Valorization of American Barrel-Shoot Wastes: Effect of Post Fermentative Addition and Readdition on Phenolic Composition and Chromatic Quality of Syrah Red Wines
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compound Analysis
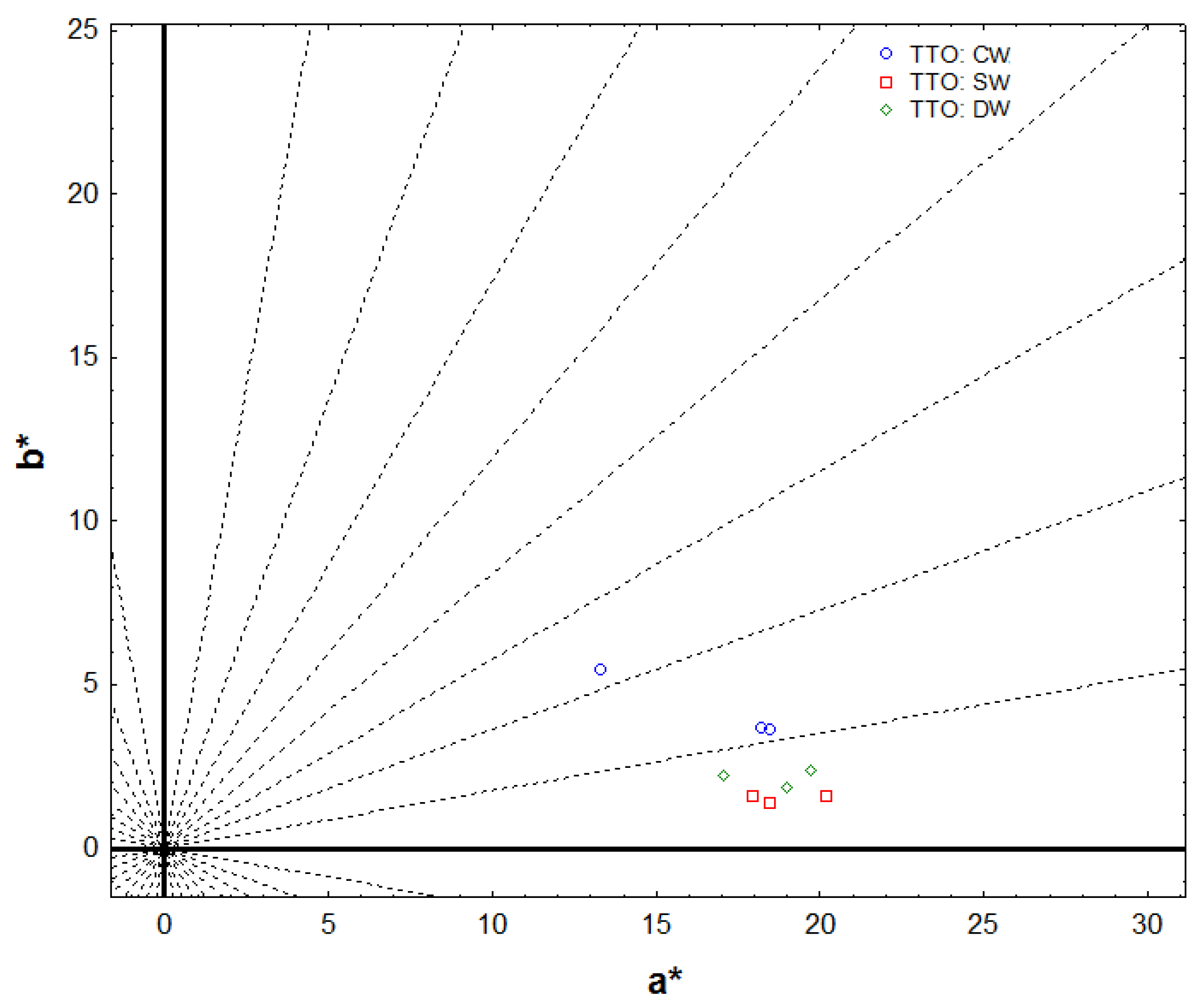
2.2. Color Analysis
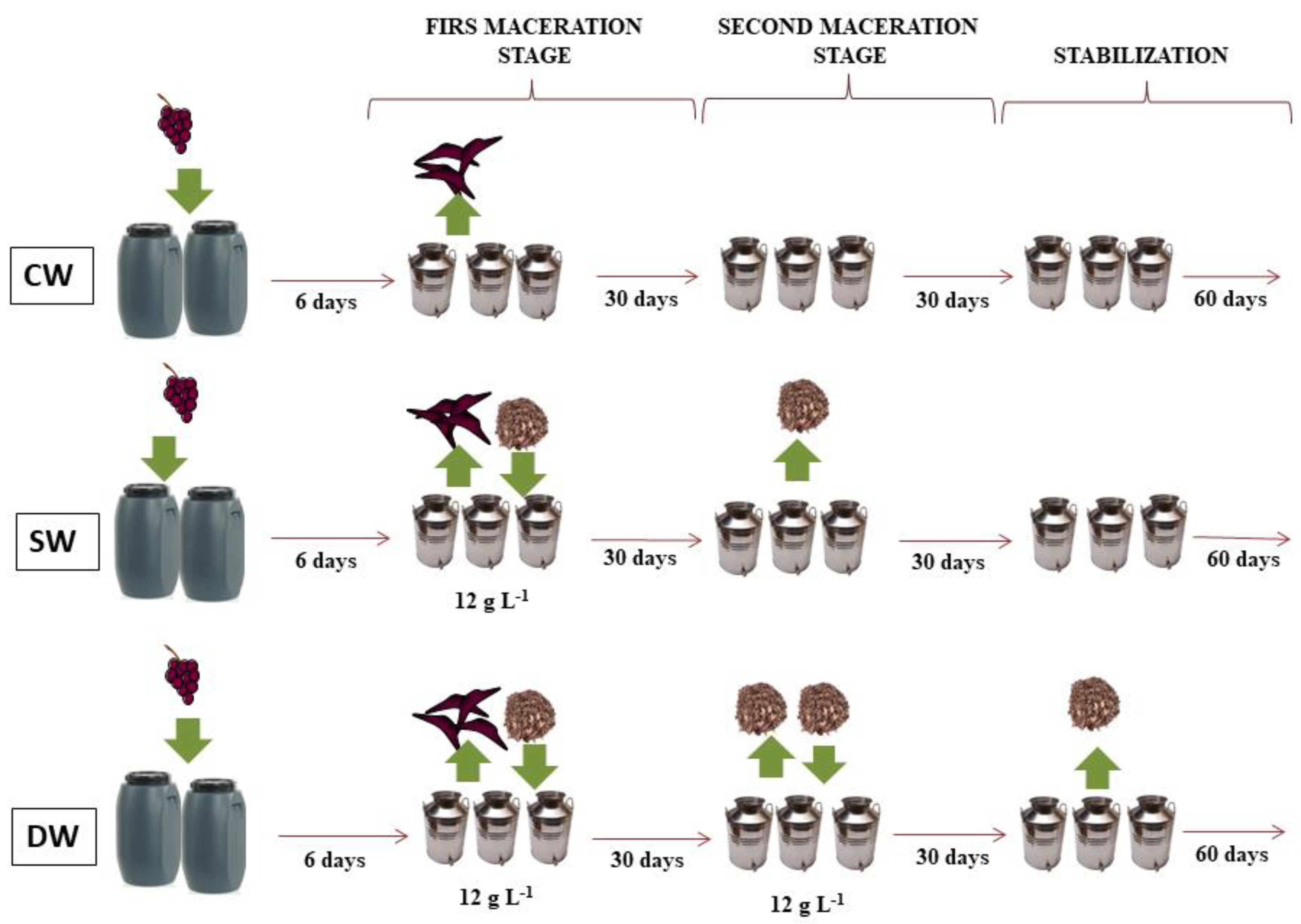
3. Material and Methods
3.1. Oak Wood Byproducts
3.2. Winemaking
3.3. Phenolic Compound Analysis
3.4. Color Analysis
3.5. Statistical analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wines. A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Gordillo, B.; Cejudo-Bastante, M.J.; Rodriguez-Pulido, F.J.; Gonzalez-Miret, M.L.; Heredia, F.J. Application of the differential colorimetry and polyphenolic profile to the evaluation of the chromatic quality of Tempranillo red wines elaborated in warm climate. Influence of the presence of oak wood chips during fermentation. Food Chem. 2013, 141, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Ferrer-Gallego, R.; Hernandez-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailon, M.T. Influence of climatic conditions on the phenolic composition of Vitis vinifera L. cv. Graciano. Anal. Chim. Acta 2012, 732, 73–77. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate change associated effects on grape and wine quality and production. Food. Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Ryan, J.M.; Revilla, E. Anthocyanin composition of Cabernet Sauvignon and Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2003, 51, 3372–3378. [Google Scholar] [CrossRef]
- Gordillo, B.; Rodriguez-Pulido, F.J.; Mateus, N.; Escudero-Gilete, M.L.; Gonzalez-Miret, M.L.; Heredia, F.J.; de Freitas, V. Application of LC-MS and tristimulus colorimetry to assess the ageing aptitude of Syrah wine in the Condado de Huelva D.O. (Spain), a typical warm climate region. Anal. Chim. Acta 2012, 732, 162–171. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Jiménez-Pascual, E.; Busse-Valverde, N.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Effect of Wine Maceration Enzymes on the Extraction of Grape Seed Proanthocyanidins. Food Bioproc. Tech. 2013, 6, 2207–2212. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Gordillo, B.; Hernanz, D.; Escudero-Gilete, M.L.; Gonzalez-Miret, M.L.; Heredia, F.J. Effect of the time of cold maceration on the evolution of phenolic compounds and colour of Syrah wines elaborated in warm climate. Int. J. Food Sci. Tech. 2014, 49, 1886–1892. [Google Scholar] [CrossRef]
- Gonzalez-Neves, G.; Favre, G.; Gil, G.; Ferrer, M.; Charamelo, D. Effect of cold pre-fermentative maceration on the color and composition of young red wines cv. Tannat. J. Food Sci. Tech. Mys. 2015, 52, 3449–3457. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; Tesfaye, W.; Loira, I.; Palomero, F.; Benito, S.; Callejo, M.J.; Villa, A.; González, M.C.; Suárez-Lepe, J.A. Electron Beam Irradiation of Wine Grapes: Effect on Microbial Populations, Phenol Extraction and Wine Quality. Food Bioproc Tech. 2015, 8, 1845–1853. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; Bañuelos, M.A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape Processing by High Hydrostatic Pressure: Effect on Microbial Populations, Phenol Extraction and Wine Quality. Food Bioproc. Tech. 2015, 8, 277–286. [Google Scholar] [CrossRef]
- Chassaing, S.; Lefeuvre, D.; Jacquet, R.; Jourdes, M.; Ducasse, L.; Galland, S.; Grelard, A.; Saucier, C.; Teissedre, P.-L.; Dangles, O.; et al. Physicochemical studies of new anthocyano-ellagitannin hybrid pigments: about the origin of the influence of oak C-glycosidic ellagitannins on wine color. Eur. J. Org. Chem. 2010, 1, 55–63. [Google Scholar] [CrossRef]
- García-Estévez, I.; Gavara, R.; Alcalde-Eon, C.; Rivas-Gonzalo, J.C.; Quideau, S.; Escribano-Bailón, M.T.; Pina, F. Thermodynamic and Kinetic Properties of a New Myrtillin–Vescalagin Hybrid Pigment. J. Agric. Food Chem. 2013, 61, 11569–11578. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Jacquet, R.; Alcalde-Eon, C.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Quideau, S. Hemisynthesis and Structural and Chromatic Characterization of Delphinidin 3-O-Glucoside-Vescalagin Hybrid Pigments. J. Agric. Food Chem. 2013, 61, 11560–11568. [Google Scholar] [CrossRef]
- García-Estévez, I.; Alcalde-Eon, C.; María Martínez-Gil, A.; Rivas-Gonzalo, J.C.; Teresa Escribano-Bailón, M.; Nevares, I.; del Alamo-Sanza, M. An Approach to the Study of the Interactions between Ellagitannins and Oxygen during Oak Wood Aging. J. Agric. Food Chem. 2017, 65, 6369–6378. [Google Scholar] [CrossRef]
- Vivas, N.; Glories, Y. Role of oak wood ellagitannins in the oxidation process of red wines during aging. Am. J. Enol. Vitic. 1996, 47, 103–107. [Google Scholar]
- Baca-Bocanegra, B.; Nogales-Bueno, J.; García-Estévez, I.; Escribano-Bailón, M.T.; Hernández-Hierro, J.M.; Heredia, F.J. Screening of wine extractable total phenolic and ellagitannin contents in revalorized cooperage by-products: evaluation by Micro-NIRS technology. Food Bioproc. Tech. 2019, 12, 477–485. [Google Scholar] [CrossRef]
- Baca-Bocanegra, B.; Nogales-Bueno, J.; Hernandez-Hierro, J.M.; Heredia, F.J. Evaluation of extractable polyphenols released to wine from cooperage byproduct by near infrared hyperspectral imaging. Food Chem. 2018, 244, 206–212. [Google Scholar] [CrossRef]
- Rivero, F.J.; Jara-Palacios, M.J.; Gordillo, B.; Heredia, F.J.; Gonzalez-Miret, M.L. Impact of a post-fermentative maceration with overripe seeds on the color stability of red wines. Food Chem. 2019, 272, 329–336. [Google Scholar] [CrossRef]
- Gordillo, B.; Baca-Bocanegra, B.; Rodríguez-Pulido, F.J.; Gonzalez-Miret, M.L.; García Estévez, I.; Quijada-Morin, N.; Heredia, F.J.; Escribano-Bailón, M.T. Optimisation of an oak chips-grape mix maceration process. Influence of chip dose and maceration time. Food Chem. 2016, 206, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, M.J.; Rivero-Granados, F.J.; Heredia, F.J. Improving the color and aging aptitude of Syrah wines in warm climate by wood-grape mix maceration. Eur. Food Res. Technol. 2017, 243, 575–582. [Google Scholar] [CrossRef]
- Cano-Lopez, M.; Lopez-Roca, J.M.; Pardo-Minguez, F.; Gomez Plaza, E. Oak barrel maturation vs. micro-oxygenation: Effect on the formation of anthocyanin-derived pigments and wine colour. Food Chem. 2010, 119, 191–195. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P.; Hermosin-Gutierrez, I. Formation of hydroxyphenyl-pyranoanthocyanins in Grenache wines: Precursor levels and evolution during aging. J. Agric. Food Chem. 2007, 55, 4883–4888. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Visual and Instrumental Color Evaluation in Red Wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Colares, C.J.G.; Pastore, T.C.M.; Coradin, V.T.R.; Marques, L.F.; Moreira, A.C.O.; Alexandrino, G.L.; Poppi, R.J.; Braga, J.W.B. Near infrared hyperspectral imaging and MCR-ALS applied for mapping chemical composition of the wood specie Swietenia Macrophylla King (Mahogany) at microscopic level. Microchem. J. 2016, 124, 356–363. [Google Scholar] [CrossRef]
- Del Álamo Sanza, M.; Nevares Domínguez, I. Wine aging in bottle from artificial systems (staves and chips) and oak woods. Anal. Chim. Acta 2006, 563, 255–263. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E.; Del Álamo Sanza, M.; Nevares, I. Effect of size, seasoning and toasting in the volatile compounds in toasted oak wood and in a red wine treated with them. Anal. Chim. Acta 2010, 660, 211–220. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Santis, D.D.; Ceccarelli, A. Influence of oak woods of different geographical origins on quality of wines aged in barriques and using oak chips. Food Chem. 2007, 103, 46–54. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Vivas, N.; Glories, Y.; Lagune, L.; Saucier, C.; Augustin, M. Estimation du degré de polymérisation des procyanidines du raisin et du vin par la méthode au p-dimethylaminocinnamaldéhyde. J. Int. Sci. Vigne Vin 1994, 28, 319–336. [Google Scholar] [CrossRef]
- García-Marino, M.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Colour and pigment composition of red wines obtained from co-maceration of Tempranillo and Graciano varieties. Anal. Chim. Acta 2010, 660, 134–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández-Hierro, J.M.; Nogales-Bueno, J.; Rodríguez-Pulido, F.J.; Heredia, F.J. Feasibility study on the use of near-infrared hyperspectral imaging for the screening of anthocyanins in intact grapes during ripening. J. Agric. Food Chem. 2013, 61, 9804–9809. [Google Scholar] [CrossRef] [PubMed]
- CIE. Colorimetry, 3rd ed.; CIE: Vienna, Austria, 2004. [Google Scholar]
- Heredia, F.J.; Alvarez, C.; Gonzalez-Miret, M.L.; Ramirez, A. CromaLab, Análisis de Color; Sevilla, Spain, 2004. [Google Scholar]
Sample Availability: Not available. |
| CW30 | SW30 | CW60 | DW60 | CW120 | SW120 | DW120 | |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Total Phenols | 2019.19a ± 36.07 | 2466.32b ± 218.38 | 2174.45a ± 157.95 | 2503.72b ± 119.89 | 2304.90a ± 292.92 | 1984.79a ± 221.36 | 2052.87a ± 165.94 |
| Total flavan-3-ols | 141.21a ± 7.24 | 136.55a ± 8.24 | 129.11a ± 9.28 | 102.24b ± 9.31 | 137.66a ± 16.91 | 70.43b ± 4.17 | 88.03b ± 8.87 |
| Total Anthocyanins | 150.20a ± 11.12 | 141.17a ± 4.84 | 132.44a ± 19.04 | 68.74b ± 3.49 | 113.57a ± 40.77 | 15.77b ± 1.10 | 23.63b ± 6.70 |
| Total non acylated anthocyanins | 67.83a ± 2.00 | 66.02a ± 3.62 | 62.99a ± 7.05 | 35.67b ± 2.15 | 54.66a ± 18.63 | 6.85b ± 0.56 | 11.33b ± 3.50 |
| Total acetyls anthocyanins | 46.53a ± 2.34 | 45.27a ± 0.61 | 43.50a ± 5.47 | 24.14b ± 1.28 | 36.94a ± 12.60 | 6.70b ± 0.39 | 9.77b ± 2.44 |
| Total coumaroyls anthocyanins | 36.51a ± 7.28 | 30.55a ± 1.16 | 26.63a ± 6.64 | 9.60b ± 0.21 | 22.64a ± 9.55 | 2.89b ± 0.35 | 3.21b ± 0.77 |
| Total acylated anthocyanins | 82.71a ± 9.20 | 75.49a ± 1.40 | 69.79a ± 12.10 | 33.41b ± 1.34 | 59.25a ± 22.14 | 9.25b ± 0.55 | 12.64b ± 3.22 |
| Delphinidin-3-O-glucoside | 2.90a ± 0.19 | 2.91a ± 0.13 | 2.81a ± 0.36 | 1.61b ± 0.07 | 2.53a ± 0.68 | 0.60b ± 0.04 | 0.78b ± 0.13 |
| Petunidin-3-O-glucoside | 5.56a ± 0.15 | 5.39a ± 0.16 | 5.20a ± 0.65 | 3.00b ± 0.12 | 4.63a ± 1.46 | 1.00b ± 0.04 | 1.38b ± 0.28 |
| Peonidin-3-O-glucoside | 5.53a ± 0.88 | 4.62a ± 0.96 | 4.50a ± 0.61 | 2.35b ± 0.10 | 3.46a ± 1.11 | 0.52b ± 0.06 | 0.66b ± 0.13 |
| Malvidin-3 O-glucoside | 54.85a ± 1.14 | 54.12a ± 2.43 | 51.49a ± 5.92 | 29.72b ± 1.86 | 45.05a ± 15.38 | 5.74b ± 0.45 | 9.53b ± 2.96 |
| Delphinidin-3-O-(6′acetyl)-glucoside | 1.56a ± 0.70 | 1.48a ± 0.72 | 1.60a ± 0.19 | 1.18b ± 0.07 | 1.42a ± 0.30 | 0.78b ± 0.02 | 0.83b ± 0.06 |
| Petunidin-3-O-(6′acetyl)-glucoside | 2.18a ± 0.25 | 2.08a ± 0.06 | 1.84a ± 0.30 | 1.13b± 0.04 | 1.74a ± 0.53 | 0.72b ± 0.02 | 0.79b ± 0.09 |
| Peonidin-3-O-(6′acetyl)-glucoside | 1.03a ± 0.19 | 1.06a ± 0.09 | 1.04a ± 0.25 | 0.44b ± 0.03 | 0.83a ± 0.28 | 0.64b ± 0.02 | 0.60b ± 0.03 |
| Malvidin-3-O-(6′acetyl)-glucoside | 4.42a ± 0.13 | 4.61a ± 0.56 | 4.44a ± 0.31 | 2.50b ± 0.20 | 3.65a ± 1.05 | 0.83b ± 0.04 | 1.09b ± 0.22 |
| Delphinidin-3-O-(6′-p-coumaroyl)glucoside (trans) | 38.70a ± 2.63 | 37.39a ± 0.93 | 35.93a ± 4.87 | 20.24b ± 1.03 | 30.65a ± 10.54 | 5.08b ± 0.32 | 7.80b ± 2.13 |
| Malvidin- 3-O-(6′ caffeoyl)-glucoside (trans) | 1.42a ± 0.23 | 1.11a ± 0.08 | 0.77a ± 0.39 | 0.50a ± 0.07 | 0.70a ± 0.23 | 0.62a ± 0.02 | 0.59a ± 0.02 |
| Cyanidin-3-O-(6′-p-coumaroyl)glucoside | 1.48a ± 0.16 | 1.36a ± 0.17 | 0.95a ± 0.37 | 0.66a ± 0.18 | 0.98a ± 0.26 | 0.37b ± 0.01 | 0.39b ± 0.02 |
| Petunidin-3-O-(6′-p-coumaroyl)glucoside (trans) | 3.48a ± 1.28 | 2.66a ± 0.25 | 2.07a ± 0.67 | 0.88b ± 0.22 | 1.80a ± 0.60 | 0.41b ± 0.01 | 0.39b ± 0.01 |
| Malvidin-3-O-(6′-p-coumaroyl)glucoside (cis) | 2.91a ± 1.88 | 1.21a ± 0.51 | 0.96a ± 0.19 | 0.45b ± 0.04 | 0.66a ± 0.12 | 0.65a ± 0.05 | 0.61a ± 0.07 |
| Peonidin-3-O-(6′-p-coumaroyl)glucoside (trans) | 2.86a ± 0.05 | 2.90a ± 0.70 | 2.98a ± 1.30 | 1.32b ± 0.09 | 2.44a ± 0.60 | 0.56 b± 0.09 | 0.56b ± 0.07 |
| Malvidin-3-O-(6′-p-coumaroyl)glucoside (trans) | 26.05a ± 3.93 | 23.00a ± 0.41 | 20.59a ± 3.78 | 7.47b ± 0.59 | 17.77a ± 8.29 | 1.88b ± 0.23 | 2.37b ± 0.70 |
| Stage | CW | SW | DW | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Initial (0 day) | L * | 74.34 ± 0.53 | 74.83 ± 0.87 | 73.90 ± 0.76 |
| a * | 27.18 ± 0.80 | 28.88 ± 0.88 | 28.51 ± 0.55 | |
| b * | −0.99 ± 0.25 | −1.72 ± 0.25 | −1.68 ± 0.14 | |
| C * ab | 27.20 ± 0.79 | 28.93 ± 0.89 | 28.23 ± 0.54 | |
| h ab | −2.10 ± 0.57 | −3.40 ± 0.43 | −3.53 ± 0.37 | |
| Maceration (30 days) | L * | 79.17a ± 0.54 | 83.44b ± 0.22 | |
| a * | 19.18a ±0.72 | 15.19b ± 0.17 | ||
| b * | 2.41a ±0.31 | 3.39b ± 0.18 | ||
| C * ab | 20.22a ± 0.61 | 15.57b ± 0.13 | ||
| h ab | 6.29a± 1.36 | 12.57b ± 0.77 | ||
| Maceration (60 days) | L * | 79.96a ± 1.24 | 81.31a ± 0.45 | |
| a * | 20.25a ± 0.89 | 19.49a ± 0.25 | ||
| b * | 1.00a ± 0.15 | 1.55b ± 0.06 | ||
| C * ab | 20.77a± 0.68 | 19.55a ± 0.26 | ||
| h ab | 2.74a ± 0.54 | 4.54b ± 1.14 | ||
| Stabilization (120 days) | L * | 80.81a ± 0.52 | 80.54a ± 1.76 | 80.92a ± 1.33 |
| a * | 18.01a ± 0.63 | 18.89a ± 1.17 | 18.60a ± 1.35 | |
| b * | 3.91a ± 0.45 | 1.50b ± 0.10 | 2.14b ± 0.26 | |
| C * ab | 17.87a ± 1.32 | 18.95a ± 1.17 | 18.73a ± 1.35 | |
| h ab | 10.94a ± 0.71 | 4.55b ± 0.37 | 6.59c ± 0.92 |
| CW | SW | DW | ||
|---|---|---|---|---|
| Stage | Mean ± SD | Mean ± SD | Mean ± SD | |
| Stabilization (120 days) | ΔE *ab | 13.50a ± 3.20 | 12.00a ± 2.65 | 12.76a ± 1.90 |
| ΔL * | 6.47a ± 0.58 | 5.71a ± 2.24 | 7.02a ± 1.48 | |
| ΔC ab | −9.99a ± 3.21 | −9.99a ± 1.90 | −9.51a ± 1.38 | |
| Δh ab | 13.04a ± 1.24 | 7.94b ± 0.75 | 10.12b ± 1.28 |
| Post-Maceration Treatment | |||
|---|---|---|---|
| SW | DW | ||
| Stage | Mean ± SD | Mean ± SD | |
| Global | ΔE * ab | 4.51a ± 2.69 | 3.76a ± 2.56 |
| ΔL * | −0.36a ± 2.08 | 0.10a ± 1.14 | |
| ΔC ab | 2.12a ± 2.88 | 1.52a ± 3.16 | |
| Δh ab | −6.33a ± 0.51 | −4.34b ± 0.61 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baca-Bocanegra, B.; Nogales-Bueno, J.; Hernández-Hierro, J.M.; Heredia, F.J. Valorization of American Barrel-Shoot Wastes: Effect of Post Fermentative Addition and Readdition on Phenolic Composition and Chromatic Quality of Syrah Red Wines. Molecules 2020, 25, 774. https://doi.org/10.3390/molecules25040774
Baca-Bocanegra B, Nogales-Bueno J, Hernández-Hierro JM, Heredia FJ. Valorization of American Barrel-Shoot Wastes: Effect of Post Fermentative Addition and Readdition on Phenolic Composition and Chromatic Quality of Syrah Red Wines. Molecules. 2020; 25(4):774. https://doi.org/10.3390/molecules25040774
Chicago/Turabian StyleBaca-Bocanegra, Berta, Julio Nogales-Bueno, José Miguel Hernández-Hierro, and Francisco José Heredia. 2020. "Valorization of American Barrel-Shoot Wastes: Effect of Post Fermentative Addition and Readdition on Phenolic Composition and Chromatic Quality of Syrah Red Wines" Molecules 25, no. 4: 774. https://doi.org/10.3390/molecules25040774
APA StyleBaca-Bocanegra, B., Nogales-Bueno, J., Hernández-Hierro, J. M., & Heredia, F. J. (2020). Valorization of American Barrel-Shoot Wastes: Effect of Post Fermentative Addition and Readdition on Phenolic Composition and Chromatic Quality of Syrah Red Wines. Molecules, 25(4), 774. https://doi.org/10.3390/molecules25040774









