosum) Affects Yield and the Nutritional Quality of Spinach In Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A. Arbuscular mycorrhizal fungi as support systems for seedling establishment in grassland. Ecol. Lett. 2004, 7, 293–303. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Yildirim, E.; Karlidag, H.; Turan, M.; Dursun, A.; Goktepe, F. Growth, nutrient uptake, and yieldpromotion of broccoli by plantgrowth promoting rhizobacteriawith manure. HortScience 2011, 46, 932–936. [Google Scholar] [CrossRef]
- Traon, D.; Amat, L.; Zotz, F.; du Jardin, P. A Legal Framework for Plant Biostimulants and Agronomic Fertiliser Additives in the EU; European Commission: Brussels, Belgium, 2014.
- Lötze, E.; Hoffman, E. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2015, 28, 1379–1386. [Google Scholar] [CrossRef]
- Aharoni, A.; Galili, G. Metabolic engineering of the plant primary-secondary metabolism interface. Curr. Opin. Biotechnol. 2011, 22, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.S.; Lyons, G.; McRoberts, C.; McCall, D.; Carmichael, E.; Andrews, F.; McCormack, R. Brown seaweed species from Strangford Lough: Compositional analyses of seaweed species and biostimulant formulations by rapid instrumental methods. J. Appl. Phycol. 2012, 24, 1141–1157. [Google Scholar]
- Petrova, I.; Tolstorebrov, I.; Eikevik, T.M. Production of fish protein hydrolysates step by step: Technological aspects, equipment used, major energy costs and methods of their minimizing. Int. Aquat. Res. 2018, 10, 223–241. [Google Scholar] [CrossRef]
- Di Pasquale, M.G. Amino Acids and Proteins for the Athlete: The Anabolic Edge, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780429138782. [Google Scholar]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- He, S.; Franco, C.; Zhang, W. Functions, applications and production of protein hydrolysates from fish processing co-products (FPCP). Food Res. Int. 2013, 50, 289–297. [Google Scholar] [CrossRef]
- Abejón, R.; Abejón, A.; Belleville, M.P.; Sánchez-Marcano, J.; Garea, A.; Irabien, Á. Multiobjective Optimization of Membrane Networks for Fractionation of Protein Hydrolysate from Fish By-Products. In 26 European Symposium on Computer Aided Process Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 415–420. ISBN 1570-7946. [Google Scholar]
- Pasupuleti, V.K.; Braun, S. State of the Art Manufacturing of Protein Hydrolysates BT—Protein Hydrolysates in Biotechnology. In Protein Hydrolysates in Biotechnology; Pasupuleti, V.K., Demain, A.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 11–32. ISBN 978-1-4020-6674-0. [Google Scholar]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Ravazzolo, L.; Franceschi, C.; Quaggiotti, S. mRNA-Sequencing Analysis Reveals Transcriptional Changes in Root of Maize Seedlings Treated with Two Increasing Concentrations of a New Biostimulant. J. Agric. Food Chem. 2017, 65, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- Wilson, H.T.; Xu, K.; Taylor, A.G. Transcriptome Analysis of Gelatin Seed Treatment as a Biostimulant of Cucumber Plant Growth. Sci. World J. 2015, 2015, 391234. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General Principles to Justify Plant Biostimulant Claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Biostimulants Market Size, Share and Industry Analysis by Source (Microbial and Non-Microbial), By Active Ingredients (Seaweed Extracts, Humic Substances, Vitamins & Amino Acids, Microbial Amendments, and Others), By Application (Foliar Treatmen); Fortune Business Insights: Pune, India, 2019.
- 115th Congress H.R.2-Agriculture Improvement Act of 2018. Available online: https://www.congress.gov/bill/115th-congress/house-bill/2 (accessed on 23 December 2019).
- Draft Guidance for Plant Regulator Label Claims Including; United States Environmental Protection Agency: Washington, DC, USA, 2018.
- Haufe, H.; Muschter, K.; Siegert, J.; Böttcher, H. dd_EndNote. Sci. Hortic. (Amsterdam) 2018, 239, 26–34. [Google Scholar]
| Biostimulant | Origin | Active Compounds | Application Methods | Plant | Main Activity |
|---|---|---|---|---|---|
| C Fish | White fish/mixed fish composition autolysates and hydrolysates | Peptides, amino acids | Foliar, irrigation, pre-planting | Vegetables, fruits | Increase plant’s resistance to insect pressure, disease and heat or drought stress |
| Radifarm | Commercial formulation | Amino acids, peptides, saponins, betaines, polysaccharides, vitamins, microelements | Irrigation, soil drench, foliar application | Fruits and vegetables | Promotes the formation of an extensive root system by speeding up the elongation of lateral and adventitious roots |
| Megafol | Commercial formulation | Amino acids, betaines, proteins, vitamins, auxin, gibberellin, cytokine | Irrigation, soil drench, foliar application | Fruits and vegetables | Promotes balanced vegetative development and productivity, and plant resistance to stress (frost, root asphyxia, weeding, hail) |
| Biozyme | Ascophyllum nodosum | Algae extract, plant hormones, chelated micronutrients | Irrigation, foliar, pre-planting, soil drench | Fruits, vegetables, legumes, | Increase nutrient uptake and activity of chlorophyll and photosynthesis |
| Algreen | Seaweed | Seaweed extract, plant hormones, vitamins, free amino acids, alginic acid | Promotes growth and yield parameters, enhance vitamin C and dry matter content | ||
| BioRoot | Plant derived protein hydrolysates | Plant and mineral-derived organic acids and humates, alfalfa and soybean meal, brewer’s yeast, K-sulfate, rock phosphate, sea kelp | Irrigation, foliar, soil drench | Fruits and vegetables | Increase rooting ability and chlorophyll and protein contents |
| Kelpak | Ecklonia maxima, | Seaweed extract | Drip irrigation, Soil drench, Seed treatment, foliar | Fruits and vegetables | Stimulate plant’s natural hormones, root initiation and germination |
| Biplantol Universal | Commercial formulation | Macro-and microelements, germanium, uronic acids, medicinal herbs, worm humus | Foliar, soil drench | Fruits, vegetables, flowers | Resistance to fungal diseases and insect pests |
| Grow-plex SP | Humic acids | Humic acids | Irrigation, foliar | Fruits, vegetables | Stimulate soil bacteria, root and shoot growth, iron and zinc uptake |
| Tablet | Microorganism | Rhizophagus intraradices and Trychoderma atroviride spores | Soil drench | vegetables | Stimulate root system architecture (higher total root length and surface), improve chlorophyll synthesis and increase proline accumulation |
| Ergonfill | Animal derived protein hydrolysates | Animal protein hydrolysates, cysteine, folic acid, keratin derivatives | Foliar | Fruits and vegetables | Promotes indolacetic acid and chlorophyll synthesis, improves translocation and chelation of macro and trace elements |
| Benefit | Commercial formulation | Amino acids, nucleotides, free enzymatic proteins, vitamins | Irrigation, foliar, soil drench | Fruits and vegetables | Stimulates cell division and increase in the number of cells per fruit |
| Source | Plant | Growth Media | Method of Application | Bioactive Compounds | Biostimulant Activity | Reference |
|---|---|---|---|---|---|---|
| Fish-derived protein hydrolysate | Lettuce | Field soil | Exogenous (during watering) | Peptides, amino acids | Increased leaf number and root biomass Enhanced chlorophyll content, photosynthetic rate | [42] |
| Commercial amino acids preparation | Lettuce | Field soil | Foliar | Glycine and glutamine | Increased yield, leaf chlorophyll and vitamin C content | [54] |
| Meat flour protein hydrolysate | Maize | Hoagland solution | Seedlings immersed in solution | Small peptides and amino acids | Stimulation of root and leaf biomass Induced nitrate conversion to organic nitrogen Stimulate efficient nutrient utilization by plants | [47] |
| Commercial preparation of chicken feather hydrolysate | Wheat | Field soil | Foliar | Short peptides and amino acids | Increased yield and nutrient content of grains | [45] |
| Chicken feather hydrolysates | Maize | Field soil | Foliar | Peptides and amino acids | Increased micro- and macronutrient concentration of leaves Increased yield and grain protein content | [55] |
| Commercial amino acid preparation | Coriander | Hoagland nutrient solution | Dissolved into growth media | Glycine | Increased growth of roots and shoots Increased micronutrient content of leaves | [56] |
| Fe-amino acid chelates preparation | Tomato | Nutrient solution | Dissolved into nutrient solution | Arginine, glycine and histidine | Increased uptake of Fe and improved root and shoot growth | [57] |
| Commercial animal-derived calcium protein hydrolysate | Rojo Brillante | Field soil | Irrigation | Peptides, amino acids and metal elements | Lower chloride uptake and reduction in leaf necrosis | [58] |
| Commercial animal-derived amino acids product | Tomato | Nutrient solution | Foliar and root application | Amino acids | No effects on Iron nutrition Caused severe plant depression | [59] [60] |
| Commercial preparation of amino acids and peptides | Passion fruit | Commercial growing medium | Foliar | Amino acids and peptides | Promotes the photosynthetic process in plants Improved transplanting successes | [61] |
| Animal derived gelatin | Cucumber, pepper, broccoli, tomato, arugula, and field corn | Field soil | Exogenous (adjacent to seeds) | Amino acids and peptides | Increased shoot dry weight Increased root N assimilation | [23] |
| Protein Hydrolysate Source | Hydrolysis Method | Bioactive Compounds | Bioactivity | Reference |
|---|---|---|---|---|
| Pollock | Alcalase and flavourzyme | Short peptides | Growth by growth hormone stimulation | [62] |
| Pollock | Chemical (formic acid) and Enzymatic | Short peptides | Induce immune-modulatory effects enhancing survival | [63] |
| Commercial fish protein hydrolysate | Enzymatic | Glutamic acid, other amino acids and peptides | Contain opioid-like compounds that may have anti-stress effects | [64] |
| Barbel | Alcalase | Short peptides | Could be used as antimicrobials or antibiotic adjuvants | [65] |
| Half-Fin anchovy | Enzymatic | Bioactive peptides | Antibacterial activity | [66] |
| Pacific hake | Flavourzyme | Amino acids and peptides | Cryoprotectant | [67] |
| Catshark | Enzyme | Peptides | Emulsifying | [68] |
| Shark Capelin | Alcalase | Short peptides | Foaming | [69] |
| Salmon | Enzymes (Alcalase, Flavourzyme, Corolase) | Peptides and amino acids | Water binding | [70] |
| Sardine Tuna | Enzymes Alcalase, neutrase, papain, pepsin | Peptides and amino acids | Antioxidative | [71] [72] |
| Tuna | Alcalase, neutrase, Protamex | Peptides | Antihypertensive ACE-inhibitory | [73] |
| Yellow fin tuna | Protamex | Lower molecular peptides | Antimicrobial | [74] |
| Slender lizard fish | Papain | Peptides | Antianemia | [39] |
| Cod and Saithe fish meal | Protamex | Bioactive peptides | ACE inhibitory | [75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madende, M.; Hayes, M. Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules 2020, 25, 1122. https://doi.org/10.3390/molecules25051122
Madende M, Hayes M. Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules. 2020; 25(5):1122. https://doi.org/10.3390/molecules25051122
Chicago/Turabian StyleMadende, Moses, and Maria Hayes. 2020. "Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA" Molecules 25, no. 5: 1122. https://doi.org/10.3390/molecules25051122
APA StyleMadende, M., & Hayes, M. (2020). Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules, 25(5), 1122. https://doi.org/10.3390/molecules25051122