Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters
Abstract
1. Introduction
2. Results and Discussion
Catalytic Performance
3. Experimental
3.1. Catalyst Development
3.2. Catalytic Reaction
3.3. Catalyst Description
3.3.1. Nitrogen Physical Adsorption
3.3.2. Temperature Programmed Reduction (TPR)
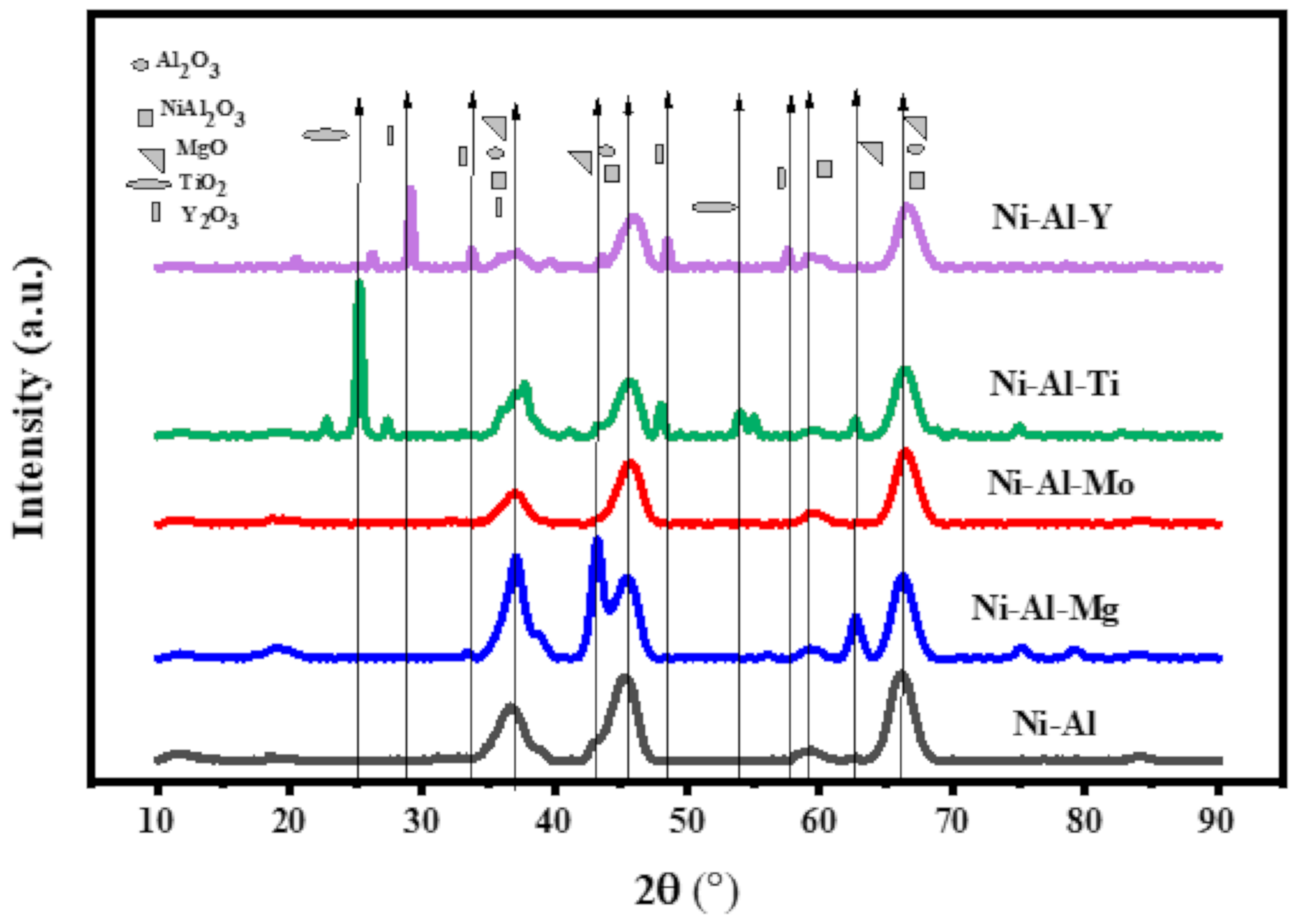
3.3.3. X-ray Diffractogram (XRD)
3.3.4. Thermo-Gravimetric Analysis (TGA)
3.3.5. Raman Spectroscopy
3.3.6. CO2-TPD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaddeche, D.; Djaidja, A.; Barama, A. Partial oxidation of methane on co-precipitated Ni–Mg/Al catalysts modified with copper or iron. Int. J. Hydrog. Energy 2017, 42, 15002–15009. [Google Scholar] [CrossRef]
- Silva, C.R.B.; da Conceição, L.; Ribeiro, N.F.P.; Souza, M.M.V.M. Partial oxidation of methane over Ni–Co perovskite catalysts. Catal. Commun. 2011, 12, 665–668. [Google Scholar] [CrossRef]
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Pantaleo, G.; Parola, V.L.; Deganello, F.; Singha, R.K.; Bal, R.; Venezia, A.M. Ni/CeO2 catalysts for methane partial oxidation: Synthesis driven structural and catalytic effects. Appl. Catal. B Environ. 2016, 189, 233–241. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Atia, H.; Abu-Dahrieh, J.K.; Ibrahim, A.A.; Eckelt, R.; Armbruster, U.; Abasaeed, A.E.; Fakeeha, A.H. Hydrogen production from CH4 dry reforming over Sc promoted Ni/MCM-41. Int. J. Hydrog. Energy 2019, 44, 20770–20781. [Google Scholar] [CrossRef]
- Vahid Shahed, G.; Taherian, Z.; Khataee, A.; Meshkani, F.; Orooji, Y. Samarium-impregnated nickel catalysts over SBA-15 in steam reforming of CH4 process. J. Ind. Eng. Chem. 2020, 86, 73–80. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, H.Y.; Cho, J.M.; Choi, J.-H.; Bae, J.W. Ni/M-Al2O3 (M=Sm, Ce or Mg) for combined steam and CO2 reforming of CH4 from coke oven gas. J. CO2 Util. 2017, 21, 211–218. [Google Scholar] [CrossRef]
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-art catalysts for CH4 steam reforming at low temperature. Int. J. Hydrog. Energy 2014, 39, 1979–1997. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Yoon, W.-L.; Rhee, Y.-W.; Seo, Y.-S. The surfactant-assisted Ni–Al2O3 catalyst prepared by a homogeneous precipitation method for CH4 steam reforming. Int. J. Hydrog. Energy 2012, 37, 9340–9350. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Atia, H.; Fakeeha, A.H.; Armbruster, U.; Abasaeed, A.E.; Frusteri, F. Evaluation of Co-Ni/Sc-SBA–15 as a novel coke resistant catalyst for syngas production via CO2 reforming of methane. Appl. Catal. A Gen. 2018, 567, 102–111. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Chen, Y.; Ma, S.; Li, Q.; Hu, X.; Wang, Z.; Buckley, C.E.; Dong, D. Highly stable nanofibrous La2NiZrO6 catalysts for fast methane partial oxidation. Fuel 2020, 265, 116861. [Google Scholar] [CrossRef]
- Shareei, M.; Taghvaei, H.; Azimi, A.; Shahbazi, A.; Mirzaei, M. Catalytic DBD plasma reactor for low temperature partial oxidation of methane: Maximization of synthesis gas and minimization of CO2. Int. J. Hydrog. Energy 2019, 44, 31873–31883. [Google Scholar] [CrossRef]
- Shishido, T.; Sukenobu, M.; Morioka, H.; Kondo, M.; Wang, Y.; Takaki, K.; Takehira, K. Partial oxidation of methane over Ni/Mg-Al oxide catalysts prepared by solid phase crystallization method from Mg-Al hydrotalcite-like precursors. Appl. Catal. A Gen. 2002, 223, 35–42. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ruckenstein, E. Partial Oxidation of Methane to Synthesis Gas over Alkaline Earth Metal Oxide Supported Cobalt Catalysts. J. Catal. 2001, 199, 309–317. [Google Scholar] [CrossRef]
- Khine, M.S.S.; Chen, L.; Zhang, S.; Lin, J.; Jiang, S.P. Syngas production by catalytic partial oxidation of methane over (La0.7A0.3) BO3 (A = Ba, Ca, Mg, Sr, and B = Cr or Fe) perovskite oxides for portable fuel cell applications. Int. J. Hydrog. Energy 2013, 38, 13300–13308. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Jiménez-González, C.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Partial oxidation of methane to syngas on bulk NiAl2O4 catalyst. Comparison with alumina supported nickel, platinum and rhodium catalysts. Appl. Catal. A Gen. 2012, 437–438, 53–62. [Google Scholar] [CrossRef]
- Araújo, J.C.S.; Oton, L.F.; Oliveira, A.C.; Lang, R.; Otubo, L.; Bueno, J.M.C. On the role of size controlled Pt particles in nanostructured Pt-containing Al2O3 catalysts for partial oxidation of methane. Int. J. Hydrog. Energy 2019, 44, 27329–27342. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Duquesne, S.; Carrazán, S.R.G.; Soria, M.A.; Ruíz, P. Influence of the products of the partial oxidation of methane (POM) on the catalytic performances of Rh/Ti-modified support catalysts. Appl. Catal. A Gen. 2011, 394, 245–256. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.-Z.; Lin, J.-D.; Chen, Z.-Q.; Wang, Y. Crucial support effect on the durability of Pt/MgAl2O4 for partial oxidation of methane to syngas. Appl. Catal. B Environ. 2018, 231, 292–298. [Google Scholar] [CrossRef]
- Ding, C.; Wang, J.; Guo, S.; Ma, Z.; Li, Y.; Ma, L.; Zhang, K. Abundant hydrogen production over well dispersed nickel nanoparticles confined in mesoporous metal oxides in partial oxidation of methane. Int. J. Hydrog. Energy 2019, 44, 30171–30184. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Ran, R.; Park, H.J.; Kwak, C.; Shao, Z. Physically mixed LiLaNi- Al2O3 and copper as conductive anode catalysts in a solid oxide fuel cell for methane internal reforming and partial oxidation. Int. J. Hydrog. Energy 2011, 36, 5632–5643. [Google Scholar] [CrossRef]
- Ding, C.; Wang, J.; Jia, Y.; Ai, G.; Liu, S.; Liu, P.; Zhang, K.; Han, Y.; Ma, X. Anti-coking of Yb-promoted Ni/Al2O3 catalyst in partial oxidation of methane. Int. J. Hydrog. Energy 2016, 41, 10707–10718. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, G.; Sheng, S.; Yang, W. Deactivation studies over NiO/γ-Al2O3 catalysts for partial oxidation of methane to syngas. Catal. Today 2000, 63, 517–522. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Zhang, Z.; Verykios, X.E. Catalytic partial oxidation of methane to synthesis gas over Ni-based catalysts: I. Catalyst performance characteristics. J. Catal. 1998, 179, 283–291. [Google Scholar] [CrossRef]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Park, K.S.; Son, M.; Park, M.J.; Kim, D.H.; Kim, J.H.; Park, S.H.; Choi, J.H.; Bae, J.W. Adjusted interactions of nickel nanoparticles with cobalt-modified MgAl2O4-SiC for an enhanced catalytic stability during steam reforming of propane. Appl. Catal. A Gen. 2018, 549, 117–133. [Google Scholar] [CrossRef]
- Min, J.E.; Lee, Y.J.; Park, H.G.; Zhang, C.; Jun, K.W. Carbon dioxide reforming of methane on Ni-MgO-Al2O3 catalysts prepared by sol-gel method: Effects of Mg/Al ratios. J. Ind. Eng. Chem. 2015, 26, 375–383. [Google Scholar] [CrossRef]
- Jilei, Y.; Zengxi, L.; Huachao, D.; Yuan, L. Lanthanum Modified Ni/y-A12O3 Catalysts for Partial Oxidation of Methane. J. Rare Earths 2006, 24, 302–308. [Google Scholar]
- Zhang, R.J.; Xia, G.F.; Li, M.F.; Wu, Y.; Nie, H.; Li, D.D. Effect of support on catalytic performance of Ni-based catayst in methane dry reforming. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Jalali, R.; Rezaei, M.; Nematollahi, B.; Baghalha, M. Preparation of Ni/MeAl2O4-MgAl2O4 (Me = Fe, Co, Ni, Cu, Zn, Mg) nanocatalysts for the syngas production via combined dry reforming and partial oxidation of methane. Renew. Energy 2020, 149, 1053–1067. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Roldan Cuenya, B.; Fierro, J.L.G. Partial Oxidation of Methane to Syngas Over Nickel-Based Catalysts: Influence of Support Type, Addition of Rhodium, and Preparation Method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef]
- Dias, J.A.C.; Assaf, J.M. Influence of calcium content in Ni/CaO/γ-Al2O3 catalysts for CO2-reforming of methane. In Proceedings of the Catalysis Today; Elsevier: Amsterdam, The Netherlands, 2003; Volume 85, pp. 59–68. [Google Scholar]
- Claude, V.; Mahy, J.G.; Tilkin, R.G.; Lambert, S.D. Enhancement of the catalytic performances and lifetime of Ni/γ- Al2O3 catalysts for the steam toluene reforming via the combination of dopants: Inspection of Cu, Co, Fe, Mn, and Mo species addition. Mater. Today Chem. 2020, 15, 100229. [Google Scholar] [CrossRef]
- Jing, Z.; Zhang, T.; Shang, J.; Zhai, M.; Yang, H.; Qiao, C.; Ma, X. Influence of Cu and Mo components of γ-Al2O3 supported nickel catalysts on hydrodeoxygenation of fatty acid methyl esters to fuel-like hydrocarbons. J. Fuel Chem. Technol. 2018, 46, 427–440. [Google Scholar] [CrossRef]
- Shah, M.; Bordoloi, A.; Nayak, A.K.; Mondal, P. Effect of Ti/Al ratio on the performance of Ni/TiO2-Al2O3 catalyst for methane reforming with CO2. Fuel Process. Technol. 2019, 192, 21–35. [Google Scholar] [CrossRef]
- Cheng, L.J.; Liu, Z.; Yuan, S.L.; Hu, X.; Zhang, B.; Jiang, Y. Preparation of Ag-Mn/γ-Al2O3-TiO2 catalysts by complexation-impregnation process with citric acid and its application in propane catalytic combustion. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2019, 47, 1379–1385. [Google Scholar] [CrossRef]
- Zhang, P.; Mu, F.; Zhou, Y.; Long, Y.; Wei, Q.; Liu, X.; You, Q.; Shan, Y.; Zhou, W. Synthesis of highly ordered TiO2-Al2O3 and catalytic performance of its supported NiMo for HDS of 4, 6-dimethyldibenzothiophene. Catal. Today 2020. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Haghighi, M.; Babaluo, A.A.; Talkhoncheh, S.K. Sono-synthesis and characterization of bimetallic Ni-Co/Al2O3-MgO nanocatalyst: Effects of metal content on catalytic properties and activity for hydrogen production via CO2 reforming of CH4. Ultrason. Sonochem. 2016, 31, 173–183. [Google Scholar] [CrossRef]
- Alabi, W.O. CO2 reforming of CH4 on Ni-Al-Ox catalyst using pure and coal gas feeds: Synergetic effect of CoO and MgO in mitigating carbon deposition. Environ. Pollut. 2018, 242, 1566–1576. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Cold plasma dielectric barrier discharge reactor for dry reforming of methane over Ni/ɤ-Al2O3-MgO nanocomposite. Fuel Process. Technol. 2018, 178, 166–179. [Google Scholar] [CrossRef]
- Jang, W.J.; Jung, Y.S.; Shim, J.O.; Roh, H.S.; Yoon, W.L. Preparation of a Ni-MgO-Al2O3 catalyst with high activity and resistance to potassium poisoning during direct internal reforming of methane in molten carbonate fuel cells. J. Power Sources 2018, 378, 597–602. [Google Scholar] [CrossRef]
- Özdemir, H.; Faruk Öksüzömer, M.A. Synthesis of Al2O3, MgO and MgAl2O4 by solution combustion method and investigation of performances in partial oxidation of methane. Powder Technol. 2020, 359, 107–117. [Google Scholar] [CrossRef]
- Xu, L.; Yin, X.L.; Wang, N.; Chen, M. Effect of Y2O3 addition on the densification, microstructure and mechanical properties of MgAl2O4[sbnd]CaAl4O7[sbnd]CaAl12O19composites. J. Alloys Compd. 2017, 702, 472–478. [Google Scholar] [CrossRef]
- Rittidech, A.; Somrit, R.; Tunkasiri, T. Effect of adding Y2O3 on structural and mechanical properties of Al2O3-ZrO2 ceramics. In Proceedings of the Ceramics International; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. S433–S436. [Google Scholar]
- Santos, D.C.R.M.; Madeira, L.; Passos, F.B. The effect of the addition of Y2O3 to Ni/α-Al2O3 catalysts on the autothermal reforming of methane. Catal. Today 2010, 149, 401–406. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, R.; Huang, S.; Chen, W.; Shi, Q. Ni/Y2O3-Al2O3 catalysts for hydrogen production from steam reforming of ethanol at low temperature. J. Rare Earths 2012, 30, 683–690. [Google Scholar] [CrossRef]
- Sun, L.; Tan, Y.; Zhang, Q.; Xie, H.; Song, F.; Han, Y. Effects of Y2O3-modification to Ni/γ-Al2O3 catalysts on autothermal reforming of methane with CO2 to syngas. Int. J. Hydrog. Energy 2013, 38, 1892–1900. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef]
- Costa, D.S.; Gomes, R.S.; Rodella, C.B.; da Silva, R.B.; Fréty, R.; Teixeira Neto, É.; Brandão, S.T. Study of nickel, lanthanum and niobium-based catalysts applied in the partial oxidation of methane. Catal. Today 2020, 344, 15–23. [Google Scholar] [CrossRef]
- Cheephat, C.; Daorattanachai, P.; Devahastin, S.; Laosiripojana, N. Partial oxidation of methane over monometallic and bimetallic Ni-, Rh-, Re-based catalysts: Effects of Re addition, co-fed reactants and catalyst support. Appl. Catal. A Gen. 2018, 563, 1–8. [Google Scholar] [CrossRef]





| Samples | SBET (m2/g) | VP (cm3/g) | dp (nm) |
|---|---|---|---|
| Ni-Al | 173.1 | 0.613 | 12.20 |
| Ni-Al-Mo | 161.0 | 0.558 | 12.42 |
| Ni-Al-Ti | 165.0 | 0.586 | 12.71 |
| Ni-Al-Mg | 172.8 | 0.603 | 12.46 |
| Ni-Al-Y | 176.3 | 0.630 | 12.35 |
| Sample | Mass mg | Methane/Oxygen | Space Velocity (ml/min) | Test Temperature (°C) | Methane Conversion (%) | Reference |
|---|---|---|---|---|---|---|
| La Ni0.5Nb0.5 O3 | 30 | 2:1 | 100 | 750 | 64 | [50] |
| 10% Ni/NiAl2O4-MgAl2O4 | 100 | CH4: CO2: O2 2:1:0.5 | 140 | 700 | 70 | [29] |
| Ni0.05Cu0.05Mg0.9/Al0.5 | 200 | 2:1 | 60 | 750 | 88 | [1] |
| 5%Ni/Al2O3 | 100 | 2:1 | 40.6 | 750 | 85 | [31] |
| 10%Ni+0.1%Rh/Al2O3 | 100 | 2:1 | 40.6 | 750 | 88 | [31] |
| 10%Ni+1%Re/γ-Al2O3 | 100 | 2:1 | 100 | 600 | 66.2 | [51] |
| 10%Ni/Al2O3+Mg | 100 | 2:1 | 32.5 | 650 | 92 | This work |
| Sample Name | Sample Formation |
|---|---|
| Ni-Al | 10%Ni/90% Al2O3 |
| Ni-Al-Mo | 10%Ni/10%Mo+80% Al2O3 |
| Ni-Al-Mg | 10%Ni/10%Mg+80% Al2O3 |
| Ni-Al-Ti | 10%Ni/10%Ti+80%Al2O3 |
| Ni-Al-Y | 10%Ni/10%Y+80%Al2O3 |
Sample Availability: Structure of the compounds and trajectories are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.A.; Khan, W.U.; Al-Mubaddel, F.; Al-Fatesh, A.S.; Kasim, S.O.; Mahmud, S.L.; Al-Zahrani, A.A.; Siddiqui, M.R.H.; Fakeeha, A.H. Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters. Molecules 2020, 25, 5029. https://doi.org/10.3390/molecules25215029
Ibrahim AA, Khan WU, Al-Mubaddel F, Al-Fatesh AS, Kasim SO, Mahmud SL, Al-Zahrani AA, Siddiqui MRH, Fakeeha AH. Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters. Molecules. 2020; 25(21):5029. https://doi.org/10.3390/molecules25215029
Chicago/Turabian StyleIbrahim, Ahmed A., Wasim U. Khan, Fahad Al-Mubaddel, Ahmed S. Al-Fatesh, Samsudeen O. Kasim, Sofiu L. Mahmud, Ateyah A. Al-Zahrani, M. Rafiq H. Siddiqui, and Anis H. Fakeeha. 2020. "Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters" Molecules 25, no. 21: 5029. https://doi.org/10.3390/molecules25215029
APA StyleIbrahim, A. A., Khan, W. U., Al-Mubaddel, F., Al-Fatesh, A. S., Kasim, S. O., Mahmud, S. L., Al-Zahrani, A. A., Siddiqui, M. R. H., & Fakeeha, A. H. (2020). Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters. Molecules, 25(21), 5029. https://doi.org/10.3390/molecules25215029









