Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea garcinii
Abstract
1. Introduction
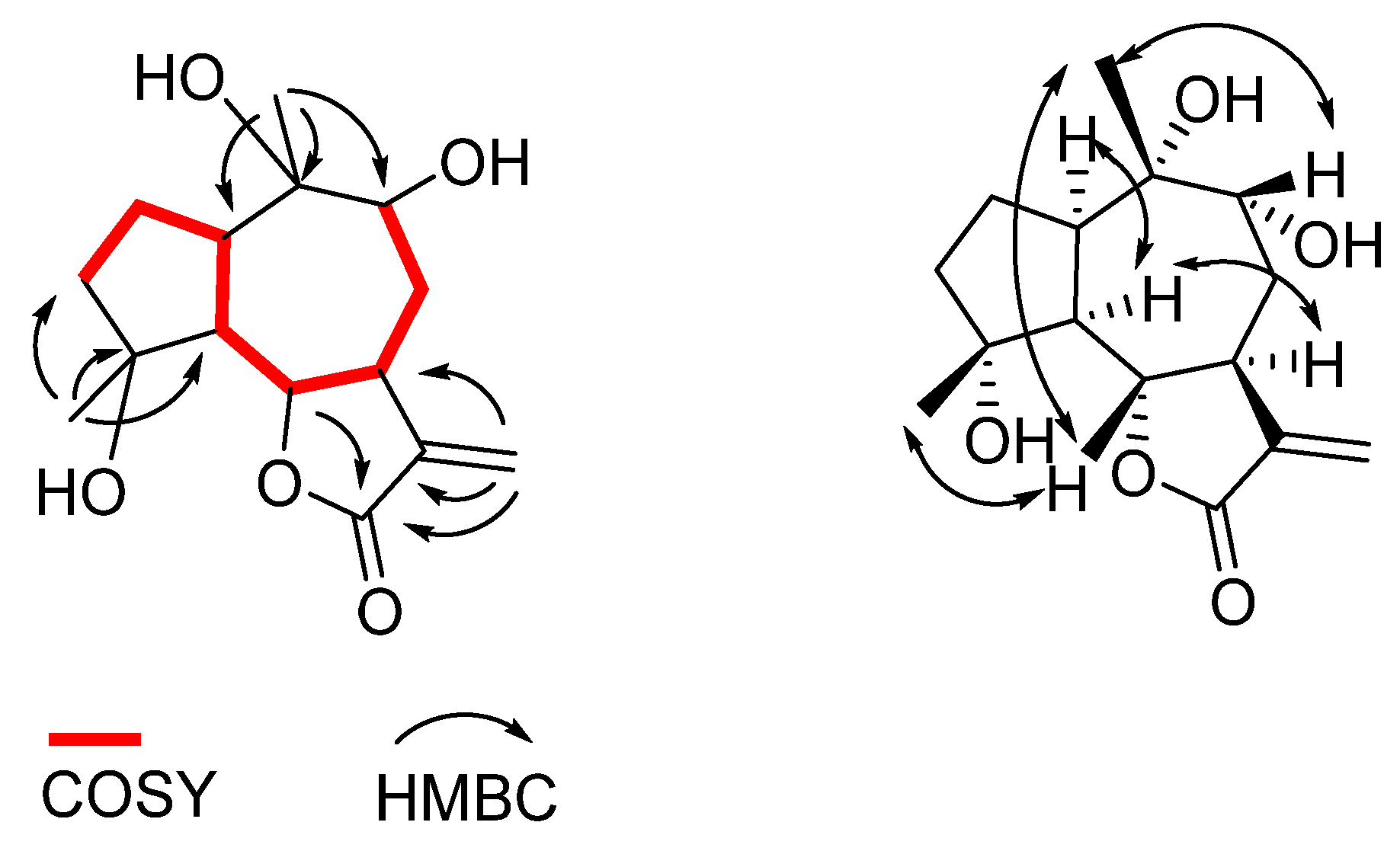
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of 2
3.5. Antibacterial Bioassay
3.6. Antifungal Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aati, H.; El-Gamal, A.; Shaheen, H.; Kayser, O. Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed. 2019, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Bitsindou, M.; Lejoly, J. Plants used in hepatoprotective remedies in traditional African medicine. In Proceedings of the WOCMAP I-Medicinal and Aromatic Plants Conference, Maastrcht, The Netherland, 19 July 1992; part 2 of 4 332. pp. 73–80. [Google Scholar]
- Oshkondali, S.T.; Elshili, M.M.; Almunir, N.; Rashed, A.; Kushlaf, N.; EL-mahmoudy, A.M.; Shaeroun, A.; Alqamoudy, H.; Ahmed, B.A.; Mohamed, K.S. Therapeutic potentials of bioactive compounds in some species (Amberboa Tubiflore, Anacyclus Clavatus and Anvillea Garcinii) in the family Asteraceae. Sch. Acad. J. Pharm. 2019, 8, 456–460. [Google Scholar] [CrossRef]
- El Hassany, B.; El Hanbali, F.; Akssira, M.; Mellouki, F.; Haidour, A.; Barrero, A.F. Germacranolides from Anvillea radiata. Fitoterapia 2004, 75, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Tyson, R.L.; Chang, C.J.; McLaughlin, J.L.; Cassady, J.M. A novel sesquiterpene lactone from Anvillea garcinii (Burm.). J. Nat. Prod. 1979, 42, 680–681. [Google Scholar]
- Tyson, R.L.; Chang, C.J.; McLaughlin, J.L.; Aynehchi, Y.; Cassady, J.M. 9-α-hydroxyparthenolide, a novel antitumor sesquiterpene lactone from Anvillea garcinii (Burm.) DC. Experientia 1981, 37, 441–442. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Dabiri, M.; Jakupovic, J. Germacranolides from Anvillea garcinii. Phytochemistry 1986, 25, 1229–1230. [Google Scholar] [CrossRef]
- Khan, M.; Saeed Abdullah, M.M.; Mousa, A.A.; Alkhathlan, H.Z. Chemical composition of vegetative parts and flowers essential oils of wild Anvillea garcinii grown in Saudi Arabia. Rec. Nat. Prod. 2016, 10, 251–256. [Google Scholar]
- Sattar, E.A.; Galal, A.M.; Mossa, G.S. Antitumor germacranolides from Anvillea garcinii. J. Nat. Prod. 1996, 59, 403–405. [Google Scholar] [CrossRef]
- Ulubelen, A.; Mabry, T.J.; Aynehchi, Y. Flavonoids of Anvillea garcinii. J. Nat. Prod. 1980, 42, 624–626. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A.M.; Yusufoglu, H.S.; Fawzy, G.A.; Foudah, A.; Abdel-Kader, M.S. Hepatoprotective and cytotoxic activities of Anvillea garcinii and isolation of four new secondary metabolites. J. Nat. Med. 2018, 72, 106–117. [Google Scholar] [CrossRef]
- Perveen, S.; Fawzi, G.A.; Al-Taweel, A.M.; Orfali, R.S.; Yusufoglu, H.S.; Abdel-Kader, M.S.; Al-Sabbagh, R.M. Antiulcer activity of different extract of Anvillea garcinii and isolation of two new secondary metabolites. Open Chem. 2018, 16, 437–445. [Google Scholar] [CrossRef]
- Perveen, S.; Alqahtani, J.; Orfali, R.; Al-Taweel, A.M.; Yusufoglu, H.S.; Abdel-Kader, M.S.; Taglialatela-Scafati, O. Antimicrobial guaianolide sesquiterpenoids from leaves of the Saudi Arabian plant Anvillea garcinii. Fitoterapia 2019, 134, 129–134. [Google Scholar] [CrossRef]
- Chae, Y.K.; Bobin, K.; Jungil, H.; Hyeon-Son, C. Parthenolide inhibits lipid accumulation via activation of Nrf2/ Keap1 signaling during adipocyte differentiation. Food Sci. Biotechnol. 2020, 29, 431–440. [Google Scholar]
- Abdel-Sattar, E.; McPhail, A.T. Cis-Parthenolid-9-one from Anvillea garcinia. J. Nat. Prod. 2000, 63, 1587–1589. [Google Scholar] [CrossRef]
- Drew, D.P.; Krichau, N.; Reichwald, K.; Simonsen, H.T. Guaianolides in Apiaceae: Perspectives on pharmacology and biosynthesis. Phytochem. Rev. 2009, 8, 581–599. [Google Scholar] [CrossRef]
- Galal, A.M. Minor guaianolides from Anvillea garcinia. Al Azhar J. Pharm. Sci. 1997, 19, 30–33. [Google Scholar]
- Tan, R.X.; Tang, H.Q.; Hu, J.; Shuai, B. Lignans and sesquiterpene lactones from Artemisia sieversiana and Inula racemosa. Phytochemistry 1998, 49, 157–161. [Google Scholar] [CrossRef]
- Tosovic, J.; Markovic, S. Structural and antioxidative features of chlorogenic acid. Croat. Chem. Acta 2016, 89, 535–541. [Google Scholar] [CrossRef]
- Erel, S.B.; Karaalp, C.; Bedir, E.; Kaehlig, H.; Glasl, S.; Khan, S.; Krenn, L. Secondary metabolites of Centaurea calolepis and evaluation of cnicin for anti-inflammatory, antioxidant, and cytotoxic activities. Pharm. Biol. 2011, 49, 840–849. [Google Scholar] [CrossRef]
- Azam, F.; Chaudhry, B.A.; Ijaz, H.; Qadir, M.I. Caffeoyl-β-D-glucopyranoside and 1,3-dihydroxy-2-tetracosanoylamino-4-(E)-nonadecene isolated from Ranunculus muricatus exhibit antioxidant activity. Sci. Rep. 2019, 9, 15613. [Google Scholar] [CrossRef]
- Delazar, A.; Nazemiyeh, H.; Afshar, F.H.; Barghi, N.; Esnaashari, S.; Asgharian, P. Chemical compositions and biological activities of Scutellaria pinnatifida A. Hamilt aerial parts. Res. Pharm. Sci. 2017, 12, 187–195. [Google Scholar]
- Aisah, L.S.; yun, Y.F.; Herlina, T.; Julaeha, E.; Zainuddin, A.; Nurfarida, I.; Hidayat, A.T.; Supratman, U.; Shiono, Y. Flavonoid compounds from the leaves of Kalanchoe prolifera and their cytotoxic activity against P-388 murine leukemia cells. Nat. Prod. Sci. 2017, 23, 139–145. [Google Scholar] [CrossRef]
- Lee, S.B.; Shin, J.S.; Han, H.S.; Lee, H.H.; Park, J.C.; Lee, K.T. Kaempferol 7-O-β-D-glucoside isolated from the leaves of Cudrania tricuspidata inhibits LPS-induced expression of pro-inflammatory mediators through inactivation of NF-κB, AP-1, and JAK-STAT in RAW 264.7 macrophages. Chem. Biol. Interact. 2018, 284, 101–111. [Google Scholar] [CrossRef]
- Achoub, H.; Mencherini, T.; Esposito, T.; Rastrelli, L.; Aquino, R.; Gazzerro, P.; Zaiter, L.; Benayache, F.; Benayache, S. New sesquiterpenes from Asteriscus graveolens. Nat. Prod. Res. 2019, in press. [Google Scholar] [CrossRef]
- Meng, J.C.; Hu, Y.F.; Chen, J.H.; Tan, R.X. Antifungal highly oxygenated guaianolides and other constituents from Ajania fruticulosa. Phytochemistry 2002, 58, 1141–1145. [Google Scholar] [CrossRef]
- Ebrahim, W.; El-Neketi, M.; Lewald, L.; Orfali, R.; Lin, W.; Rehberg, N.; Kalscheuer, R.; Daletos, G.; Proksch, P. Metabolites from the fungal endophyte Aspergillus austroafricanus in axenic culture and in fungal−bacterial mixed cultures. J. Nat. Prod. 2016, 79, 914–922. [Google Scholar] [CrossRef]
- Berghe, V.; Vlietinck, A. Screening methods for antibacterial and antiviral agents from higher plants. Methods Plant Biochem. 1991, 6, 47–68. [Google Scholar]
- Gong, L.; Guo, S. Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Afr. J. Biotechnol. 2009, 8, 731. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| 1 | 2 | |||
|---|---|---|---|---|
| Pos. | δH (mult., J in Hz) | δC | δH (mult., J in Hz) | δC |
| 1 | 2.80 (m) | 40.5 | 5.56 (dd, 9.5, 11.5) | 129.4 |
| 2α | 1.64 (dddd, 1.5, 3.5, 7.5, 12.5) | 24.6 | 2.61 (m) | 23.5 |
| 2β | 1.49 (tdd, 2.0, 6.5, 12.5) | 2.22 (m) | ||
| 3 | 1.53 (m) | 40.4 | 2.13 (m) | 35.9 |
| 1.22 (m) | ||||
| 4 | - | 79.6 | - | 61.8 |
| 5 | 2.00 (t, 11.0) | 54.6 | 2.79 (d, 9.0) | 65.9 |
| 6 | 4.17 (dd, 9.5, 11.0) | 82.6 | 4.04 (t, 9.0) | 81.4 |
| 7 | 2.87 (ddd, 9.5, 3.5, 1.5) | 40.7 | 2.11 (m) | 48.2 |
| 8 α | 1.99 (ddd, 2.5, 3.5, 9.2) | 31.6 | 2.09 (dd, 2.1, 9.2) | 35.0 |
| 8β | 1.53 (ddd, 1.0, 1.5, 9.2) | - | 1.98 (bdd, 9.2, 7.5) | - |
| 9 | 3.58 (dd, 2.5, 1.0) | 76.0 | 4.37 (dd, 7.5, 2.1) | 83.1 |
| 10 | - | 76.6 | - | 133.9 |
| 11 | - | 139.8 | 2.49 (dt, 2.5, 7.0) | 41.7 |
| 12 | - | 170.8 | - | 178.7 |
| 13a | 5.90 (brs) | 118.7 | 1.27 (d, 7.0) | 12.0 |
| 13b | 5.36 (brs) | - | ||
| 14 | 0.95 (s) | 21.8 | 1.76 (s) | 10.0 |
| 15 | 1.10 (s) | 22.0 | 1.36 (s) | 16.3 |
| 1′ | 4.11 (d, 7.5) | 98.7 | ||
| 2′ | 3.23 (t, 7.5) | 73.5 | ||
| 3′ | 3.17 (m) | 76.5 | ||
| 4′ | 3.29 (m) | 70.3 | ||
| 5′ | 3.31 (m) | 76.7 | ||
| 6′a | 3.67 (m) | 61.4 | ||
| 6′b | 3.86 (m) | |||
| Compound | Growth Inhibition (%, mean ± SD) * | MIC (µg mL−1) | ||
|---|---|---|---|---|
| C. albicans | C. parapsilosis | C. albicans | C. parapsilosis | |
| 1 | 83.4 ± 3.3 | 81.3 ± 2.6 | 0.21 ± 0.04 | 0.25 ± 0.05 |
| 2 | 79.8 ± 5.3 | 76.5 ± 4.5 | 0.26 ± 0.07 | 0.31 ± 0.02 |
| 3 | 85.0 ± 3.4 | 80.0 ± 2.7 | 0.38 ± 0.03 | 0.34 ± 0.06 |
| 4 | 61.2 ± 3.3 | 69.5 ± 2.4 | 0.89 ± 0.02 | 0.61 ± 0.08 |
| 5 | 23.6 ± 5.2 | 18.9 ± 3.7 | 0.68 ± 0.01 | 0.79 ± 0.03 |
| 6 | 19.5 ± 2.9 | 21.7 ± 3.4 | 0.73 ± 0.08 | 0.86 ± 0.07 |
| 7 | 15.8 ± 3.2 | 10.9 ± 4.7 | 0.97 ± 0.12 | 0.79 ± 0.06 |
| 8 | 42.7 ± 4.4 | 51.8 ± 2.5 | 0.74 ± 0.05 | 0.62 ± 0.03 |
| 9 | 45.3 ± 3.7 | 49.9 ± 4.8 | 0.68 ± 0.08 | 0.74 ± 0.02 |
| Itraconazole | 54.7 ± 2.6 | 51.5 ± 4.1 | 0.29 ± 0.06 | 0.33 ± 0.04 |
| Compound | MIC (µg mL−1) | ||||
|---|---|---|---|---|---|
| Staphilococcus aureus | Bacillus licheniformis | Escherichia xiangfangensis | Escherichia fergusonii | Pseudomonas aeruginosa | |
| 1 | 2.3 | 2.3 | >25 | 5.7 | >25 |
| 2 | 3.4 | 3.1 | >25 | 6.3 | >25 |
| 3 | 5.2 | 4.4 | 3.8 | >25 | >25 |
| 4 | >25 | >25 | 5.2 | 4.6 | >25 |
| 5 | >25 | >25 | >25 | >25 | >25 |
| 6 | >25 | >25 | >25 | >25 | >25 |
| 7 | >25 | >25 | >25 | >25 | >25 |
| 8 | 9.4 | >25 | >25 | 6.8 | >25 |
| 9 | >25 | 7.5 | >25 | 8.4 | >25 |
| Amikacin | 0.523 | 0.523 | 0.523 | 0.523 | 0.523 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perveen, S.; Alqahtani, J.; Orfali, R.; Aati, H.Y.; Al-Taweel, A.M.; Ibrahim, T.A.; Khan, A.; Yusufoglu, H.S.; Abdel-Kader, M.S.; Taglialatela-Scafati, O. Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea garcinii. Molecules 2020, 25, 1730. https://doi.org/10.3390/molecules25071730
Perveen S, Alqahtani J, Orfali R, Aati HY, Al-Taweel AM, Ibrahim TA, Khan A, Yusufoglu HS, Abdel-Kader MS, Taglialatela-Scafati O. Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea garcinii. Molecules. 2020; 25(7):1730. https://doi.org/10.3390/molecules25071730
Chicago/Turabian StylePerveen, Shagufta, Jawaher Alqahtani, Raha Orfali, Hanan Y. Aati, Areej M. Al-Taweel, Taghreed A. Ibrahim, Afsar Khan, Hasan S. Yusufoglu, Maged S. Abdel-Kader, and Orazio Taglialatela-Scafati. 2020. "Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea garcinii" Molecules 25, no. 7: 1730. https://doi.org/10.3390/molecules25071730
APA StylePerveen, S., Alqahtani, J., Orfali, R., Aati, H. Y., Al-Taweel, A. M., Ibrahim, T. A., Khan, A., Yusufoglu, H. S., Abdel-Kader, M. S., & Taglialatela-Scafati, O. (2020). Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea garcinii. Molecules, 25(7), 1730. https://doi.org/10.3390/molecules25071730







