Abstract
In our continuous search for new, relatively simple 2-alkylidene-1-oxoheterocycles as promising anticancer drug candidates, herein we report an efficient synthesis of 2,2,6-trisubstituted 5-methylidenetetrahydropyran-4-ones. These compounds were obtained in a four step reaction sequence, in which starting diethyl 2-oxopropylphosphonate was transformed into 2,2-disubstituted 5-diethoxyphosphoryldihydropyran-4-ones, followed by Michael addition of various Grignard reagents and Horner-Wadsworth-Emmons reaction of the obtained adducts with formaldehyde. Stereochemistry of the intermediate Michael adducts is also discussed. Final 5-methylidenetetrahydropyran-4-ones were tested for their possible antiproliferative effect against three cancer cell lines and 6-isopropyl-2,2-dimethyl-5-methylidenetetrahydropyran-4-one (11c), which showed very high cytotoxic activity against HL-60 human leukemia cells and was three times more active than known anticancer drug carboplatin, was selected for further biological evaluation, in order to disclose its mechanism of action. The obtained results indicated that 11c induced apoptosis in HL-60 cells and caused the arrest of the cell cycle in the G2/M phase, which may suggest its cytotoxic and cytostatic activity.
1. Introduction
In the continuation of our search for relatively simple structural motifs with anticancer potential [1,2,3], we turned our attention to 3-alkylidenetetrahydropyran-4-ones 1. Compounds containing this skeleton are common in nature and display interesting biological activities. This structural motif is present in a number of biologically active and medicinally important natural products. For example, norperovskatone 2, recently isolated from Perovskia atriplicifolia, possesses remarkable anti-HBV (Hepatitis B Virus) activity [4] and (+)-okilactomycin 3 [5] exhibits in vitro cytotoxicity against a number of human cancer cell lines, including lymphoid leukemia L1210 or leukemia P388. This compound also shows in vivo activity against Ehrlich ascites carcinoma. Chrolactomycin 4 displays significant cytotoxicity in vitro against several human cancer cell lines: ACHN, A431, MCF-7, and T24 [6]. The structures of natural compounds containing 3-alkylidenetetrahydropyran-4-one motif are shown in Figure 1.
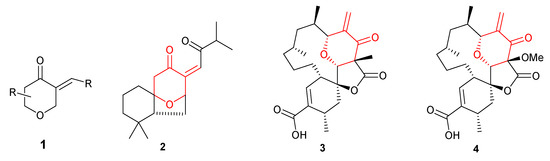
Figure 1.
Natural compounds containing 3-alkylidenetetrahydropyran-4-one skeleton.
However, literature search revealed that synthetic methodologies that can be applied to the preparation of 3-alkylidenetetrahydropyran-4-ones 1 with diverse substitution patterns are limited. There are several methods which enable the introduction of arylidene group onto the plain tetrahydropyran-4-one ring using a reaction with aromatic aldehydes in the presence of catalytic amount of TMSNMe2 and MgBr2 Et2O [7] or pyrrolidine [8]. There are also two literature reports which describe preparation of specific 3-methylidenetetrahydropyran-4-ones, and thus 8-methylidene-9-oxo-6-oxaspiro [5,6] decane was prepared by carbocyclization of unsaturated thioesters under palladium catalysis [9] and 2-butyl-6,6-dimethyl-3-methylidenetetrahydropyran-4-one was obtained in a three-step reaction sequence starting from 6-ethoxy-2-methyl-5-diphenylphosphorylhex-3-yn-5-en-2-ol [10].
In this report, we present a novel, general, and efficient synthesis of 2,2,6-trisubstituted 5-methylidenetetrahydropyran-4-ones 11 using Horner-Wadsworth-Emmons methodology [11,12]. The obtained compounds have been evaluated for their possible cytotoxic activity against three human cancer cell lines: NALM-6 and HL-60 leukemia and the MCF-7 breast cancer cell line.
2. Results and Discussion
2.1. Chemistry
We start our research from the synthesis of diethyl 4-hydroxy-2-oxoalkylphosphonates 7a–d using a procedure described by Wada and coworkers [13]. In this respect, diethyl 2-oxopropylphosphonate (5) was reacted with 1,1 equiv. of sodium hydride at r.t. and next with 1,1 equiv. of 2,5 M n-BuLi solution in hexane at −40 °C to obtain dianion 6, which, after cooling to −70 °C was treated with symmetric ketones, such as acetone, cyclohexanone, benzophenone, and fluoren-9-one (Scheme 1). After standard work-up, expected phosphonates 7a–d were obtained in moderate to good yields (Table 1). Next, 7a–d were subjected to a reaction with dimethylformamide dimethyl acetal (DMF-DMA) in the presence of trifluoroborate diethyl etherate BF3·Et2O, applying toluene as a solvent. Initial results, using one equiv. of DMF-DMA and BF3·Et2O and performing the reaction at r.t. for 24 h were unsatisfactory with 15–20% conversion to the desired pyran-4-ones 9. To our delight, the optimization of this reaction was fully successful and carrying out the reaction with 3 equiv. of DMF-DMA and 1 equiv. of BF3 Et2O for 3 h at 100 °C (for pyran-4-ones 9a–c) or at r.t. (for pyran-4-one 9d) gave desired 3-diethoxyphosphoryldihydropyran-4-ones 9a–d in good yields (Table 1). Apparently, this reaction proceeds via the Knoevenagel type condensation, with the formation of dimethylaminovinylphosphonates 8a–d as intermediates, followed by intramolecular oxy-Michael cyclization. When progress of these reactions was monitored by 31P NMR technique, signals with chemical shift ~25 ppm initially appeared, which could be attributed to intermediate vinylphosphonates 8. Only when BF3·Et2O was added to the reaction mixture did these signals gradually disappear with the simultaneous appearance of pyran-4-ones 9 signals.
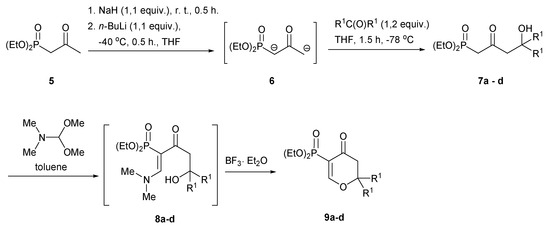
Scheme 1.
Synthesis of diethyl 4-hydroxy-2-oxoalkylphosphonates 7a–d and 3-diethoxyphosphoryldihydropyran-4-ones 9a–d.

Table 1.
Yields of diethyl 4-hydroxy-2-oxoalkylphosphonates 7a–d and 3-diethoxyphosphoryldihydropyran-4-ones 9a–d.
With 3-diethoxyphosphoryldihydropyran-4-ones 9a–d in hand, we used them as Michael acceptors in the reaction with Grignard reagents, to introduce various substituents into position 2. The addition of ethyl-, n-butyl- and i-propylmagnesium chlorides proceeded smoothly when three equiv. of Grignard reagent were used, while the addition of phenylmagnesium chloride was effective only in the presence of 3 equiv. of copper iodide (I). Standard work-up of the reaction mixtures followed by column chromatography purification furnished 6-substituted 5-diethoxyphosphoryltetrahydropyran-4-ones 10a–p in good yields (Scheme 2, Table 2). Interestingly, pyran-4-ones 10a–p were formed as mixtures of diasteroisomers trans-10a–p and cis-10a–p along with the enol form, enol-10a–p.
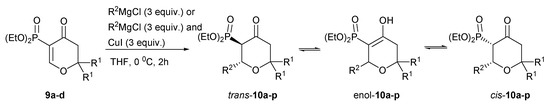
Scheme 2.
Synthesis of 6-substituted 5-diethoxyphosphoryldihydropyran-4-ones 10a–p.

Table 2.
Adducts 10a–p and 5-methylidenetetrahydropyran-4-ones 11a–p obtained.
Careful analysis of 1H and 13C NMR spectra allowed us not only to propose the relative configuration of both diastereoisomers but also their preferred conformation. For example, coupling constants 3JH5-H6, 3JH6-P and 3JC1′-P for trans-10f and cis-10f (Figure 2) unambiguously indicated diequatorial and axial/equatorial position of n-Bu and phosphoryl groups in these diastereoisomers, respectively. Because similar sets of coupling constants were observed for other pairs of diastereoisomers we believe that this assignment is valid for all 6-substituted 5-diethoxyphosphoryltetrahydropyran-4-ones 10a–p. In turn enol form of pyran-4-ones 10a–p had characteristic doublets of hydroxyl group with chemical shift in the range of 11–12 ppm and coupling constant 4JH-P ~1 Hz. Presence of such coupling constant is yet another confirmation of the existence of resonance assisted hydrogen bond (RAHB) in organophosphorus compounds [14]. Relative ratios of trans-, cis- and enol-10a–p, taken from the corresponding 31P NMR spectra, are given in Table 2.
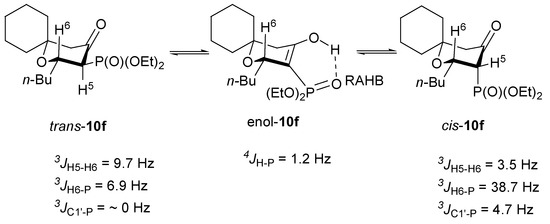
Figure 2.
Preferred conformations of trans-, cis- and enol-10f and characteristic coupling constants.
In the final step, the target 2,2,6-triisubstituted-5-methylidenetetrahydropyran-4-ones 11a–p were obtained by olefination of formaldehyde using 5-diethoxyphosphoryltetrahydropyran-4-ones 10a–p as Horner-Wadsworth-Emmons reagents (Scheme 3). Very good yields were obtained when formalin and potassium carbonate were used and the reaction mixture was stirred at 0 °C for 2 h (Table 2). However, to get 2,2-disubstituted 6-isopropyl-5-methylidenetetrahydropyran-4-ones 11c,g,k,o in reasonable yields (60–70%), stirring of the reaction mixture at r.t. for 3 h was necessary.
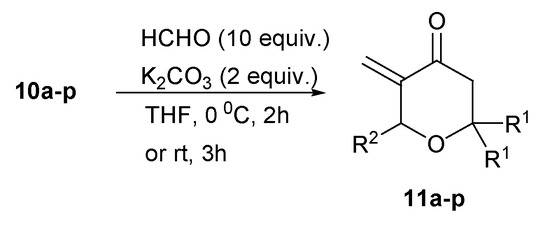
Scheme 3.
Synthesis of 2,2,6-trisubstituted-5-methylidenetetrahydropyran-4-ones 11a–p.
2.2. Biological Screening of Novel Pyranones
Novel 2,2,6-trisubstituted 5-methylidenetetrahydropyran-4-ones 11a–p were tested for their possible antiproliferative activity on two human leukemia cell lines, acute promyelocytic leukemia HL-60 and lymphoblastic leukemia NALM-6, and on a solid tumor-derived human breast adenocarcinoma cell line MCF-7, using the tetrazolium derivative reduction assay (MTT) (Table 3). An anticancer drug, carboplatin, and a sesquiterpene lactone, parthenolide, containing the same α, β-unsaturated carbonyl function as analogs 11a–p, were used as reference compounds. Concentration-response analysis was performed to determine drug concentrations required to inhibit the growth of cancer cells by 50% (IC50) after 48 h incubation.

Table 3.
In vitro cytotoxic activity of 5-methylidenetetrahydropyran-4-ones 11a–p tested on three cancer cell lines, HL-60, NALM-6, and MCF-7.
All 16 analogs were found to possess antiproliferative activity in the tested cell lines in the µM range. The IC50 values below 5 µM were obtained for 4 compounds (11b,c,k,o) on HL-60, 8 compounds (11a,b,c,f,g,k,m,o) on NALM-6, and 1 analog (11o) on the MCF-7 cell line. Carboplatin inhibited the growth of all three types of cells with IC50 values below 5 µM and the potency order NALM-6 > HL-60 > MCF-7. Parthenolide was about 2-3-fold less cytotoxic than carboplatin on these cell lines. The obtained data indicate that methylidenepyranones 11a–p were more cytotoxic against leukemia cells than against breast cancer cells. Analog 11c was the most cytotoxic, with a three-fold lower IC50 than carboplatin on HL-60 and NALM-6 cells (1.02 and 0.27 µM as compared to 2.9 and 0.7 µM for carboplatin, respectively). None of the analogs were more cytotoxic than carboplatin on MCF-7 cells.
Analysis of the structure-activity relationship revealed that the cytotoxicity of 5-methylidenetetrahydropyran-4-ones 11a–p strongly depended on the nature of the substituent in position 6 (R2) and, to a much lesser extent, on the structure of geminal substituents in position 2 (R1, R1). This observation can be rationalized with the reasonable assumption that the conjugated methylidene group in pyranones 11 acts as an effective Michael acceptor towards various bionucleophiles through a reaction with mercapto groups (-SH) of cysteine residues in proteins and intracellular glutathione [15]. Such alkylation of cellular thiols disrupts key biological processes and affects multiple targets in cancer cells [16]. Therefore, R2 substituents that are much closer to the reaction site (methylidene group) than R1 substituents can influence the effectiveness of the alkylation to a much greater extent.
Among position 2 substituents (R1, R1), two phenyl groups (11i-l) or a fluorenyl moiety (11m-p) were more advantageous for growth inhibition on all three tested cell lines than the non-aromatic substituents. Among analogs with various R2 alkyl groups introduced into position 6, those having isopropyl were the most active. The only exception was pyran-4-one 11k (R1 = Ph, R2 = i-Pr, IC50 = 11.9 μM), which was less active than pyran-4-one 11j (R1 = Ph, R2 = n-Bu, IC50 = 6.9 μM) against the MCF-7 cell line. As opposed to position 2, phenyl moiety in position 6 (as R2) was the one contributing less to the compounds’ cytotoxicity.
Evidently, the most cytotoxic compound of the series was pyran-4-one 11c (R1 = Me, R2 = i-Pr), which had the highest activity among all tested analogs on HL-60 and NALM-6 cell lines (IC50 = 1.02 μM and 0.27 μM, respectively). Therefore, analog 11c was selected for further evaluation of its anticancer activity. Since the mortality rates for acute myeloid leukemia are very high [17], necessitating the search for novel chemotherapeutic candidates, additional tests were performed on the HL-60 cell line.
We wanted to determine whether the effect of 11c on cell viability was due to apoptosis. DNA damage and the subsequent induction of apoptosis is a primary cytotoxic mechanism of many anticancer agents.
Cytotoxicity of 11c in HL-60 cells was once again measured after a shorter incubation time (24 h) using a broad range of analog concentrations (0–25 µM) and the obtained IC50 value was 2.2 µM (Figure 3A). Phosphorylation of H2AX histone was used as a marker of DNA double-strand breaks. To assess the ability of 11c to phosphorylate H2AX, HL-60 cells were incubated with this compound for 24 h at 2.2 and 4.4 µM concentration (IC50 and 2 IC50, respectively). At 4.4 µM, 11c caused a significant H2AX phosphorylation, indicative of DNA damage (Figure 3B). The induction of apoptosis was assessed by detection of the 89 kDa-cleaved fragment of PARP (poly (ADP-ribose) polymerase). The tested analog caused about 4-fold increase in the number of cleaved PARP positive (apoptotic) cells as compared to the control (Figure 3C).
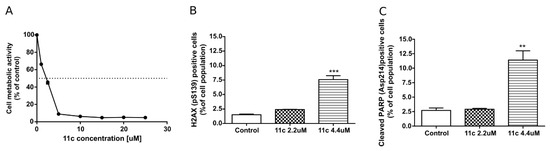
Figure 3.
Influence of 11c on HL-60 cells after 24 h incubation (A): Metabolic activity, (B): DNA damage measured by H2AX phosphorylation changes and (C): apoptosis induction measured by PARP cleavage. (B,C): Results are expressed as mean ± SEM of a triplicate assay; *** p < 0.001, ** p < 0.01.
Analysis of the cell cycle phases was then performed using flow cytometry. After 24 h incubation with 11c, HL-60 cells were treated with a fluorescent dye (4,6-diamidino-2-phenylindole, DAPI) that quantitatively stains DNA. The fluorescence intensity of the stained cells correlates with the amount of DNA they contain and identifies cell cycle position in the major phases (G0/G1 versus S versus G2/M phase). Representative profiles are shown in Figure 4. Analog 11c at a concentration of 4.4 µM caused a significant increase of cells in the G2/M phase (from 18.9% in control to 31.4%), suggesting an antimitotic effect. Together, the obtained data may indicate that the novel pyranone 11c produced both cytotoxic and cytostatic effects.
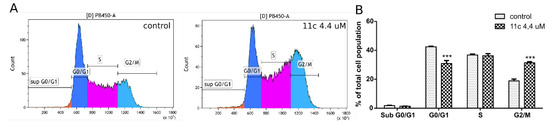
Figure 4.
Effect of 11c on the cell cycle progression in HL-60 cells. (A): Representative flow cytometry results of the cell cycle changes. (B): Percentage quantification of the total cell populations in each of the cell cycle phases expressed as mean ± SEM of a triplicate assay; *** p < 0.001.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
NMR spectra were recorded on a Bruker DPX 250 (Billerica, MA, USA) or Bruker Avance II instrument (Billerica, MA, USA) at 250.13 MHz or 700 MHz for 1H, 62.9 MHz or 176 MHz for 13C, and 101.3 MHz for 31P NMR with tetramethylsilane used as an internal and 85% H3PO4 as an external standard. 31P NMR spectra were recorded using broadband proton decoupling. IR spectra were recorded on a Bruker Alpha ATR spectrophotometer. Melting points were determined in open capillaries and are uncorrected. Column chromatography was performed on silica gel 60 (230–400 mesh) (Aldrich, Saint Louis, MO, US). Thin-layer chromatography was performed on the pre-coated TLC sheets of silica gel 60 F254 (Aldrich). The purity of the synthesized compounds was confirmed by combustion elemental analyses (CH, elemental analyzer EuroVector 3018, Elementar Analysensysteme GmbH, Langenselbold, Germany. MS spectra of intermediates were recorded on Waters 2695-Waters ZQ 2000 LC/MS apparatus. EI mass spectra of the final compounds were recorded on a GCMS-QP2010 ULTR A instrument (Shimadzu, Kyoto, Japan). Mass spectra were obtained using the following operating conditions: electron energy of 70 eV and ion source temperature of 200 °C. Samples were introduced via a direct insertion probe heated from 30 °C to 300 °C. All reagents and starting materials were purchased from commercial vendors and used without further purification. Organic solvents were dried and distilled prior to use. Standard syringe techniques were used for transferring dry solvents.
General procedures and characterization data for diethyl 4-hydroxy-2-oxoalkylphosphonates 7a–d, 3-diethoxyphosphoryldihydropyran-4-ones 9a–d and 2,2-dialkyl(diaryl)-6-alkyl(aryl)-5-methylenetetrahydro -4H-pyran-4-ones 10a–p are given in Supplementary Materials.
3.1.2. General Procedure for the Synthesis of 2,2,6-Triisubstituted-5-Methylidenetetrahydropyran-4-Ones 11a–p
To a vigorously stirred solution of 5-diethoxyphosphoryltetrahydropyran-4-ones 10a–p (0.2 mmol) in dry THF (2 mL), formaldehyde (36–38% solution in water, 0.167 mL, ca. 2.00 mmol) was added at 0 °C, followed by addition of K2CO3 (55.3 mg, 0.40 mmol) in water (0.56 mL). The resulting mixture was stirred vigorously at 0 °C for 2 h (for pyran-4-ones 10a,b,d–f,h–j,l–n,p) or at r.t. for 3 h (for pyran-4-ones 10c,g,k,o). Next Et2O (10 mL) was added and the layers were separated. The water fraction was washed with Et2O (5 mL). Organic fractions were combined, washed with brine (10 mL), and dried over MgSO4. The solvents were evaporated under reduced pressure and the resulting crude product was purified by column chromatography (eluent DCM).
6-Ethyl-2,2-dimethyl-5-methylidenetetrahydro-4H-pyran-4-one (11a) (32.6 mg, 97%) Colorless oil. 1H NMR (700 MHz, Chloroform-d) δ 0.97 (t, J = 7.3 Hz, 3H), 1.28 (s, 3H), 1.29 (s, 3H), 1.60–1.69 (m, 1H), 1.79–1.89 (m, 1H), 2.39–2.51 (m, 2H), 4.47 (ddd, J = 5.6, 2.0, 2.0 Hz, 1H), 5.27 (dd, J = 2.0, 1.0 Hz, 1H), 6.14 (dd, J = 2.0, 1.0 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 9.3, 25.3, 28.0, 30.8, 51.1, 72.3, 72.9, 119.6, 144.9, 197.9. EI-MS [M]+ = 168.0. Anal. Calcd. for C10H16O2: C, 71.39; H, 9.59. Found: C, 71.21; H, 9.61.
6-Butyl-2,2-dimethyl-5-methylidenetetrahydro-4H-pyran-4-one (11b) (37.7 mg, 96%) Colorless oil. 1H NMR (700 MHz, Chloroform-d) δ 0.91 (t, J = 7.2 Hz, 3H), 1.27 (s, 3H), 1.28 (s, 3H), 1.30–1.42 (m, 3H), 1.43–1.52 (m, 1H), 1.62 (dddd, J = 14.1, 10.2, 7.8, 4.7 Hz, 1H), 1.77 (dddd, J = 14.1, 10.6, 5.4, 3.7 Hz, 1H), 2.46 (d, J = 16.7 Hz, 2H), 4.50 (dddd, J = 7.8, 3.9, 2.0, 2.0 Hz, 1H), 5.27 (dd, J = 2.0, 1.0 Hz, 1H), 6.11 (dd, J = 2.1, 1.0 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 14.2, 22.8, 25.4, 27.3, 30.8, 34.7, 51.1, 71.8, 72.4, 119.4, 145.3, 197.9. EI-MS [M]+ = 196.0. Anal. Calcd. for C12H20O2: C, 73.43; H, 10.27. Found: C, 73.60; H, 10.30.
6-Isopropyl-2,2-dimethyl-5-methylidenetetrahydro-4H-pyran-4-one (11c) (18.6 mg, 51%) Colorless oil. 1H NMR (700 MHz, Chloroform-d) δ 0.86 (d, J = 6.8 Hz, 3H), 1.01 (d, J = 6.8 Hz, 3H), 1.26 (s, 3H), 1.29 (s, 3H), 1.96 (heptd, J = 6.8, 3.2 Hz, 1H), 2.37–2.48 (m, 2H), 4.44 (ddd, J = 3.2, 2.0, 2.0 Hz, 1H), 5.27 (dd, J = 2.0, 1.1 Hz, 1H), 6.20 (dd, J = 2.0, 1.1 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 15.7, 19.4, 25.0, 30.8, 33.7, 51.0, 71.8, 76.8, 120.6, 144.1, 198.1. EI-MS [M]+ = 182.0. Anal. Calcd. for C11H18O2: C, 72.49; H, 9.95. Found: C, 72.71; H, 9.92.
2,2-Dimethyl-5-methylidene-6-phenyltetrahydro-4H-pyran-4-one (11d) (32.9 mg, 76%) Colorless oil. 1H NMR (700 MHz, Chloroform-d) δ 1.40 (s, 3H), 1.42 (s, 3H), 2.65 (s, 2H), 4.82 (dd, J = 2.2, 1.2 Hz, 1H), 5.52 (dd, J = 2.2, 2.2 Hz, 1H), 6.15 (dd, J = 2.2, 1.2 Hz, 1H), 7.30 – 7.34 (m, 1H), 7.35 – 7.40 (m, 4H). 13C NMR (176 MHz, Chloroform-d) δ 25.5, 30.8, 51.5, 73.5, 76.0, 123.0, 128.0 (2 × C), 128.4, 128.7 (2 × C), 140.5, 145.4, 197.1. EI-MS [M]+ = 216.0. Anal. Calcd. for C14H16O2: C, 77.75; H, 7.46. Found: C, 77.71; H, 7.47.
2-Ethyl-3-methylidene-1-oxaspiro [5.5]undecan-4-one (11e) (41.2 mg, 99%) Colorless oil. 1H NMR (700 MHz, Chloroform-d) δ 1.05 (t, J = 7.3 Hz, 3H), 1.25–1.92 (m, 12H), 2.38 (d, J = 16.7 Hz, 1H), 2.44 (d, J = 16.7 Hz, 1H), 4.37 (dddd, J = 7.8, 3.6, 2.1, 2.1 Hz, 1H), 5.25 (dd, J = 2.1, 1.0 Hz, 1H), 6.11 (dd, J = 2.1, 1.0 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 9.9, 21.8, 21.8, 25.5, 28.1, 33.2, 39.3, 50.9, 71.7, 73.1, 119.3, 145.6, 198.2. EI-MS [M]+ = 208.0. Anal. Calcd. for C13H20O2: C, 74.96; H, 9.68. Found: C, 74.76; H, 9.70.
2-Butyl-3-methylidene-1-oxaspiro[5.5]undecan-4-one (11f) (43.5 mg, 92%) Colorless oil. 1H NMR (700 MHz, Chloroform-d) δ 0.92 (t, J = 7.2 Hz, 3H), 1.18–1.91 (m, 16H), 2.37 (d, J = 16.7 Hz, 1H), 2.43 (d, J = 16.7 Hz, 1H), 4.32–4.49 (m, 1H), 5.25 (dd, J = 2.1, 1.0 Hz, 1H), 6.08 (dd, J = 2.1, 1.0 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 14.2, 21.8, 21.8, 22.8, 25.5, 27.7, 33.2, 34.8, 39.3, 50.9, 70.6, 73.1, 119.1, 145.9, 198.2. EI-MS [M]+ = 236.0. Anal. Calcd. for C15H24O2: C, 76.23; H, 10.24. Found: C, 76.18; H, 10.25.
2-Isopropyl-3-methylidene-1-oxaspiro[5.5]undecan-4-one (11g) (24.5 mg, 55%) Colorless oil. 1H NMR (700 MHz, Chloroform-d) δ 0.86 (d, J = 6.8 Hz, 3H), 1.07 (d, J = 6.8 Hz, 3H), 1.16–1.91 (m, 10H), 2.00 (hd, J = 6.8, 3.0 Hz, 1H), 2.32 (d, J = 17.1 Hz, 1H), 2.38 (d, J = 17.1 Hz, 1H), 4.37 (ddd, J = 3.0, 2.2, 2.2 Hz, 1H), 5.24 (dd, J = 2.2, 1.1 Hz, 1H), 6.18 (dd, J = 2.2, 1.1 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 15.3, 19.7, 21.7, 21.9, 25.6, 32.9, 33.4, 39.2, 50.8, 72.4, 75.2, 120.1, 144.7, 198.3. EI-MS [M]+ = 222.0. Anal. Calcd. for C14H22O2: C, 75.63; H, 9.97. Found: C, 75.80; H, 9.96.
3-Methylidene-2-phenyl-1-oxaspiro[5.5]undecan-4-one (11h) (38.5 mg, 75%) White solid mp 128 – 130 °C. 1H NMR (700 MHz, Chloroform-d) δ 1.04–2.12 (m, 10H), 2.57 (d, J = 16.5 Hz, 1H), 2.64 (d, J = 16.5 Hz, 1H), 4.82 (dd, J = 2.2, 1.2 Hz, 1H), 5.48 (dd, J = 2.2, 2.2 Hz, 1H), 6.14 (dd, J = 2.2, 1.2 Hz, 1H), 7.31–7.35 (m, 1H), 7.36–7.40 (m, 4H). 13C NMR (176 MHz, Chloroform-d) δ 21.8, 21.9, 25.5, 33.7, 39.2, 50.6, 74.4, 74.9, 122.7, 128.0 (2 × C), 128.2, 128.6 (2 × C), 140.8, 145.8, 197.4. EI-MS [M]+ = 256.0. Anal. Calcd. for C17H20O2: C, 79.65; H, 7.86. Found: C, 79.87; H, 7.85.
6-Ethyl-5-methylidene-2,2-diphenyltetrahydro-4H-pyran-4-one (11i) (55.6 mg, 95%) Pale yellow oil. 1H NMR (700 MHz, Chloroform-d) δ 1.14 (t, J = 7.3 Hz, 3H), 1.79–1.87 (m, 1H), 1.89–1.98 (m, 1H), 2.90 (d, J = 17.1 Hz, 1H), 3.58 (d, J = 17.2 Hz, 1H), 4.21 (dddd, J = 7.8, 3.9, 2.2, 2.2 Hz, 1H), 5.19 (dd, J = 2.2, 1.0 Hz, 1H), 6.13 (dd, J = 2.2, 1.0 Hz, 1H), 7.26 (m, 10H). 13C NMR (176 MHz, Chloroform-d) δ 9.7, 28.0, 50.98, 73.5, 80.1, 120.2, 125.1 (2 × C), 127.0, 127.9 (2 × C), 127.9, 128.3 (2 × C), 128.8 (2 × C), 142.2, 144.4, 147.6, 196.5. EI-MS [M]+ = 292.0. Anal. Calcd. for C20H20O2: C, 82.16; H, 6.90. Found: C, 82.37; H, 6.89.
6-Butyl-5-methylidene-2,2-diphenyltetrahydro-4H-pyran-4-one (11j) (55.8 mg, 91%) White solid 141 – 143 °C. 1H NMR (700 MHz, Chloroform-d) δ 0.88–0.98 (m, 1H), 1.02 (t, J = 7.3 Hz, 3H), 1.42–1.48 (m, 1H), 1.52–1.61 (m, 1H), 1.71–1.79 (m, 1H), 1.85–1.92 (m, 2H), 2.94 (d, J = 17.1 Hz, 1H), 3.63 (d, J = 17.1 Hz, 1H), 4.28 (dddd, J = 6.9, 4.4, 2.1, 2.1 Hz, 1H), 5.25 (dd, J = 2.1, 1.1 Hz, 1H), 6.16 (dd, J = 2.1, 1.1 Hz, 1H), 7.26 (m, 10H). 13C NMR (176 MHz, Chloroform-d) δ 14.2, 22.8, 27.4, 34.7, 51.0, 72.4, 80.1, 120.1, 125.1 (2 × C), 127.0, 127.9 (2 × C), 127.9, 128.3 (2 × C), 128.8 (2 × C), 142.2, 144.8, 147.6, 196.6. EI-MS [M]+ = 320.0. Anal. Calcd. for C22H24O2: C, 82.46; H, 7.55. Found: C, 82.66; H, 7.53.
6-Isopropyl-5-methylidene-2,2-diphenyltetrahydro-4H-pyran-4-one (11k) (34.9 mg, 57%) Pale yellow oil. 1H NMR (700 MHz, Chloroform-d) δ 1.09 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.9 Hz, 3H), 2.10 (heptd, J = 6.8, 2.8 Hz, 1H), 2.90 (d, J = 17.5 Hz, 1H), 3.59 (d, J = 17.5 Hz, 1H), 4.16–4.29 (m, 1H), 5.17 (dd, J = 2.0, 1.0 Hz, 1H), 6.21 (dd, J = 2.0, 1.0 Hz, 1H), 7.18–7.51 (m, 10H). 13C NMR (176 MHz, Chloroform-d) δ 15.7, 19.9, 33.5, 50.8, 77.0, 79.6, 121.2, 125.0 (2 × C), 127.0, 127.89 (3 × C), 128.3 (2 × C), 128.8 (2 × C), 142.0, 143.3, 147.7, 196.5. EI-MS [M]+ = 306.0. Anal. Calcd. for C21H22O2: C, 82.32; H, 7.24. Found: C, 82.07; H, 7.22.
5-Methylidene-2,2,6-triphenyltetrahydro-4H-pyran-4-one (11l) (57.9 mg, 85%) Pale yellow oil. 1H NMR (700 MHz, Chloroform-d) δ 3.12 (d, J = 17.0 Hz, 1H), 3.70 (d, J = 17.0 Hz, 1H), 4.76 (dd, J = 2.2, 1.1 Hz, 1H), 5.20 (dd, J = 2.2, 2.2 Hz, 1H), 6.13 (dd, J = 2.2, 1.1 Hz, 1H), 7.17–7.46 (m, 15H). 13C NMR (176 MHz, Chloroform-d) δ 51.5, 76.6, 81.3, 123.3, 125.2 (2 × C), 127.2, 128.0 (2 × C), 128.2 (3 × C), 128.4 (2 × C), 128.5, 128.7 (2 × C), 129.0 (2 × C), 140.3, 142.0, 145.2, 147.2, 196.0. EI-MS [M]+ = 340.0. Anal. Calcd. for C24H20O2: C, 84.68; H, 5.92. Found: C, 84.45; H, 5.90.
6′-Ethyl-5′-methylidene-5′,6′-dihydrospiro[fluorene-9,2′-pyran]-4′(3′H)-one (11m) (47.6 mg, 82%) Pale yellow oil. 1H NMR (700 MHz, Chloroform-d) δ 0.99 (t, J = 7.4 Hz, 3H), 1.78–1.88 (m, 1H), 1.96 (ddt, J = 14.6, 7.3, 3.6 Hz, 1H), 2.87 (d, J = 16.7 Hz, 1H), 3.12 (d, J = 16.7 Hz, 1H), 5.00 (dddd, J = 6.1, 4.0, 2.1, 2.1 Hz, 1H), 5.53 (dd, J = 2.1, 1.2 Hz, 1H), 6.49 (dd, J = 2.1, 1.2 Hz, 1H), 7.24 (dd, J = 8.4, 7.5 Hz, 1H), 7.33 (dd, J = 8.4, 7.3 Hz, 1H), 7.39 (dd, J = 8.4, 7.6 Hz, 2H), 7.41 (d, J = 7.5 Hz, 1H), 7.46 (d, J = 7.4 Hz, 1H), 7.64 (d, J = 7.5 Hz, 1H), 7.68 (d, J = 7.6 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 9.0, 27.8, 47.3, 75.5, 82.6, 120.1, 120.8, 121.0, 123.9, 124.7, 127.6, 128.5, 129.5, 129.5, 139.0, 140.3, 144.7, 145.3, 147.4, 196.3. EI-MS [M]+ = 290.0. Anal. Calcd. for C20H18O2: C, 82.73; H, 6.25. Found: C, 83.01; H, 6.23.
6′-Butyl-5′-methylidene-5′,6′-dihydrospiro[fluorene-9,2′-pyran]-4′(3′H)-one (11n) (63.04 mg, 99%) Pale yellow oil. 1H NMR (700 MHz, Chloroform-d) δ 0.87 (t, J = 7.3 Hz, 3H), 1.26–1.49 (m, 4H), 1.79 (dddd, J = 14.0, 10.5, 7.1, 4.8 Hz, 1H), 1.90 (dddd, J = 14.3, 10.7, 5.7, 3.8 Hz, 1H), 2.88 (d, J = 16.6 Hz, 1H), 3.09 (d, J = 16.7 Hz, 1H), 5.03 (dddd, J = 7.2, 4.0, 2.1, 2.1 Hz, 1H), 5.53 (dd, J = 2.1, 1.2 Hz, 1H), 6.46 (dd, J = 2.1, 1.2 Hz, 1H), 7.24 (dd, J = 8.6, 7.5 Hz, 1H), 7.33 (dd, J = 8.6, 7.4 Hz, 1H), 7.37–7.40 (m, 2H), 7.41 (d, J = 7.5 Hz, 1H), 7.44 (d, J = 7.5 Hz, 1H), 7.63 (d, J = 7.6 Hz, 1H), 7.68 (d, J = 7.5 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 14.1, 22.8, 26.9, 34.6, 47.3, 74.7, 82.7, 120.1, 120.8, 120.8, 124.0, 124.7, 127.7, 128.5, 129.5, 129.5, 139.0, 140.3, 145.22, 145.4, 147.5, 196.4. EI-MS [M]+ = 318.0. Anal. Calcd. for C22H22O2: C, 82.99; H, 6.96. Found: C, 82.75; H, 6.97.
6′-Isopropyl-5′-methylidene-5′,6′-dihydrospiro[fluorene-9,2′-pyran]-4′(3′H)-one (11o) (35.3 mg, 58%) Pale yellow oil. 1H NMR (700 MHz, Chloroform-d) δ 0.99 (d, J = 6.9 Hz, 3H), 1.01 (d, J = 6.8 Hz, 3H), 2.11–2.17 (m, 1H), 2.71 (d, J = 17.1 Hz, 1H), 3.15 (d, J = 17.1 Hz, 1H), 4.86 (ddd, J = 3.2, 2.1 Hz, 1H), 5.55 (dd, J = 2.1, 1.2 Hz, 1H), 6.56 (dd, J = 2.2, 1.2 Hz, 1H), 7.21 (dd, J = 8.6, 7.5 Hz, 1H), 7.34 (dd, J = 8.5, 7.6 Hz, 1H), 7.36–7.43 (m, 3H), 7.47 (d, J = 7.5 Hz, 1H), 7.63 (d, J = 7.5 Hz, 1H), 7.68 (d, J = 7.5 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 15.7, 19.6, 33.9, 47.7, 79.3, 82.4, 120.0, 120.8, 122.0, 124.0, 124.9, 127.7, 128.5, 129.4, 129.5, 139.0, 140.4, 144.3, 145.2, 147.6, 196.3. EI-MS [M]+ = 304.0. Anal. Calcd. for C21H20O2: C, 82.86; H, 6.62. Found: C, 82.92; H, 6.61.
5′-Methylidene-6′-phenyl-5′,6′-dihydrospiro[fluorene-9,2′-pyran]-4′(3′H)-one (11p) (66.3 mg, 98%) Pale yellow oil. 1H NMR (700 MHz, Chloroform-d) δ 3.02 (d, J = 16.6 Hz, 1H), 3.30 (d, J = 16.5 Hz, 1H), 5.05 (dd, J = 2.1, 1.4 Hz, 1H), 5.99 (dd, J = 2.1, 2.1 Hz, 1H), 6.47 (dd, J = 2.1, 1.4 Hz, 1H), 7.28–7.44 (m, 9H), 7.51 (d, J = 7.6 Hz, 1H), 7.53 (d, J = 7.4 Hz, 1H), 7.63 (d, J = 7.5 Hz, 1H), 7.69 (d, J = 7.6 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 47.6, 78.6, 83.4, 120.2, 120.9, 124.1, 124.5, 124.8, 127.8, 128.2 (2 × C), 128.6 (2 × C), 128.7 (2 × C), 129.7, 129.7, 139.2, 140.0, 140.5, 144.8, 145.71, 146.9, 195.8. EI-MS [M]+ = 338.0. Anal. Calcd. for C24H18O2: C, 85.18; H, 5.36. Found: C, 85.01; H, 5.35.
3.2. Biology
3.2.1. Cell Culture
The breast cancer MCF-7, leukemia HL-60, and NALM-6 cell lines were obtained from the European Collection of Cell Cultures (ECACC). The MCF-7 cells were cultured in Minimum Essential Medium Eagle (MEME, Sigma-Aldrich, St. Louis, MO, USA) with glutamine (2 mM), Men Non-essential amino acid solution, gentamycin (5 μg/mL) and 10% heat-inactivated fetal bovine serum (FBS). HL-60 and NALM-6 cells were maintained in RPMI 1640 + Glutamax medium (Gibco/Life Technologies, Carlsbad, CA, USA), supplemented with 10% FBS, streptomycin and penicillin. Cells were maintained in the logarithmic phase at 37 °C under a 5% carbon dioxide atmosphere.
3.2.2. Cytotoxicity Determination (MTT Assay)
The MTT assay was performed according to a known procedure [18]. The cells were incubated with the analogs for 48 or 24 h. The absorbance of the blue formazan product was measured at 560 nm using FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC, San Jose, CA, USA) and compared with the control (untreated cells). All experiments were performed in triplicate.
3.2.3. Analysis of DNA Damage and Apoptosis by Flow Cytometry
DNA damage and apoptotic cell death were determined by flow cytometry using fluorescent antibodies for phosphorylated H2AX (Alexa Fluor 647 Mouse Anti-H2AX (pS139)) and cleaved PARP (PE Mouse Anti-Cleaved PARP (Asp214) Antibody) (BD Bioscience, San Jose, CA, USA), according to the manufacturer’s guidelines, as described in detail elsewhere [19].
3.2.4. Cell Cycle Analyses by Flow Cytometry
The cell cycle kinetics were determined using DAPI staining solution (BD Bioscience), according to the manufacturer guidelines, as described in detail elsewhere [20]. Cells were analyzed by flow cytometry using CytoFLEX (Beckman Coulter, Inc., Brea, CA, USA).
3.2.5. Statistical Analysis
Statistical analyses were performed using Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA). The data were expressed as means ± SEM. Statistical comparisons were assessed by a one-way ANOVA, followed by a post-hoc multiple comparison Student-Newman-Keuls test or Student t test. A probability level of 0.05 or lower was considered statistically significant; *** p < 0.001, ** p < 0.01, * p < 0.05.
4. Conclusions
The reported results showed that biologically important 5-methylidenetetrahydropyran-4-ones 11 can be effectively synthesized using the Horner-Wadsworth-Emmons reaction of easily accessible 3-diethoxyphosphoryldihydropyran-4-ones 10 with formaldehyde. Various substituents in position 2 can be introduced to the target compounds performing the reaction of dianion 6 with both, acyclic or cyclic symmetrical ketones. Furthermore, additional substituents can be placed in position 6 by the addition of selected Grignard reagents to 3-diethoxyphosphoryldihydropyran-4-ones 9. Therefore, the described methodology has a broad scope, as a variety of substituents can be introduced in these positions. Currently, the reaction of aldehydes and unsymmetrical ketones with dianion 6 is investigated to broaden the scope of the presented methodology. The biological evaluation of the obtained 5-methylidenetetrahydropyran-4-ones 11 confirmed our assumption that these compounds possess a strong cytotoxic activity against several cancer cell lines. Investigation of structure-activity relationship revealed that the i-propyl substituent in position 6 is crucial for activity. 6-Isopropyl-2,2-dimethyl-5-methylidenetetrahydropyran-4-one 11c, which was recognized as the most active compound against the HL-60 cancer cell line, was further tested in order to disclose its mechanism of action. The preliminary tests indicated that 11c induced apoptosis in HL-60 cells and caused the arrest of the cell cycle in the G2/M phase, which may suggest its cytotoxic and cytostatic activity. Further biological experiments are necessary to fully determine the mode of action of this analog in leukemic cells.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/25/3/611/s1, General procedures and characterization data for diethyl 4-hydroxy-2-oxoalkylphosphonates 7a–d, 3-diethoxyphosphoryldihydropyran-4-ones 9a–d and 2,2-dialkyl(diaryl)-6-alkyl(aryl)-5-methylenetetrahydro -4H-pyran-4-ones 10a–p. Copies of 1H, 13C and 31P NMR spectra of all obtained compounds.
Author Contributions
Conceptualization, T.J., A.J and M.M.; Data curation, T.B., J.D.-S. and U.K.; Formal analysis, T.B., J.K. and J.D.-S.; Funding acquisition, T.J., A.J. and M.M.; Investigation, T.B., J.K., J.D.-S. and U.K.; Methodology, T.J., J.K., J.D.-S. and A.J.; Project administration, T.J. and A.J.; Resources, T.B., J.K. and J.D.-S.; Supervision, T.J. and A.J.; Validation, J.K. and J.D.-S.; Visualization, T.B. and J.D.-S.; Writing—original draft T.J., A.J. and T.B.; Writing—review & editing, T.J. and A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Young Scientists’ Fund at the Faculty of Chemistry, Lodz University of Technology, Grant W-3D/FMN/24G/2019 and by a grant from the Medical University of Łódź No. 503/1-156-02/503-11-001-19-00.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Kędzia, J.; Bartosik, T.; Drogosz, J.; Janecka, A.; Krajewska, U.; Janecki, T. Synthesis and cytotoxic evaluation of 3-methylidenechroman-4-ones. Molecules 2019, 24, 1868. [Google Scholar] [CrossRef] [PubMed]
- Pięta, M.; Kędzia, J.; Kowalczyk, D.; Wojciechowski, J.; Wolf, W.M.; Janecki, T. Enantioselective synthesis of 5-methylidenedihydrouracils as potential anticancer agents. Tetrahedron 2019, 75, 2495–2505. [Google Scholar] [CrossRef]
- Jakubowski, R.; Pomorska, D.K.; Długosz, A.; Janecka, A.; Krajewska, U.; Różalski, M.; Mirowski, M.; Bartosik, T.; Janecki, T. Synthesis of 4,4-disubstituted 3-methylidenechroman-2-ones as potent anticancer agents. ChemMedChem 2017, 12, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Y.; Zhou, J.; Huang, C.-G.; Hu, Q.-F.; Huang, X.-Z.; Wang, W.; Zhang, L.-Z.; Li, G.-P.; Xia, F.-T. Two novel antiviral terpenoids from the cultured Perovskia atriplicifolia. Tetrahedron 2015, 71, 3844–3849. [Google Scholar] [CrossRef]
- Imai, H.; Suzuki, K.-I.; Morioka, M.; Numasaki, Y.; Kadota, S.; Nagai, K.; Sato, T.; Iwanami, M.; Saito, T. Okilactomycin, a novel antibiotic produced by a Streptomyces species. J. Antibiot. 1987, 40, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Nakai, R.; Kakita, S.; Asai, A.; Chiba, S.; Akinaga, S.; Mizukami, T.; Yamashita, Y. Chrolactomycin, a novel antitumor antibiotic produced by Streptomyces sp. J. Antibiot. 2001, 54, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, M.M.; Abaee, M.S.; Khakbaz, M.; Alishiri, T.; Samianifard, M.; Mesbah, A.W.; Harms, K. An efficient procedure for the synthesis of α,β-unsaturated ketones and its application to heterocyclic systems. Synthesis 2011, 23, 3821–3826. [Google Scholar] [CrossRef]
- Gu, X.; Wang, X.; Wang, F.; Sun, H.; Liu, J.; Xie, Y.; Xiang, M. Pyrrolidine-mediated direct preparation of (E)-monoarylidene derivatives of homo- and heterocyclic ketones with various aldehydes. Molecules 2014, 19, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Thottumkara, A.P.; Kurokawa, T.; Du Bois, J. Carbocyclization of unsaturated thioesters under palladium catalysis. Chem. Sci. 2013, 4, 2686–2689. [Google Scholar] [CrossRef]
- Okauchi, T.; Nagamori, H.; Kouno, R.; Ichikawa, J.; Minami, T. Synthesis of 5-diphenylphosphinoyl-2,3-dihydropyran-4-ones. Heterocycles 2000, 52, 1393–1398. [Google Scholar] [CrossRef]
- Janecki, T. Organophosphorus reagents as versatile tool in the synthesis of α-alkylidene-γ-butyrolactones and α-alkylidene-γ-butyrolactams. In Targets in Heterocyclic Systems-Chemistry and Properties; Orazio, A., Attanasi, D., Eds.; Spinelli, Italian Chemical Society: Roma, Italy, 2006; Volume 10, pp. 301–320. [Google Scholar]
- Albrecht, Ł.; Albrecht, A.; Janecki, T. α-Alkylidene-γ- and δ lactones and lactams in natural lactones and lactams. In Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity; Janecki, T., Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014; pp. 147–192. [Google Scholar]
- Wada, E.; Kanemasa, S.; Tsuge, O. New synthesis of 2-oxo-3-alkenylphosphonates and hetero diels-Alder reactions with vinyl ethers leading to 5-substituted 2-phosphinyl-2-cyclohexen-1-ones. Bull. Chem. Soc. Jpn. 1989, 62, 866–868. [Google Scholar] [CrossRef]
- Koszuk, J.; Bartosik, T.; Wojciechowski, J.; Wolf, W.M.; Janecka, A.; Drogosz, J.; Długosz, A.; Krajewska, U.; Mirowski, M.; Janecki, T. Synthesis of 3-methylidene-1-tosyl-2,3-dihydroquinolin-4-(1H)-ones as potent cytotoxic agents. Chem. Biodivers. 2018, 15, e1800242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Won, Y.; Ong, C.; Shen, H. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Pati, H.N.; Das, U.; Sharma, R.K.; Dimmock, J.R. Cytotoxic thiol alkylators. Mini Rev. Med. Chem. 2007, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.M. Will new agents impact survival in AML? Best Pr. Res. Clin. Haematol. 2019, 32, 101094. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gach, K.; Modranka, J.; Szymański, J.; Pomorska, D.; Krajewska, U.; Mirowski, M.; Janecki, T.; Janecka, A. Anticancer properties of new synthetic hybrid molecules combining naphtho[2,3-b]furan-4,9-dione or benzo[f]indole-4,9-dione motif with phosphonate subunit. Eur. J. Med. Chem. 2016, 120, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Gach, K.; Szymański, J.; Pomorska, D.; Długosz, A.; Modranka, J.; Janecki, T.; Janecka, A. Combined effects of anticancer drugs and new synthetic α-methylene-δ-lactones on MCF-7 cells. Tumour Biol. 2015, 36, 5971–5977. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 7a–d, 9a–d and 10a–n,p are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).