The Impact of Herbal Infusion Consumption on Oxidative Stress and Cancer: The Good, the Bad, the Misunderstood
Abstract
1. Introduction
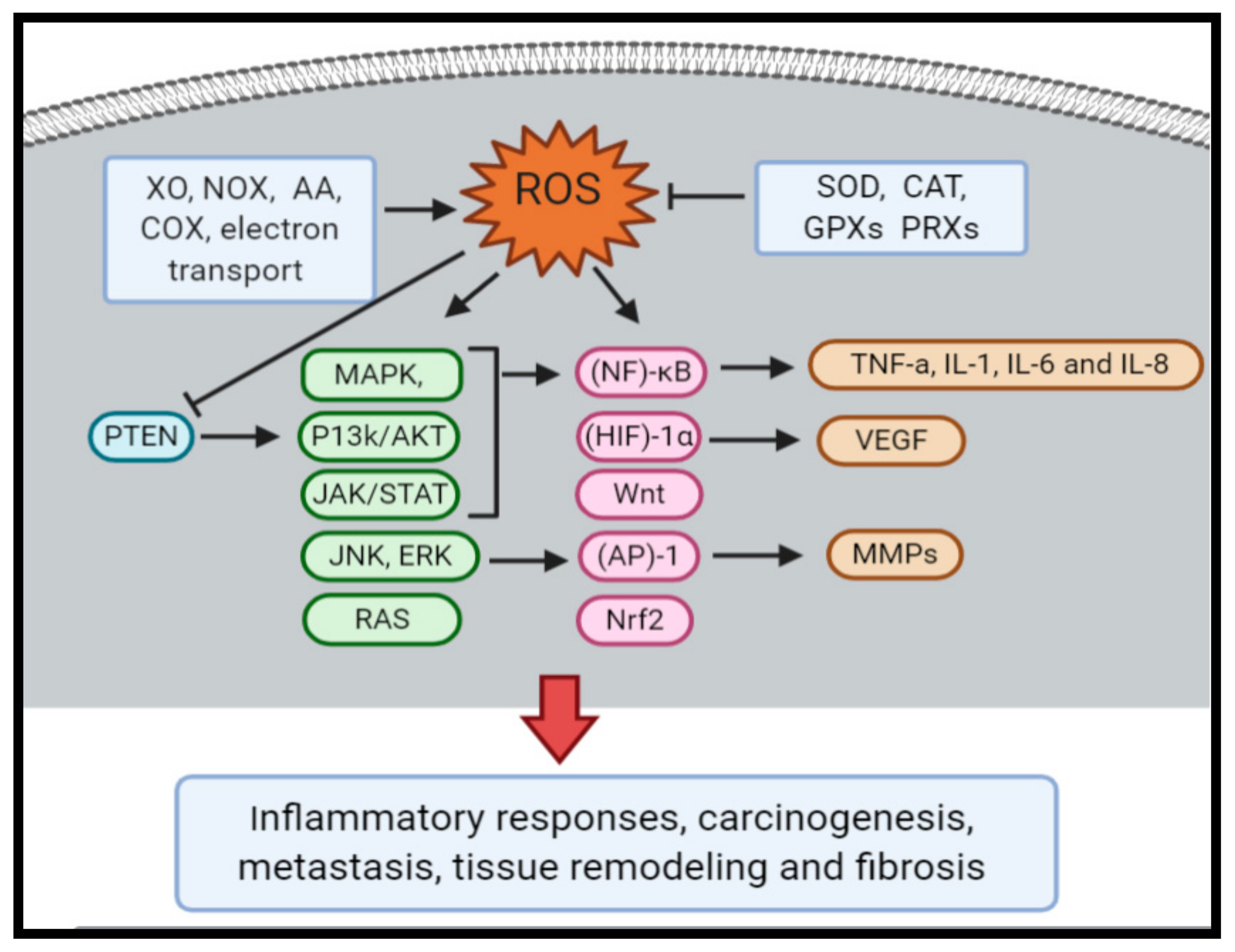
Oxidative Stress and Cancer
2. Herbal Infusions Antioxidant and Anticancer Capacities
2.1. Lemon and Ginger Combination
2.2. Wild Thyme (Thymus Serpyllum)
2.3. Marjoram (Organum Majorana)
2.4. Palestinian Herbal Mix
2.5. Lebanese Herbal Mix
2.6. Roselle (Hibiscus sabdariffa L.f)
2.7. Pomegranate (Punica granatum)
2.8. Anise Seeds (Pimpinella anisum L.)
2.9. Cumin (Cuminum cyminum)
2.10. Lemon Balm (Mellissa officinalis L.)
2.11. Rosemary (Rosmarinus officinalis L.)
3. Herbal Infusions in Human Clinical Trials
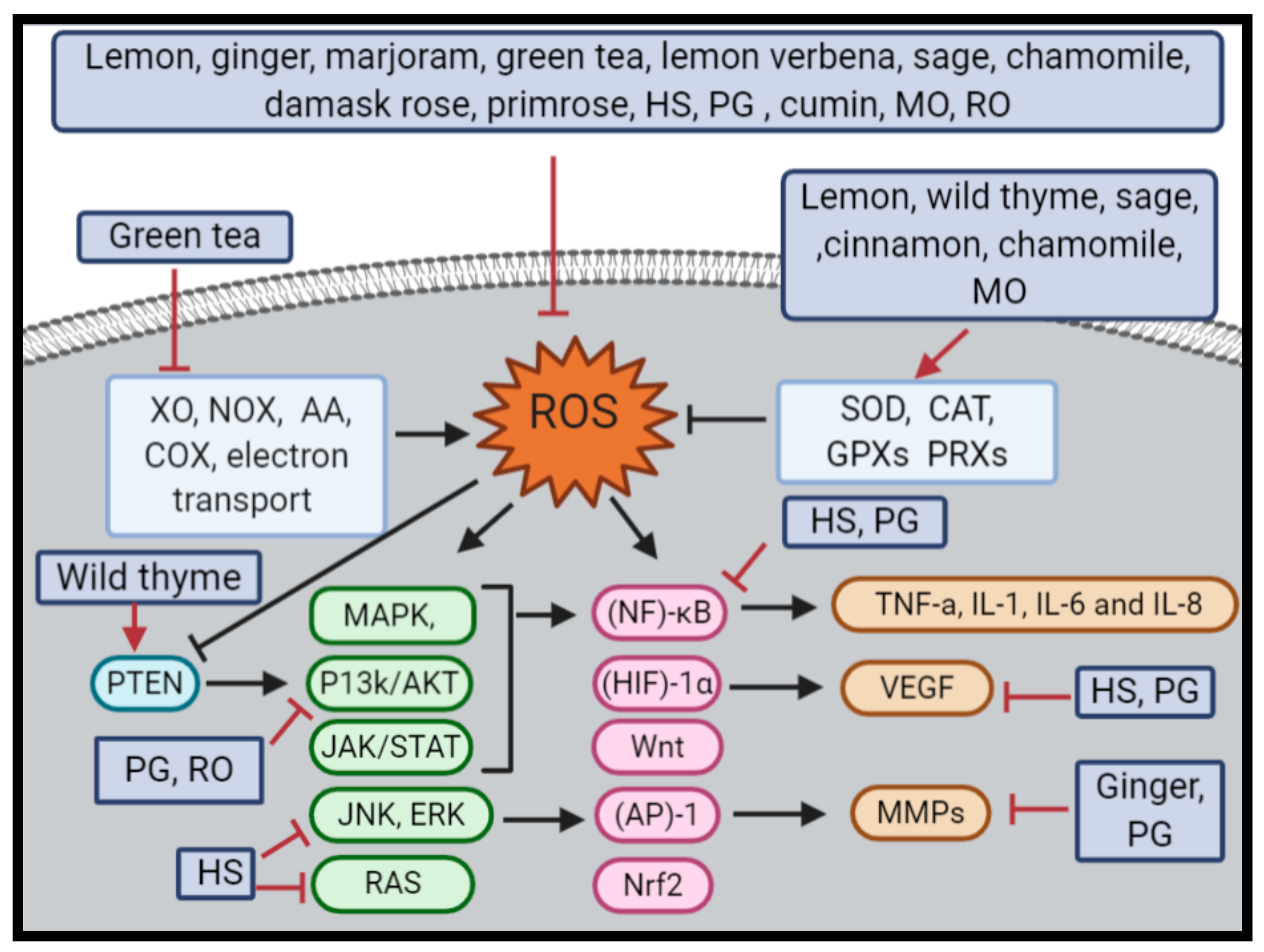
4. Herbal Infusion Contradictory Effects
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-Hydroxynonenal |
| 4T1 | Breast cancer (mammary gland) cell line |
| A2780 | Human ovarian cancer cell line |
| A375 | Human melanoma cell line |
| A431 | Epidermoid carcinoma |
| A549 | Adenocarcinomic human alveolar basal epithelial cell line |
| AA | Arachidonic Acid |
| ABTS assay | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AGS | Human gastric carcinoma cell line |
| AKT | Protein kinase B |
| ALT | Alanine transaminase |
| AMP | Adenosine monophosphate-activated protein kinase |
| AP-1 | Activator Protein -1 |
| AST | Aspartate transaminase |
| BHT | Butylated hydroxytoluene |
| Caco-2 | Colon cancer cell line |
| CAT | Catalase |
| CC1(4)) | Carbon tetrachloride |
| CD31 | Cluster of Differentiation 31 |
| Cdks | Cyclin-dependent kinase complex |
| c-met | Tyrosine-protein kinase Met |
| CoA | Coenzyme A reductase |
| COX | Cyclooxygenase |
| DJ-1 | Protein deglycase |
| DNA | Deoxyriboncleic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EAC | Ehrlich-ascites-carcinoma |
| eNOS | Endothelial Nitric Oxide Synthase |
| ERK | Extracellular signal-regulated kinases |
| ES-2 | Human ovarian cancer cell line |
| Fe-NTA | Ferric nitrilotriacetate |
| FPG | Fasting plasma glucose |
| FRAP | Ferric ion reducing antioxidant power |
| GC-FID | Gas Chromatography with Flame Ionization Detection |
| GC-MS analysis | Gas chromatography–mass spectrometry analysis |
| GPX | Glutathione peroxidase |
| Gr | Glutathione reductase |
| GSH | Glutathione |
| GST | Glutathione-S-transferase |
| H2O2 | Hydrogen peroxide |
| HCC | Hepatocellular carcinoma |
| HCT-116 | Human Colon Tumour Cell line 116 |
| HDL | High-density lipoprotein |
| HEK293 | Human embryonic kidney 293 |
| HeLa | Human epithelioid cervix carcinoma |
| HepG2 | Hepatocellular cancer cell lines |
| HIF-1α | Hypoxia Inducible Factor -1α |
| HL-60 | Human cancer promyelocytic leukemia |
| HS | Hibiscus sabdariffa L. |
| HT-29 | Human colon cancer cell line |
| HUVEC | Human umbilical vein endothelial cell |
| IMR32 | Human neuroblastoma cell lines |
| JAK/STAT | Janus kinase/signal transducer and activator of Transcription proteins |
| JNK | Mitogen-activated protein kinase 8 |
| KB | Oral squamous cell carcinoma |
| LC–ESI-MS/MS analysis | Liquid chromatography positive ion electrospray ionization tandem mass spectrometry |
| LDL | Low-density lipoproteins |
| LNCaP | Androgen-sensitive human prostate adenocarcinoma cells |
| LNM35 | Human lung cancer cells |
| MAPK | Mitogen-activated protein kinase |
| MCF7 | Michigan Cancer Foundation-7 (breast cancer cells) |
| MDA | Malondialdehyde |
| MDA-MB 231 | Epithelial, human breast cancer cell line |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP9 | Matrix metallopeptidase 9 |
| MMPs | Matrix metalloproteinases |
| mTOR | The mammalian target of rapamycin |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| PH oxidase (NOX) | Nicotinamide Adenine Dinucleotide Phosphate oxidase |
| NCI-H460 | Human Lung Carcinoma |
| NF | Nuclear Factor |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIH3T3 | Mouse embryonic fibroblast cells |
| NOS | Nitric oxide synthases |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ovo CAM model | In Ovo Chick Chorioallantoic Membrane |
| p15 | Tumor suppressor protein |
| p16 | Tumor suppressor protein |
| p21 | Cyclin-dependent kinase inhibitor 1 |
| P38 | P38 mitogen-activated protein kinases |
| p38MAPK | P38 mitogen-activated protein kinases |
| P450 complex | Cytochromes P450 |
| P53 | Tumor suppressor protein |
| p70S6K | Ribosomal protein S6 kinase beta-1 |
| p90Rsk | 90 kDa ribosomal s6 kinases |
| PC3 | Prostate cancer cell line |
| Pfrap | Potassium ferricyanide reducing power |
| PI3K | Phosphoinositide 3-kinases |
| PN | Punicalagin |
| PPAR-γ | Peroxisome proliferator-activated receptor |
| PRXs | Peroxiredoxins |
| PTEN | Phosphatase and tensin homolog |
| PTP-1B | Protein-tyrosine phosphatase 1B |
| RA | Rosmarinic acid |
| RAS | Rat sarcoma |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| S phase | Synthesis Phase |
| SiHa | Human cervical carcinoma |
| SK-N-MC | Human Neuroblastoma Cell Line |
| SOD | Superoxide dismutase |
| SW480 | Human colorectal carcinoma |
| T47D | Human breast cancer cell line |
| T-47-D | Human breast cancer cell line |
| TAC | Total anthocyanin content |
| TC | Total cholesterol |
| TG | Triglyceride |
| THP-1 | Human monocytic cell line |
| tNOX | Tumor-associated NADH oxidase |
| TPC | Total phenolic content |
| TFC | Total flavonoids concentration |
| TRX | Thioredoxin reductase |
| UPLC-PDA-MS | Ultra-performance liquid chromatography (UPLC) coupled to photodiode array detection (PDA) and electrospray ionization (ESI) tandem mass spectrometry (MS) |
| UV radiation | Ultraviolet radiation |
| VEGF | Vascular endothelial growth factor |
| WRL-68 | Human hepatic cell line |
| XO | Xanthine oxidase |
| ZR75 | Human breast cancer cell line |
| β-catenin/Wnt | β-Catenin/Wingless-related integration |
References
- Kennedy, E. Nutrition policy in the US: 50 years in review. Asia Pac. J. Clin. Nutr. 2008, 17, 340–342. [Google Scholar] [PubMed]
- Benzie, I.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kaliora, A.; Kogiannou, D.A.; Kefalas, P.; Papassideri, I.S.; Kalogeropoulos, N. Phenolic profiles and antioxidant and anticarcinogenic activities of Greek herbal infusions; balancing delight and chemoprevention? Food Chem. 2014, 142, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Al Obaydi, M.F.; Hamed, W.M.; Al Kury, L.T.; Talib, W.H. Terfezia boudieri: A Desert Truffle With Anticancer and Immunomodulatory Activities. Front. Nutr. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Ipek, E.; Zeytinoğlu, H.; Okay, S.; Tuylu, B.A.; Kurkcuoglu, M.; Başer, K.H.C. Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem. 2005, 93, 551–556. [Google Scholar] [CrossRef]
- Talib, W.H. Consumption of garlic and lemon aqueous extracts combination reduces tumor burden by angiogenesis inhibition, apoptosis induction, and immune system modulation. Nutrition 2017, 43, 89–97. [Google Scholar] [CrossRef]
- Talib, W.H. Regressions of Breast Carcinoma Syngraft Following Treatment with Piperine in Combination with Thymoquinone. Sci. Pharm. 2017, 85, 27. [Google Scholar] [CrossRef]
- Talib, W.H.; Al Kury, L.T. Parthenolide inhibits tumor-promoting effects of nicotine in lung cancer by inducing P53-dependent apoptosis and inhibiting VEGF expression. Biomed. Pharmacother. 2018, 107, 1488–1495. [Google Scholar] [CrossRef]
- Talib, W.H.; Al-Hadid, S.A.; Ali, M.B.W.; Al-Yasari, I.H.; Ali, M.R.A. Role of curcumin in regulating p53 in breast cancer: An overview of the mechanism of action. Breast Cancer (Dove. Med. Press) 2018, 10, 207–217. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Jamous, R.M. Herbal preparation use by patients suffering from cancer in Palestine. Complement. Ther. Clin. Pr. 2011, 17, 235–240. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shadboorestan, A. Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr. Cancer 2015, 68, 29–39. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Boil. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; De Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Boil. 2016, 37, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Boil. 2017, 11, 240–253. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free. Radic. Boil. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Saed, G.M.; Diamond, M.P.; Fletcher, N.M. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 2017, 145, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, D.; Terashvili, M.; Wickramasekera, N.; Zhang, D.X.; Rau, N.; Miura, H.; Harder, D.R. Redox Signaling via Oxidative Inactivation of PTEN Modulates Pressure-Dependent Myogenic Tone in Rat Middle Cerebral Arteries. PLoS ONE 2013, 8, e68498. [Google Scholar] [CrossRef]
- Niecknig, H.; Tug, S.; Reyes, B.D.; Kirsch, M.; Fandrey, J.; Berchner-Pfannschmidt, U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free. Radic. Res. 2012, 46, 705–717. [Google Scholar] [CrossRef]
- Coso, S.; Harrison, I.; Harrison, C.B.; Vinh, A.; Sobey, C.G.; Drummond, G.R.; Williams, E.D.; Selemidis, S. NADPH Oxidases as Regulators of Tumor Angiogenesis: Current and Emerging Concepts. Antioxid. Redox Signal. 2012, 16, 1229–1247. [Google Scholar] [CrossRef]
- Toyokuni, S. Oxidative stress as an iceberg in carcinogenesis and cancer biology. Arch. Biochem. Biophys. 2016, 595, 46–49. [Google Scholar] [CrossRef]
- Bagati, A.; Moparthy, S.; Fink, E.E.; Bianchi-Smiraglia, A.; Yun, D.H.; Kolesnikova, M.; Udartseva, O.; Wolff, D.W.; Roll, M.V.; Lipchick, B.C.; et al. KLF9-dependent ROS regulate melanoma progression in stage-specific manner. Oncogene 2019, 38, 3585–3597. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef] [PubMed]
- Wiel, C.; Le Gal, K.; Ibrahim, M.X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T.; et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell 2019, 178, 330–345.e22. [Google Scholar] [CrossRef] [PubMed]
- Cordani, M.; Butera, G.; Pacchiana, R.; Masetto, F.; Mullappilly, N.; Riganti, C.; Donadelli, M. Mutant p53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules 2020, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Fu, J.; Yu, T.; Xu, A.; Qin, W.; Yang, Z.; Chen, Y.; Wang, H. Contradictory effects of mitochondria- and non-mitochondria-targeted antioxidants on hepatocarcinogenesis by altering DNA repair in mice. Hepatology 2018, 67, 623–635. [Google Scholar] [CrossRef]
- Li, S.; Li, S.-K.; Gan, R.-Y.; Song, F.-L.; Kuang, L.; Li, H.-B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant Activity of Limonene on Normal Murine Lymphocytes: Relation to H2O2Modulation and Cell Proliferation. Basic Clin. Pharmacol. Toxicol. 2009, 106, 38–44. [Google Scholar] [CrossRef]
- De Almeida, A.A.C.; De Carvalho, R.B.F.; Silva, O.A.; De Sousa, D.P.; De Freitas, R.M. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol. Biochem. Behav. 2014, 118, 69–78. [Google Scholar] [CrossRef]
- Moosavy, M.; Hassanzadeh, P.; Mohammadzadeh, E.; Mahmoudi, R.; Khatibi, S.; Mardani, K. Antioxidant and antimicrobial activities of essential oil of Lemon (Citrus limon) peel in vitro and in a food model. J. Food Qual. Hazards Control 2017, 4, 42–48. [Google Scholar]
- Guimarães, R.; Barros, L.; Barreira, J.C.M.; Sousa, M.J.; Carvalho, A.M.; Ferreira, I.C. Targeting excessive free radicals with peels and juices of citrus fruits: Grapefruit, lemon, lime and orange. Food Chem. Toxicol. 2010, 48, 99–106. [Google Scholar] [CrossRef]
- Prasanna, G.L.; Rao, B.V.D.; Reddy, A.G.; Rao, M.V.B.; Pal, M. Lemon Juice Mediated Reaction under Ultrasound Irradiation: Synthesis of Indolofuroquinoxalines as Potential Anticancer Agents. Mini Rev. Med. Chem. 2019, 19, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.-G.; Yu, H.; Liang, L.; Fu, Y.-J.; Efferth, T.; Liu, X.; Wu, N. Activities of Ten Essential Oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 Cancer Cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.V.; Srinivas, P.; Bettadaiah, B. New scalable and eco-friendly synthesis of gingerols. Tetrahedron Lett. 2012, 53, 2993–2995. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Shirin, A.P.R.; Jamuna, P.; Pilerood, S.A.; Prakash, J. Chemical composition and antioxidant properties of ginger root (Zingiber officinale). J. Med. Plants Res. 2010, 4, 2674–2679. [Google Scholar] [CrossRef]
- Idris, N.A.; Yasin, H.M.; Usman, A. Voltammetric and spectroscopic determination of polyphenols and antioxidants in ginger (Zingiber officinale Roscoe). Heliyon 2019, 5, e01717. [Google Scholar] [CrossRef]
- Azeem, A.A.; Hegazy, A.; Ibrahim, K.S.; Farrag, A.-R.H.; El-Sayed, E.M. Hepatoprotective, Antioxidant, and Ameliorative Effects of Ginger (Zingiber officinale Roscoe) and Vitamin E in Acetaminophen Treated Rats. J. Diet. Suppl. 2013, 10, 195–209. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, E.Y.; E Kang, N.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Shukla, Y.; Prasad, S.; Tripathi, C.; Singh, M.; George, J.; Kalra, N. In vitro andin vivomodulation of testosterone mediated alterations in apoptosis related proteins by [6]-gingerol. Mol. Nutr. Food Res. 2007, 51, 1492–1502. [Google Scholar] [CrossRef]
- Cheng, X.-L.; Liu, Q.; Peng, Y.; Li, P.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Sáez, F.; Stahl-Biskup, E. Essential oil polymorphism in the genus Thymus. In Thyme—The genus Thymus; CRC Press: London, UK, 2002; pp. 125–143. [Google Scholar]
- Bilušić, T.; Krisko, A.; Dragović-Uzelac, V.; Milos, M.; Pifat, G. The effects of essential oils and aqueous tea infusions of oregano (Origanum vulgare L. spp. hirtum), thyme (Thymus vulgaris L.) and wild thyme (Thymus serpyllum L.) on the copper-induced oxidation of human low-density lipoproteins. Int. J. Food Sci. Nutr. 2007, 58, 87–93. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Asimovic, Z.; Tufek, N. Determination of total phenols and antioxidative activity in teas of wild thyme and mint depending on duration of the extraction. In Proceedings of the 25th Scientific-Experts Congress on Agriculture and Food Industry, Izmir, Turkey, 25–27 September 2014; pp. 85–88. [Google Scholar]
- Zhang, Y.; Chen, X.; Yang, L.; Zu, Y.; Lu, Q. Effects of rosmarinic acid on liver and kidney antioxidant enzymes, lipid peroxidation and tissue ultrastructure in aging mice. Food Funct. 2015, 6, 927–931. [Google Scholar] [CrossRef]
- Jaric, S.; Mitrović, M.; Pavlović, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid. Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, J.; Yang, Y.; Wang, X.-X.; Chen, G.; Shi, A.; Lu, Y.; Jia, S.; Kang, X.; Lu, L. Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine 2020, 68, 153186. [Google Scholar] [CrossRef]
- Liao, X.-Z.; Gao, Y.; Sun, L.-L.; Liu, J.-H.; Chen, H.-R.; Yu, L.; Chen, Z.-Z.; Chen, W.-H.; Lin, L.-Z. Rosmarinic acid reverses non-small cell lung cancer cisplatin resistance by activating the MAPK signaling pathway. Phytotherapy Res. 2020, 34, 1142–1153. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kee, J.-Y.; Hong, S.-H. Rosmarinic Acid Activates AMPK to Inhibit Metastasis of Colorectal Cancer. Front. Pharmacol. 2018, 9, 68. [Google Scholar] [CrossRef]
- Baig, S.; Ahma, B.; Azizan, A.; Ali, S.; Rouhollahi, E.; Abdulla, M. Hexane extract of Thymus serpyllum L.: GC-MS profile, antioxidant potential and anticancer impact on HepG2 (liver carcinoma) cell line. Int. Sci. Index 2014, 8, 1518–1525. [Google Scholar]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical Analysis and Evaluation of Its Antimalarial, Antioxidant, and Cytotoxic Activities. J. Food Sci. 2011, 76, C512–C518. [Google Scholar] [CrossRef]
- Zandi, P.; Ahmadi, L. Antioxidant effect of plant extracts of labiatae family. J. Food Sci. Technol. 2000, 37, 436–439. [Google Scholar]
- Hossain, M.; Brunton, N.; Barry-Ryan, C.; Martin-Diana, A.B.; Wilkinson, M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J. Chem 2008, 1, 751–756. [Google Scholar]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Hossain, M.B.; Camphuis, G.; Aguiló-Aguayo, I.; Gangopadhyay, N.; Rai, D.K. Antioxidant activity guided separation of major polyphenols of marjoram (Origanum majorana L.) using flash chromatography and their identification by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2014, 37, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, N. Comparative Study of Volatile Oil of Stem and Aerial Parts of Origanum majorana Linn. J. Essent. Oil Bear. Plants 2016, 19, 2091–2099. [Google Scholar] [CrossRef]
- Makrane, H.; El Messaoudi, M.; Melhaoui, A.; El Mzibri, M.; Benbacer, L.; Aziz, M. Cytotoxicity of the Aqueous Extract and Organic Fractions from Origanum majorana on Human Breast Cell Line MDA-MB-231 and Human Colon Cell Line HT-29. Adv. Pharmacol. Sci. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Alrashedi, A.N.R. Anti-Colon Cancer Effect of Origanum Majorana Essential Oil. Master Thesis, United Arab Emirates University, Abu Dhabi, UAE, April 2018. [Google Scholar]
- Attoub, S.; Shahrazad, S.; Kholoud, A. PO-419 Use of origanum majorana oil in lung cancer therapy. ESMO Open 2018, 3, A187. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. Egyptian herbal tea infusions’ antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2014, 29, 474–479. [Google Scholar] [CrossRef]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. The aroma, taste, color and bioactive constituents of tea. J. Med. Plant Res. 2011, 5, 2110–2124. [Google Scholar] [CrossRef]
- Babu, P.A.; Liu, N.; Velayutham, A.B.P.; Babu, A. Green Tea Catechins and Cardiovascular Health: An Update. Curr. Med. Chem. 2008, 15, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Hügel, H.M.; Jackson, N. Redox chemistry of green tea polyphenols: Therapeutic benefits in neurodegenerative diseases. Mini Rev. Med. Chem. 2012, 12, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Boil. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sartippour, M.R.; Pietras, R.J.; Márquez-Garbán, D.C.; Chen, H.-W.; Heber, D.; Henning, S.M.; Sartippour, G.; Zhang, L.; Lu, M.; Weinberg, O.; et al. The combination of green tea and tamoxifen is effective against breast cancer. Carcinogenesis 2006, 27, 2424–2433. [Google Scholar] [CrossRef]
- Lee, B.-R.; Cho, J.-I.; Park, P.-S. Effects of Dietary Tea Polyphenol on Tumor Growth Inhibition by Cisplatin in EMT6 Breast Tumor-bearing Mice. J. Korean Soc. Food Sci. Nutr. 2014, 43, 47–54. [Google Scholar] [CrossRef]
- Morré, D.M.; Morré, D.J. Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett. 2006, 238, 202–209. [Google Scholar] [CrossRef]
- Tang, S.-N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.N. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5, 14. [Google Scholar] [CrossRef]
- Ebadi, M.; Azizi, M.; Sefidkon, F.; Ahmadi, N. Influence of different drying methods on drying period, essential oil content and composition of Lippia citriodora Kunth. J. Appl. Res. Med. Aromat. Plants 2015, 2, 182–187. [Google Scholar] [CrossRef]
- Carnat, A.; Fraisse, D.; Lamaison, J. The aromatic and polyphenolic composition of lemon verbena tea. Fitoterapia 1999, 70, 44–49. [Google Scholar] [CrossRef]
- Portmann, E.; Nigro, M.M.L.; Reides, C.G.; Llesuy, S.; Ricco, R.A.; Wagner, M.L.; Gurni, A.A.; Carballo, M.A. Aqueous Extracts of Lippia turbinata and Aloysia citriodora (Verbenaceae): Assessment of Antioxidant Capacity and DNA damage. Int. J. Toxicol. 2012, 31, 192–202. [Google Scholar] [CrossRef]
- Zamorano-Ponce, E.; Fernández, J.; Vargas, G.; Rivera, P.; A Carballo, M. Protective activity of cedron (Aloysia triphylla) infusion over genetic damage induced by cisplatin evaluated by the comet assay technique. Toxicol. Lett. 2004, 152, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Malekirad, A.A.; Hosseini, N.; Bayrami, M.; Hashemi, T.; Rahzani, K. Benefit of Lemon Verbena in Healthy Subjects; Targeting Diseases Associated with Oxidative Stress. Asian J. Anim. Veter. Adv. 2011, 6, 953–957. [Google Scholar] [CrossRef]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Vamvakias, M.; Bardouki, H.; Galanis, A.; Chlichlia, K.; Kourkoutas, Y.; I Panayiotidis, M.; Pappa, A. Chemical Composition and Evaluation of the Biological Properties of the Essential Oil of the Dietary Phytochemical Lippia citriodora. Molecules 2018, 23, 123. [Google Scholar] [CrossRef] [PubMed]
- Fotsis, T.; Pepper, M.S.; Aktas, E.; Breit, S.; Rasku, S.; Adlercreutz, H.; Wähälä, K.; Montesano, R.; Schweigerer, L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997, 57, 2916–2921. [Google Scholar] [PubMed]
- Zervoudakis, G.; Salahas, G.; Kaspiris, G.; Konstantopoulou, E. Influence of light intensity on growth and physiological characteristics of common sage (Salvia officinalis L.). Braz. Arch. Boil. Technol. 2012, 55, 89–95. [Google Scholar] [CrossRef]
- Mohamed, A.Y.; Mustafa, A.A. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oil Salvia Officinalis in Sudan. J. Multidis. Res. Rev. 2019, 1, 43–45. [Google Scholar] [CrossRef]
- Zimmermann, B.F.; Walch, S.G.; Tinzoh, L.N.; Stühlinger, W.; Lachenmeier, D.W. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L. (sage tea). J. Chromatogr. B 2011, 879, 2459–2464. [Google Scholar] [CrossRef]
- Lima, C.F.; Andrade, P.B.; Seabra, R.M.; Fernandes-Ferreira, M.; Pereira-Wilson, C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J. Ethnopharmacol. 2005, 97, 383–389. [Google Scholar] [CrossRef]
- Amin, A.; Hamza, A.A. Hepatoprotective effects of Hibiscus, Rosmarinus and Salvia on azathioprine-induced toxicity in rats. Life Sci. 2005, 77, 266–278. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Albayrak, S.; Sagdic, O. In vitro antioxidant and antimicrobial activity of some Lamiaceae species. Iran J. Sci. Technol. A. 2013, 37, 1–9. [Google Scholar]
- Shahrzad, K.; Mahya, N.; Fatemeh, T.B.; Maryam, K.; Mohammadreza, F.B.; Jahromy, M.H. Hepatoprotective and Antioxidant Effects of Salvia officinalis L. Hydroalcoholic Extract in Male Rats. Chin. Med. 2014, 5, 130–136. [Google Scholar] [CrossRef]
- Veličković, D.T.; Karabegović, I.T.; Stojičević, S.S.; Lazić, M.L.; Marinković, V.D.; Veljković, V. Comparison of antioxidant and antimicrobial activities of extracts obtained from Salvia glutinosa L. and Salvia officinalis L. Chem. Ind. 2011, 65, 599–605. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Shen, H.-M.; Ong, C.N. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG(2) cells. Cancer Lett. 2000, 153, 85–93. [Google Scholar] [CrossRef]
- Sertel, S.; Eichhorn, T.; Plinkert, P.; Efferth, T. Anticancer activity of Salvia officinalis essential oil against HNSCC cell line (UMSCC1). HNO 2011, 59, 1203–1208. [Google Scholar] [CrossRef]
- Ebadi, M. Pharmacodynamic Basis of Herbal Medicine, 2nd ed.; CRC press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef]
- Bernard, D.; Kwabena, A.I.; Osei, O.D.; Daniel, G.A.; Elom, S.A.; Sandra, A. The Effect of Different Drying Methods on the Phytochemicals and Radical Scavenging Activity of Ceylon Cinnamon (Cinnamomum zeylanicum) Plant Parts. Eur. J. Med. Plants 2014, 4, 1324–1335. [Google Scholar] [CrossRef]
- Lee, J.-S.; Jeon, S.-M.; Park, E.-M.; Huh, T.-L.; Kwon, O.-S.; Lee, M.-K.; Choi, M.-S. Cinnamate Supplementation Enhances Hepatic Lipid Metabolism and Antioxidant Defense Systems in High Cholesterol-Fed Rats. J. Med. Food 2003, 6, 183–191. [Google Scholar] [CrossRef]
- Juliani, H.R.; Simon, J.E.; Ramboatiana, M.M.R.; Behra, O.; Garvey, A.; Raskin, I. Malagasy aromatic plants: Essential oils, antioxidant and antimicrobial activities. Acta Hortic. 2004, 629, 77–81. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Ohnishi-Kameyama, M.; Ono, H.; Yoshida, M.; Jaganmohan Rao, L. Phenolic constituents in the fruits of Cinnamomum zeylanicum and their antioxidant activity. J. Agri. Food Chem. 2006, 54, 1672–1679. [Google Scholar] [CrossRef]
- Moselhy, S.S.; Ali, H.K.H. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Boil. Res. 2009, 42, 93–98. [Google Scholar] [CrossRef]
- Herdwiani, W.; Soemardji, A.; Elfahmi, I.; Tan, M. A review of cinnamon as a potent anticancer drug. Asian J. Pharm. Clin. Res. 2016, 9, 8–13. [Google Scholar]
- Packiaraj, R. Antimicrobial and cytotoxic activities of endophytic fungus Colletotrichum gloeosporioides isolated from endemic tree Cinnamomum malabatrum. Stud. Fungi 2016, 1, 104–113. [Google Scholar] [CrossRef]
- Singh, R.; Koppikar, S.J.; Paul, P.; Gilda, S.; Paradkar, A.; Kaul-Ghanekar, R. Comparative analysis of cytotoxic effect of aqueous cinnamon extract from Cinnamomum zeylanicum bark with commercial cinnamaldehyde on various cell lines. Pharm. Boil. 2009, 47, 1174–1179. [Google Scholar] [CrossRef]
- Mirzaei, M.; Sefidkon, F.; Ahmadi, N.; Shojaeiyan, A.; Hosseini, H. Damask rose (Rosa damascena Mill.) essential oil is affected by short-and long-term handling. Ind. Crop. Prod. 2016, 79, 219–224. [Google Scholar] [CrossRef]
- Salman, S.Y.; Erbas, S. Contact and repellency effects of Rosa damascena Mill. essential oil and its two major constituents against Tetranychus urticae Koch (Acari: Tetranychidae). Türk. Entomol. Derg. 2014, 38, 365–376. [Google Scholar] [CrossRef]
- Himesh, S.; Nanda, S.; Singhai, A.; Jitender, M. Radical scavenging activities and natural indicator activity of aqueous and ethanolic extract of Rosa damascena. Int. J. Pharm. Pharm. Sci. 2012, 4, 581–586. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascene Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011, 25, 1577–1584. [Google Scholar] [CrossRef]
- Senol, F.S.; Orhan, I.E.; Kurkcuoglu, M.; Khan, M.T.H.; Altintas, A.; Sener, B.; Başer, K.H.C. A mechanistic investigation on anticholinesterase and antioxidant effects of rose (Rosa damascena Mill.). Food Res. Int. 2013, 53, 502–509. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crop. Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Ozkan, G.; Sagdic, O.; Baydar, N.G.; Baydar, H. Note: Antioxidant and Antibacterial Activities of Rosa damascena Flower Extracts. Food Sci. Technol. Int. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Habibi, E.; Modanloo, M. Cytotoxic and genotoxic studies of essential oil from Rosa damascene Mill., Kashan, Iran. Med. Glas. (Zenica) 2017, 14, 152–157. [Google Scholar] [PubMed]
- Stanojević, L.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojević, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Gupta, V.; Mittal, P.; Bansal, P.; Khokra, S.L.; Kaushik, D. Pharmacological potential of Matricaria recutita—A review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 12–16. [Google Scholar]
- Al-Ismail, K.; Talal, A. A study of the effect of water and alcohol extracts of some plants as antioxidants and antimicrobial on long-term storage of anhydrous butter fat. Dirasat. Agric. Sci. 2003, 30, 330–337. [Google Scholar]
- Sazegar, M.; Banakar, A.; Bahrami, N.; Bahrami, A.; Baghbani, M.; Nematolahi, P.; Mottaghi, M. Determination of the antioxidant activity and stability of Chamomile (Matricaria chamomilla L.) extract in sunflower oil. World App. Sci. J. 2011, 12, 1500–1504. [Google Scholar]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P.A. Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria chamomilla L.) and Sage (Salvia officinalis L.) Prepared at Different Temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef]
- Zemestani, M.; Rafraf, M.; Jafarabadi, M.A.; Information, P.E.K.F.C. Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nutrients 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Demir, N.; Gungor, A.A.; Nadaroglu, H.; Demir, Y. The antioxidant and radical scavenging activities of Primrose (Primula vulgaris). Eur. J. Exp. Biol. 2014, 4, 395–401. [Google Scholar]
- Ozkan, M.T.; Aliyazicioglu, R.; Demir, S.; Misir, S.; Turan, I.; Yildirmis, S.; Aliyazicioglu, Y. Phenolic characterisation and antioxidant activity of Primula vulgaris and its antigenotoxic effect on fibroblast cells. Jundishapur J. Nat. Pharm. Prod. 2017, 12, 395–401. [Google Scholar] [CrossRef]
- Demir, S.; Turan, I.; Aliyazicioğlu, Y. Antioxidant properties of Primula vulgaris flower extract and its cytotoxic effect on human cancer cell lines. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi 2019, 22, 78–84. [Google Scholar]
- Demir, S.; Turan, I.; Aliyazicioğlu, R.; Yaman, S.O.; Aliyazicioglu, Y. Primula vulgaris extract induces cell cycle arrest and apoptosis in human cervix cancer cells. J. Pharm. Anal. 2018, 8, 307–311. [Google Scholar] [CrossRef]
- Adnan, I.; Talib, W. Do herbal drinks augment the cytotoxic effect against different cancer cells? Are they potent stimulators of innate and acquired immunity? J. Clin. Oncol. 2020, 38, e21657. [Google Scholar] [CrossRef]
- Adegunloye, B.J.; O Omoniyi, J.; A Owolabi, O.; Ajagbonna, O.P.; Sofola, O.A.; A Coker, H. Mechanisms of the blood pressure lowering effect of the calyx extract of Hibiscus sabdariffa in rats. Afr. J. Med. Med. Sci. 1996, 25, 235–238. [Google Scholar] [PubMed]
- Gaya, I.; Mohammed, O.; Suleiman, A.; Maje, M.; Adekunle, A. Toxicology and lactogenic studies on the seed of Hibiscus sabdariffa extract on serum prolactin levels of albino wistar rats. Internet J. Endocrinal 2009, 5, 2–3. [Google Scholar]
- Nkumah, O.C. Phytochemical analysis and medicinal uses of Hibiscus sabdariffa. Int. J. Herb. Med. 2015, 2, 16–19. [Google Scholar]
- Obouayeba, A.P.; Diarrassouba, M.; Soumahin, E.F.; Kouakou, T.H. Phytochemical analysis, purification and identification of Hibiscus anthocyanins. J. Pharm. Chem. Biol. Sci. 2015, 3, 156–168. [Google Scholar]
- Shen, C.-Y.; Zhang, T.-T.; Jiang, J.-G. Anti-inflammatory activities of essential oil isolated from the calyx of Hibiscus sabdariffa L. Food Funct. 2016, 7, 4451–4459. [Google Scholar] [CrossRef]
- Rassem, H.; Nour, A.H.; Yunus, R.M. GC-MS analysis of bioactive constituents of Hibiscus flower. Aust. J. Basic Appl. Sci. 2017, 11, 91–97. [Google Scholar]
- Cisse, M.; Vaillant, F.; Pallet, D.; Dornier, M. Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Res. Int. 2011, 44, 2607–2614. [Google Scholar] [CrossRef]
- Salmerón-Ruiz, M.L.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Alvarez-Parrilla, E.; Villegas-Ochoa, M.A.; Sáyago-Ayerdi, S.G.; Valenzuela-Melendez, M.; González-Aguilar, G.A. Optimization of total anthocyanin content and antioxidant activity of a Hibiscus sabdariffa infusion using response surface methodology. BIOtecnia 2019, 21, 114–122. [Google Scholar] [CrossRef][Green Version]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive Oxygen Species (ROS) Generation and Detoxifying in Plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Pham, T.N.; Binh, M.L.T.; Thuan, M.; Van, N.T.T.; Lam, T.D.; Nguyen, P.T.N. Effects of Extraction Conditions on Antioxidant Activities of Roselle (Hibiscus sabdariffa L.) Extracts. Mater. Sci. 2020, 977, 201–206. [Google Scholar] [CrossRef]
- Haron, N.; Jusoh, H.M.; Abul, N.S.B.; Rahman, A. The Total Phenolic Content and Antioxidant Activity of Roselle (Hibiscus sabdariffa) Extract. Int. J. Allied Heal. Sci. 2019, 3, 725–733. [Google Scholar]
- Fernández-Arroyo, S.; Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Pasini, F.; Joven, J.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011, 44, 1490–1495. [Google Scholar] [CrossRef]
- Khaghani, S.; Razi, F.; Yajloo, M.M.; Paknejad, M.; Shariftabrizi, A.; Pasalar, P. Selective Cytotoxicity and Apoptogenic Activity of Hibiscus Sabdariffa Aqueous Extract Against MCF-7 Human Breast Cancer Cell Line. J. Cancer Ther. 2011, 2, 394–400. [Google Scholar] [CrossRef]
- Su, C.-C.; Wang, C.-J.; Huang, K.-H.; Lee, Y.-J.; Chan, W.-M.; Chang, Y.-C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Ahirwar, B.; Ahirwar, D. In vivo and in vitro investigation of cytotoxic and antitumor activities of polyphenolic leaf extract of Hibiscus sabdariffa against breast cancer cell lines. Res. J. Pharm. Technol. 2020, 13, 615. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chen, J.-H.; Kuo, W.-H.; Wang, C.-J. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem. Biol. Interact. 2007, 165, 59–75. [Google Scholar] [CrossRef]
- Longtin, R. The Pomegranate: Nature’s Power Fruit? J. Natl. Cancer Inst. 2003, 95, 346–348. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pitonzo, R.; Novara, M.E.; Bongiorno, D.; Indelicato, S.; Gentile, C.; Avellone, G.; Bognanni, R.; Scandurra, S.; Melilli, M.G. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC-Orbitrap-MS approach. J. Sci. Food Agric. 2018, 99, 1038–1045. [Google Scholar] [CrossRef]
- Bhandary, S.K.; Bhat, V.S.; Sharmila, K.P.; Bekal, M.P. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. NITTE Univ. J. Heal. Sci. 2012, 2, 34–38. [Google Scholar] [CrossRef]
- Attia, E. Antimicrobial Activity and Bio-active compounds analysis in Ethanolic plant extracts of Punica Grantanum (Pomegranate) using GC-MS. Egypt J. Exp. Boil. 2019, 15, 325. [Google Scholar] [CrossRef]
- Kachkoul, R.; Houssaini, T.S.; Mohim, M.; El Habbani, R.; Lahrichi, A. Chemical Compounds Identification and Antioxidant and Calcium Oxalate Anticrystallization Activities of Punica granatum L. Evid. Based Complement. Altern. Med. 2020, 2020, 9424510. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Cecchi, L.; Khatib, M.; Bellumori, M.; Cairone, F.; Carradori, S.; Zengin, G.; Cesa, S.; Innocenti, M.; Mulinacci, N. Characterization of Arils Juice and Peel Decoction of Fifteen Varieties of Punica granatum L.: A Focus on Anthocyanins, Ellagitannins and Polysaccharides. Antioxidants 2020, 9, 238. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, J.K.; Chalkroborty, B.; Hazra, A.K. Comparison of different aqueous extraction methods for optimum extraction of polyphenols and in-vitro anti-oxidant activity from pomegranate peel. J. Pharmacogn. Phytochem. 2019, 8, 342–347. [Google Scholar]
- Doostan, F.; Vafafar, R.; Zakeri-Milani, P.; Pouri, A.; Afshar, R.A.; Abbasi, M.M. Effects of Pomegranate (Punica Granatum L.) Seed and Peel Methanolic Extracts on Oxidative Stress and Lipid Profile Changes Induced by Methotrexate in Rats. Adv. Pharm. Bull. 2017, 7, 269–274. [Google Scholar] [CrossRef]
- González, J.C.R.; Hernández, R.D.; Berghe, W.V. Punica granatum L. Constituents for Cancer Prevention, Chemosensitisation and Therapeutic Treatment. In Natural Products for Cancer Chemoprevention: Single Compounds and Combinations; Pezzuto, J.M., Vang, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 401–468. [Google Scholar]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Erguen, B.; Nitzsche, B.; Höpfner, M.; Jung, K.; Rabien, A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC-3 and LNCaP cells. Chem. Interactions 2017, 274, 100–106. [Google Scholar] [CrossRef]
- Uddandrao, V.V.S.; Parim, B.; Nivedha, P.R.; Swapna, K.; Rameshreddy, P.; Vadivukkarasi, S.; Begum, M.S.; Ganapathy, S. Anticancer activity of pomegranate extract: Effect on hematological and antioxidant profile against ehrlich-ascites-carcinoma in Swiss albino mice. Orient. Pharm. Exp. Med. 2018, 19, 243–250. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Z.; Wang, S.; Li, T.; Mastriani, E.; Li, Q.-H.; Bao, H.-X.; Zhou, Y.-J.; Wang, X.; Liu, Y.; et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Boil. Ther. 2017, 18, 990–999. [Google Scholar] [CrossRef]
- Özcan, M.M.; Chalchat, J.C. Chemical composition and antifungal effect of anise (Pimpinella anisum L.) fruit oil at ripening stage. Ann. Microbiol. 2006, 56, 353–358. [Google Scholar] [CrossRef]
- Al-Ismail, K.; Aburjai, T. Antioxidant activity of water and alcohol extracts of chamomile flowers, anise seeds and dill seeds. J. Sci. Food Agric. 2004, 84, 173–178. [Google Scholar] [CrossRef]
- Farzaneh, V.; Gominho, J.; Pereira, H.; Carvalho, I.S. Screening of the Antioxidant and Enzyme Inhibition Potentials of Portuguese Pimpinella anisum L. Seeds by GC-MS. Food Anal. Methods 2018, 11, 2645–2656. [Google Scholar] [CrossRef]
- Lee, J.-B.; Yamagishi, C.; Hayashi, K.; Hayashi, T. Antiviral and Immunostimulating Effects of Lignin-Carbohydrate-Protein Complexes from Pimpinella anisum. Biosci. Biotechnol. Biochem. 2011, 75, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Andallu, B.; Rajeshwari, C.U. Chapter 20—Aniseeds (Pimpinella anisum L.) in Health and Disease. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 175–181. [Google Scholar]
- Kharobi, K. Effect of Grinding the Herb and Boiling the Infusion on Total Phenolic Content and Antioxidant Capacity of Herbal Infusions. J. Pharm. Nutr. Sci. 2019, 9, 66–70. [Google Scholar] [CrossRef]
- Faried, M.A.; El-Mehi, A.E.-S. Aqueous anise extract alleviated the pancreatic changes in streptozotocin-induced diabetic rat model via modulation of hyperglycemia, oxidative stress, apoptosis and autophagy: A biochemical, histological and immunohistochemical study. Folia Morphol. 2019. [Google Scholar] [CrossRef]
- Mukunda, A.; Pynadath, M.K.; Kadar, N.; Mohan, A. Cytotoxic effect of anise seed (Pimpinella anisum) extract on KB cell line—A comparative study with CISPLATIN. Oral Maxillofac. Pathol. J. 2020, 11, 1–10. [Google Scholar]
- Asadi, H.; Rahamooz-Haghighi, S. Anti-proliferative effect of the extracts and essential oil of Pimpinella anisum on gastric cancer cells. J. HerbMed Pharm. 2016, 5, 157–161. [Google Scholar]
- Kadan, S. Anticancer Activity of Anise (Pimpinella anisum L.) Seed Extract. Open Nutraceuticals J. 2013, 6, 1–5. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Sumrra, S.H. Chapter 13—Cumin. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–178. [Google Scholar]
- Moghaddam, M.; Miran, S.N.K.; Pirbalouti, A.G.; Mehdizadeh, L.; Ghaderi, Y. Variation in essential oil composition and antioxidant activity of cumin (Cuminum cyminum L.) fruits during stages of maturity. Ind. Crop. Prod. 2015, 70, 163–169. [Google Scholar] [CrossRef]
- Rai, N.; Yadav, S.; Verma, A.; Tiwari, L.; Sharma, R.K. A monographic profile on quality specifications for a herbal drug and spice of commerce—Cuminum cyminum L. Int. J. Adv. Sci. 2012, 1, 1–12. [Google Scholar]
- Ghasemi, G.; Fattahi, M.; Alirezalu, A.; Ghosta, Y. Antioxidant and antifungal activities of a new chemovar of cumin (Cuminum cyminum L.). Food Sci. Biotechnol. 2018, 28, 669–677. [Google Scholar] [CrossRef]
- Al-Shawi, S.; Al-Younis, Z.; Al-Kareem, N. Study of cumin antibacterial and antioxidant activity of alcoholic and aqueous extracts. Pakistan J. Biotechnol. 2017, 14, 227–231. [Google Scholar]
- Demir, S.; Korukluoglu, M. A comparative study about antioxidant activity and phenolic composition of cumin (Cuminum cyminum L.) and coriander (Coriandrum sativum L.). Indian J. Tradit. Know. 2020, 19, 383–393. [Google Scholar]
- Mahalakshmi, R.; Priyanga, J.; Hari, B.N.V.; Bhakta-Guha, D.; Guha, G. Hexavalent chromium-induced autophagic death of WRL-68 cells is mitigated by aqueous extract of Cuminum cyminum L. seeds. 3 Biotech 2020, 10, 191. [Google Scholar] [CrossRef]
- Allahghadri, T.; Rasooli, I.; Owlia, P.; Nadooshan, M.J.; Ghazanfari, T.; Taghizadeh, M.; Astaneh, S.D.A. Antimicrobial Property, Antioxidant Capacity, and Cytotoxicity of Essential Oil from Cumin Produced in Iran. J. Food Sci. 2010, 75, H54–H61. [Google Scholar] [CrossRef]
- Prakash, E.; Gupta, D.K. Cytotoxic activity of ethanolic extract of Cuminum cyminum Linn against seven human cancer cell line. Univers. J. Agric. Res. 2014, 2, 27–32. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.; Gupta, S.; Abdullah, A.; Talwar, P.; Ravanan, P. Anti-Cancer and Neuro-Protective effect of Cuminum cyminum extracts on IMR32 Human Neuroblastoma Cell Lines. Res. J. Pharm. Technol. 2018, 11, 1547. [Google Scholar] [CrossRef]
- Goodarzi, S.; Tabatabaei, M.J.; Jafari, R.M.; Shemirani, F.; Tavakoli, S.; Mofasseri, M.; Tofighi, Z. Cuminum cyminum fruits as source of luteolin-7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2018, 34, 1602–1606. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.; Dueñas, M. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C. Melissa officinalis L. decoctions as functional beverages: A bioactive approach and chemical characterization. Food Funct. 2015, 6, 2240–2248. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Ismael, M.F.; Pedro, R.E.R.; Selvin, A.S.M.; Vany, P.F.; Ricardo, S.A.; Jhunior, A.M.F.; Luis, A.B.A. Chemical composition of essential oil of Melissa officinalis L. and antioxidant activity from Boa Vista-RR, Brazil. Afr. J. Pharm. Pharmacol. 2020, 14, 41–45. [Google Scholar] [CrossRef]
- Skotti, E.; Sotiropoulou, N.S.; Lappa, I.; Kaiafa, M.; Tsitsigiannis, D.I.; Tarantilis, P.A. Screening of Lemon Balm Extracts for Anti-Aflatoxigenic, Antioxidant and Other Biological Activities. Preprints 2019, 2019070005. [Google Scholar] [CrossRef]
- Yaldiz, G.; Arici, K.; Yilmaz, G. Phytochemical analysis, antioxidant and antibacterial activities of four Lamiaceae species cultivated in barnyard manure. J. Agric. Sci. 2017, 23, 95–108. [Google Scholar]
- Papoti, V.T.; Totomis, N.; Atmatzidou, A.; Zinoviadou, K.; Androulaki, A.; Petridis, D.; Ritzoulis, C. Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes 2019, 7, 88. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Voća, S.; Dobričević, N.; Pliestić, S.; Galić, A.; Boričević, A.; Borić, N. Ultrasound-assisted extraction of bioactive compounds from lemon balm and peppermint leaves. Int. Agrophys. 2016, 30, 95–104. [Google Scholar] [CrossRef]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-khodaei, M.; Sadigh-Eteghad, S. Melissa officinalis L. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp. Physiol. 2020, 105, 707–720. [Google Scholar] [CrossRef]
- Popova, A.; Dalemska, Z.; Mihaylova, D.; Hristova, I.; Alexieva, I. Melissa officinalis L.—GC profile and antioxidant activity. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 634–638. [Google Scholar]
- Martins, E.N.; Pessano, N.T.; Leal, L.; Roos, D.H.; Folmer, V.; Puntel, G.O.; Da Rocha, J.B.T.; Aschner, M.; Ávila, D.S.; Puntel, R.L. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Res. Bull. 2012, 87, 74–79. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, G.; Randy, A.; Son, Y.-J.; Hong, C.R.; Kim, S.M.; Nho, C.W. Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis. Nutrients 2020, 12, 1166. [Google Scholar] [CrossRef]
- Hassan, R.A.; Abotaleb, S.T.; Hamed, H.B.; Eldeen, M.S. Antioxidant and Antimicrobial Activities of Melissa officinalis L. (Lemon Balm) Extracts. J. Agric. Chem. Biotechnol. 2019, 10, 183–187. [Google Scholar] [CrossRef]
- Encalada, M.A.; Hoyos, K.M.; Rehecho, S.; Berasategi, I.; De Ciriano, M.G.-I.; Ansorena, D.; Astiasarán, I.; Navarro-Blasco, I.; Cavero, R.Y.; Calvo, M.I. Anti-proliferative Effect of Melissa officinalis on Human Colon Cancer Cell Line. Plant Foods Hum. Nutr. 2011, 66, 328–334. [Google Scholar] [CrossRef]
- Magalhaes, D.B.; Castro, I.; Lopes-Rodrigues, V.; Pereira, J.M.; Barros, L.; Ferreira, I.C.; Xavier, C.P.R.; Vasconcelos, M.H. Melissa officinalis L. ethanolic extract inhibits the growth of a lung cancer cell line by interfering with the cell cycle and inducing apoptosis. Food Funct. 2018, 9, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Shamseini, M.; Mohammadi, M.; Shirazi, F.H.; Andalib, S.; Gholami, S.; Hosseini, S.H.; Noubarani, M.; Kamalinejad, M.; Eskandari, M.R. Prevention of liver cancer by standardized extract of Melissa officinalis L. in a rat model of hepatocellular carcinoma: Its potential role as a chemopreventive agent. Int. Pharm. Acta 2019, 2, 2e8-1–2e8-7. [Google Scholar] [CrossRef]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon Balm Extracts Prevent Breast Cancer Progression In Vitro and In Ovo on Chorioallantoic Membrane Assay. Evid. Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.N.T.; Williams, D.B.; Head, R. Rosemary and Cancer Prevention: Preclinical Perspectives. Crit. Rev. Food Sci. Nutr. 2011, 51, 946–954. [Google Scholar] [CrossRef]
- Nie, J.-Y.; Li, R.; Jiang, Z.-T.; Wang, Y.; Tan, J.; Tang, S.-H.; Zhang, Y. Antioxidant activity screening and chemical constituents of the essential oil from rosemary by ultra-fast GC electronic nose coupled with chemical methodology. J. Sci. Food Agric. 2020, 100, 3481–3487. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Sánchez, J.C. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Christina, A.; Joseph, D.; Packialakshmi, M.; Kothai, R.; Robert, S.H.; Chidambaranathan, N.; Ramasamy, M. Anticarcinogenic activity of Withania somnifera Dunal against Dalton’s Ascitic Lymphoma. J. Ethnopharmacol. 2004, 93, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.M.; Shawush, N.A. Influence of olive and rosemary leaves extracts on chemically induced liver cirrhosis in male rats. Saudi J. Boil. Sci. 2014, 22, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Al Laham, S.A.; Al Fadel, F.M. Antibacterial efficacy of variety plants against the resistant Streptococcus which cause clinical mastitis in cows. Asian J. Pharm. Res. Health Care 2013, 5, 32–41. [Google Scholar]
- Arranz, E.; Mes, J.; Wichers, H.; Jaime, L.; Mendiola, J.A.; Reglero, G.; Santoyo, S. Anti-inflammatory activity of the basolateral fraction of Caco-2 cells exposed to a rosemary supercritical extract. J. Funct. Foods 2015, 13, 384–390. [Google Scholar] [CrossRef]
- Đilas, S.; Knez, Ž.; Četojević-Simin, D.; Šaponjac, V.T.; Škerget, M.; Čanadanović-Brunet, J.; Ćetković, G. In vitro antioxidant and antiproliferative activity of three rosemary (Rosmarinus officinalis L.) extract formulations. Int. J. Food Sci. Technol. 2012, 47, 2052–2062. [Google Scholar] [CrossRef]
- Jaglanian, A.; Tsiani, E. Rosemary Extract Inhibits Proliferation, Survival, Akt, and mTOR Signaling in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 810. [Google Scholar] [CrossRef]
- Telang, N. Anti-proliferative and pro-apoptotic effects of rosemary and constituent terpenoids in a model for the HER-2-enriched molecular subtype of clinical breast cancer. Oncol. Lett. 2018, 16, 5489–5497. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Li, X.-N.; Qi, Y.; Liu, D.-L.; Yang, Y.; Zhao, J.; Zhang, C.-Y.; Wu, K.; Zhao, S. MiR-454 promotes the progression of human non-small cell lung cancer and directly targets PTEN. Biomed. Pharmacother. 2016, 81, 79–85. [Google Scholar] [CrossRef]
- Karimi, N.; Rashedi, J.; Poor, B.M.; Arabi, S.; Ghorbani, M.; Tahmasebpour, N.; Asgharzadeh, M. Cytotoxic effect of rosemary extract on gastric adenocarcinoma (AGS) and esophageal squamous cell carcinoma (KYSE30) cell lines. Gastroenterol. Hepatol. Bed Bench 2017, 10, 102–107. [Google Scholar]
- Jang, Y.-G.; Hwang, K.-A.; Choi, K.-C. Rosmarinic Acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; García-Cañas, V.; Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Artemenko, K.A.; Micol, V.; Bergquist, J.; Cifuentes, A. Shotgun proteomic analysis to study the decrease of xenograft tumor growth after rosemary extract treatment. J. Chromatogr. A. 2017, 1499, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, G.; Petiwala, S.M.; Householter, E.; Johnson, J.J. Standardized rosemary (Rosmarinus officinalis) extract induces Nrf2/sestrin-2 pathway in colon cancer cells. J. Funct. Foods 2015, 13, 137–147. [Google Scholar] [CrossRef]
- Ashmawy, A.; Mostafa, N.; Eldahshan, O.A. GC/MS Analysis and Molecular Profiling of Lemon Volatile Oil against Breast Cancer. J. Essent. Oil Bear. Plants 2019, 22, 903–916. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Babasheikhali, S.R.; Rahgozar, S.; Mohammadi, M. Ginger extract has anti-leukemia and anti-drug resistant effects on malignant cells. J. Cancer Res. Clin. Oncol. 2019, 145, 1987–1998. [Google Scholar] [CrossRef]
- Pashaei-Asl, R.; Pashaei-Asl, F.; Gharabaghi, P.M.; Khodadadi, K.; Ebrahimi, M.; Ebrahimie, E.; Pashaiasl, M. The Inhibitory Effect of Ginger Extract on Ovarian Cancer Cell Line; Application of Systems Biology. Adv. Pharm. Bull. 2017, 7, 241–249. [Google Scholar] [CrossRef]
- Alexa, E.; Sumalan, R.-M.; Danciu, C.; Obistioiu, D.M.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C.A. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Tranquillo, E.; Sapio, L.; Illiano, M.; Caiafa, I.; Naviglio, S. Chemical analysis and anti-proliferative activity of Campania Thymus Vulgaris essential oil. J. Essent. Oil Res. 2017, 29, 461–470. [Google Scholar] [CrossRef]
- Benhalilou, N.; Alsamri, H.; Alneyadi, A.; Athamneh, K.; Alrashedi, A.; Altamimi, N.; Al Dhaheri, Y.; Eid, A.H.; Iratni, R. Origanum majorana Ethanolic Extract Promotes Colorectal Cancer Cell Death by Triggering Abortive Autophagy and Activation of the Extrinsic Apoptotic Pathway. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Fathy, S.A.; Emam, M.A.; Agwa, S.H.A.; Abu Zahra, F.A.; Youssef, F.S.; Sami, R.M. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-κB. Cell. Mol. Boil. 2016, 62, 80–84. [Google Scholar]
- Huang, J.; Chen, S.; Shi, Y.; Li, C.-H.; Wang, X.-J.; Li, F.-J.; Wang, C.-H.; Meng, Q.-H.; Zhong, J.-N.; Liu, M. Epigallocatechin gallate from green tea exhibits potent an-ticancer effects in A-549 non-small lung cancer cells by inducing apoptosis, cell cycle arrest and inhibition of cell migration. J. BUON 2017, 22, 1422–1427. [Google Scholar]
- Cádiz-Gurrea, M.D.L.L.; Olivares-Vicente, M.; Herranz-López, M.; Arráez-Román, D.; Fernández-Arroyo, S.; Micol, V.; Segura-Carretero, A. Bioassay-guided purification of Lippia citriodora polyphenols with AMPK modulatory activity. J. Funct. Foods 2018, 46, 514–520. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Shakouri, M.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Raeisi, M.; Yousefi, S.; Harikrishnan, R.; Reverter, M. Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss). Fish Shellfish. Immunol. 2020, 99, 379–385. [Google Scholar] [CrossRef]
- De Oliveira, P.F.; Munari, C.C.; Nicolella, H.D.; Veneziani, R.C.S.; Tavares, D.C. Manool, a Salvia officinalis diterpene, induces selective cytotoxicity in cancer cells. Cytotechnology 2015, 68, 2139–2143. [Google Scholar] [CrossRef]
- Nguyen, C.V. Exploring the Efficacy of Long Pepper and Sage Ethanolic Extracts for Inducing Selective Cell Death in Hodgkin Lymphoma Cell Lines. Presented at the Undergraduate Research Conference, Windsor, ON, Canada, 29 March 2016. [Google Scholar]
- Hasan, M.; Genovese, S.; Fiorito, S.; Epifano, F.; Witt-Enderby, P.A. Oxyprenylated Phenylpropanoids Bind to MT1 Melatonin Receptors and Inhibit Breast Cancer Cell Proliferation and Migration. J. Nat. Prod. 2017, 80, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Larasati, Y.A.; Putri, D.P.; Utomo, R.Y.; Hermawan, A.; Meiyanto, E. Combination of cisplatin and cinnamon essential oil inhibits HeLa cells proliferation through cell cycle arrest. J. Appl. Pharm. Sci. 2014, 4, 14–19. [Google Scholar] [CrossRef][Green Version]
- Golmakani, M.T.; Barani, S.; Alavi, N.; Tahsiri, Z. Oxidative stability of UV irradiated and X-rayed soybean oil incorporated with rose oil. Grasas y Aceites 2019, 70, 286. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytotherapy Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Ogata, I.; Seo, H.; Kawanai, T.; Hashimoto, E.; Oyama, Y. Cytotoxic action of bisabololoxide A of German chamomile on human leukemia K562 cells in combination with 5-fluorouracil. Phytomedicine 2011, 18, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Khodeer, D.M.; Mehanna, E.T.; Abushouk, A.; Abdel-Daim, M. Protective Effects of Evening Primrose Oil against Cyclophosphamide-Induced Biochemical, Histopathological, and Genotoxic Alterations in Mice. Pathogens 2020, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, K.; Hrabec, E.; Fichna, J.; Sosnowska, D.; Koziolkiewicz, M.; Szymański, J.; Lewandowska, U. Inhibition of nuclear factor-kappaB, cyclooxygenase-2, and metalloproteinase-9 expression by flavanols from evening primrose (Oenothera paradoxa) in human colon cancer SW-480 cells. J. Funct. Foods 2017, 37, 553–563. [Google Scholar] [CrossRef]
- Amran, N.; Rani, A.N.A.; Mahmud, R.; Yin, K.B. Antioxidant and Cytotoxic Effect of Barringtonia racemosa and Hibiscus sabdariffa Fruit Extracts in MCF-7 Human Breast Cancer Cell Line. Pharmacogn. Res. 2016, 8, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Fithrotunnisa, Q.; Arsianti, A.; Kurniawan, G.; Qorina, F.; Tejaputri, N.A.; Azizah, N.N. In vitro Cytotoxicity of Hibiscus sabdariffa Linn Extracts on A549 Lung Cancer Cell Line. Pharmacogn. J. 2020, 12, 14–19. [Google Scholar] [CrossRef]
- Chaves, F.M.; Pavan, I.C.B.; Da Silva, L.G.S.; De Freitas, L.B.; Rostagno, M.A.; Antunes, A.E.C.; Bezerra, R.M.N.; Simabuco, F.M. Pomegranate Juice and Peel Extracts are Able to Inhibit Proliferation, Migration and Colony Formation of Prostate Cancer Cell Lines and Modulate the Akt/mTOR/S6K Signaling Pathway. Plant Foods Hum. Nutr. 2019, 75, 54–62. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Liang, L.; Zeng, A.; Ye, T.; Yang, F.; He, H.; Yao, Y.-Q.; Xie, Y.; An, Z. Punica granatum (pomegranate) peel extract exerts potent antitumor and anti-metastasis activity in thyroid cancer. RSC Adv. 2016, 6, 84523–84535. [Google Scholar] [CrossRef]
- Díaz-Castillo, C. Junk DNA Contribution to Evolutionary Capacitance Can Drive Species Dynamics. Evol. Boil. 2016, 44, 190–205. [Google Scholar] [CrossRef]
- Khateef, R.; Khadri, H.; Almatroudi, A.; A Alsuhaibani, S.; Mobeen, S.A.; A Khan, R. Potential in-vitro anti-breast cancer activity of green-synthesized silver nanoparticles preparation against human MCF-7 cell-lines. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045012. [Google Scholar] [CrossRef]
- Dinparvar, S.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Aydogdu, M.; Aktas, D. A nanotechnology-based new approach in the treatment of breast cancer: Biosynthesized silver nanoparticles using Cuminum cyminum L. seed extract. J. Photochem. Photobiol. B Boil. 2020, 208, 111902. [Google Scholar] [CrossRef]
- Khallouki, F.; Breuer, A.; Akdad, M.; Laassri, F.E.; Attaleb, M.; Elmoualij, B.; Mzibri, M.; Benbacer, L.; Owen, R.W. Cytotoxic activity of Moroccan Melissa officinalis leaf extracts and HPLC-ESI-MS analysis of its phytoconstituents. Future J. Pharm. Sci. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Domaszewski, P.; Pokora, I.; Żebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Ursin, G.; Emory, T.H.; Lee, E.; Wang, R.; Torkelson, C.J.; Dostal, A.M.; Swenson, K.; Le, C.T.; Yang, C.S.; et al. A Randomized Controlled Trial of Green Tea Extract Supplementation and Mammographic Density in Postmenopausal Women at Increased Risk of Breast Cancer. Cancer Prev. Res. 2017, 10, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Okizaki, A.; Takahashi, K. A Randomized Controlled Trial for the Effectiveness of Aromatherapy in Decreasing Salivary Gland Damage following Radioactive Iodine Therapy for Differentiated Thyroid Cancer. BioMed Res. Int. 2016, 2016, 9509810. [Google Scholar] [CrossRef]
- Citronberg, J.; Bostick, R.; Ahearn, T.; Turgeon, D.K.; Ruffin, I.M.T.; Djuric, Z.; Sen, A.; Brenner, D.E.; Zick, S.M. Effects of Ginger Supplementation on Cell Cycle Biomarkers in the Normal-Appearing Colonic Mucosa of Patients at Increased Risk for Colorectal Cancer: Results from a Pilot, Randomized, Controlled Trial. Cancer Prev. Res. 2013, 6, 271–281. [Google Scholar] [CrossRef]
- Morovati, A.; Gargari, B.P.; Sarbakhsh, P.; Azari, H.; Lotfi-Dizaji, L. The effect of cumin supplementation on metabolic profiles in patients with metabolic syndrome: A randomized, triple blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 1182–1190. [Google Scholar] [CrossRef]
- Boldaji, R.B.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: A randomized crossover trial. J. Sci. Food Agric. 2019, 100, 846–854. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; Dávalos, A.; González-Sarrías, A.; Casas-Agustench, P.; Visioli, F.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; et al. MicroRNAs expression in normal and malignant colon tissues as biomarkers of colorectal cancer and in response to pomegranate extracts consumption: Critical issues to discern between modulatory effects and potential artefacts. Mol. Nutr. Food Res. 2015, 59, 1973–1986. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Hammouda, O.; Chtourou, H.; Trabelsi, K.; Bouaziz, M.; Abdelkarim, O.; Hoekelmann, A.; Ayadi, F.; Souissi, N.; et al. Effects of Pomegranate Juice Supplementation on Oxidative Stress Biomarkers Following Weightlifting Exercise. Nutrients 2017, 9, 819. [Google Scholar] [CrossRef]
- Urbaniak, A.; Basta, P.; Ast, K.; Wołoszyn, A.; Wołoszyn, J.K.; Latour, E.; Skarpanska-Stejnborn, A. The impact of supplementation with pomegranate fruit (Punica granatum L.) juice on selected antioxidant parameters and markers of iron metabolism in rowers. J. Int. Soc. Sports Nutr. 2018, 15, 35. [Google Scholar] [CrossRef]
- Zeraatpishe, A.; Oryan, S.; Bagheri, M.H.; Pilevarian, A.A.; Malekirad, A.A.; Baeeri, M.; Abdollahi, M. Effects of Melissa officinalis L. on oxidative status and DNA damage in subjects exposed to long-term low-dose ionizing radiation. Toxicol. Ind. Heal. 2010, 27, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Duarte, J.; Jiménez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharmacol. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef]
- Sari, A.; Selim, N.; Dilek, M. Effect of lemon juice on blood pressure. J. Exp. Clin. Med. 2012, 29, 38–41. [Google Scholar] [CrossRef][Green Version]
- Langmead, L.; Rampton, D. Herbal treatment in gastrointestinal and liver disease—Benefits and dangers. Aliment. Pharmacol. Ther. 2001, 15, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Deshpande, A.; Saxena, K.; Varma, M.; Sinha, A.R. Ginger supplementary therapy for iron absorption in iron deficiency anemia. Indian J. Tradit. Know. 2012, 11, 78–80. [Google Scholar]
- Hopkins, A.L.; Lamm, M.G.; Funk, J.L.; Ritenbaugh, C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia 2013, 85, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.; Wong, S.Y.; Chong, N.J. Effects of Hibiscus sabdariffa L. on serum lipids: A systematic review and meta-analysis. J. Ethnopharmacol. 2013, 150, 442–450. [Google Scholar] [CrossRef]
- Bule, M.H.; Albelbeisi, A.H.; Nikfar, S.; Amini, M.; Abdollahi, M. The antidiabetic and antilipidemic effects of Hibiscus sabdariffa: A systematic review and meta-analysis of randomized clinical trials. Food Res. Int. 2020, 130, 108980. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, A.; Kumar, P. Pharmacotherapeutic Botanicals for Cancer Chemoprevention; Springer: Singapore, 2020. [Google Scholar]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol. Sci. 2001, 22, 285–291. [Google Scholar] [CrossRef]
- Lakshmi, P.; Kumar, S.; Pawar, S.; Kuriakose, B.B.; Sudheesh, M.; Pawar, R.S. Targeting metabolic syndrome with phytochemicals: Focus on the role of molecular chaperones and hormesis in drug discovery. Pharmacol. Res. 2020, 159, 104925. [Google Scholar] [CrossRef]
- Mehta, R.; Lansky, E.P. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur. J. Cancer Prev. 2004, 13, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Tsatsakis, A.; Agathokleous, E.; Giordano, J.; Calabrese, V. Does Green Tea Induce Hormesis? Dose Response 2020, 18, 1559325820936170. [Google Scholar] [CrossRef] [PubMed]
- Günes-Bayir, A.; Kocyigit, A.; Guler, E.M.; Dadak, A. In Vitro Hormetic Effect Investigation of Thymol on Human Fibroblast and Gastric Adenocarcinoma Cells. Molecules 2020, 25, 3270. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jašek, K.; Vybohova, D.; Líšková, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef]
- Četojević-Simin, D.D.; Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Mrđanović, J.Ž.; Bogdanović, V.V.; Šolajić, S.V. Bioactivity of Lemon Balm Kombucha. Food Bioprocess Technol. 2010, 5, 1756–1765. [Google Scholar] [CrossRef]
| Name of the Herbal Infusion/Ref. | Extracts/Oils | Active Ingredients | Antioxidant and Anti-Tumor Mechanisms | Type of Cancer Treated | Cell Lines Used (In Vitro) |
|---|---|---|---|---|---|
| Lemon [203,204] | water extract, volatile oils, lemon juice | limonene, ascorbic acid, phenolics, flavonoids, carotenoids, reducing sugars, indolofuroquinoxaline, alkaloids, terpenoids, geranial, neral | reduced exogenous H2O2 effect, enhance the activity of catalase and SOD, inhibit DPPH, decrease the expression of BcL-2 and the proliferative marker Ki-67, downregulate of caspase 3 | myeloid leukemia, prostate, lung and breast, gastric cancer | K562 MDA-MB 231 MCF7 PC-3 A549 AGS BGC-823 SGC-7901 |
| Ginger [205,206] | aqueous extract, oil/water soluble extract | gingerols and shogaols, gallic acid, quercetin | reduce oxidative stress and raise total antioxidant capacity, represse activities of MMP-2 and MMP-9, increase p53, CASP2 and DEDD, high expression levels of ABCA2 or ABCA3 transporter genes | breast and cervical cancer, ovarian, leukemia | Hela MDA-MB-231 SKOV-3 CCRF-CEM Nalm-6 |
| Wild thyme (Thymus serpyllum) [207,208] | aqueous extract, essential oils, hexane extract | rosmarinic acid, eriocitrin, luteolin, apigenin, quercetin, luteolin7-O-glucoside, apigenin-7-O-glucoside, luteolin, apigenin, thymol, p-cymene, caryophyllene camphene eucalyptol and β-pinene | prevent oxidation of low-density lipoproteins, increases the activity of SOD, catalase, and GPXs, reduce malondialdehyde, reduce DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway, activate MAPK signaling pathway and AMP-activated protein kinase, decrease of cells in the S phase | liver carcinoma, colon, breast, prostate and lung, pancreatic cancer, osteosarcoma, melanoma | MDA-MB-231 MCF-7 HepG2 HCT-116 PC3 A549 PANC-1 U2OS A375 B164A5 |
| Marjoram (Organum Majorana) [209,210] | methanolic extracts, water extract, essential oil, ethanolic extract aqueous extract | rosmarinic, linalool, estragole | reduce ferric reducing ability, down-regulation of survivin, upregulation of cyclin-dependent kinase inhibitor 1 (p21), activate caspase-dependent extrinsic apoptotic pathway and TNFα pathway, suppress NF-kB | breast and lung cancer, colon, liver cancer | Caco-LNM35 A549 MDA-MB231 MCF-7 HT29 HepG2 |
| Green tea [211] | caffeine, theobromine, theophylline, lignin, organic acids, chloro-phylland, theanine, free amino acids, depsides, carbohydrates, alkaloids, minerals, vitamins, enzymes, polyphenols, tea catechins, epigallocatechin-3gallate, polyphenols, quercetin, epigallocatechin gallate | electron donors and efficient scavengers, interact with proteins and phospholipids in the plasma membrane and regulates signal transduction pathways, transcription factors, DNA methylation, mitochondrial function, and autophagy, prevents of tNOX activity, modulate Bax/blc-2 ratio and trigger G2/M cell cycle arrest | breast cancer, non-small lung cancer | MCF-7 ZR75 T47D A-549 | |
| Lemon verbena [212,213] | crude extract | verbascoside, luteolin 7-diglucuronide, citral or geranial, luteolin, verbascoside, gardoside | protected against lipid peroxidation and protein carbonylation, free radical scavenger, increase in the total antioxidant ability, modulate AMPK activity, decrease NF-κB, increase GST and GPx | human melanoma, human leukemia, colon, liver, brest cancer | A375 Caco2 HepG2 MCF-7 THP-1 |
| Sage [214,215] | water extracts, essential oil, methanolic extract, hydroalcoholic extract, n-hexane soluble extract | were α-terpineol, camphor, α-pinene, camphene, β-cymen, caryphyllene, β-myrcene, β-menth1-en-b-ol, bomeol, flavonoids, diterpenes, manool | prevent lipid peroxidation, increase in the liver antioxidant enzyme GST activity, increase in glutathione (GSH) level and free radical-scavenging | head and neck squamous cell carcinoma, Hodgkin lymphoma, melanoma, human breast cervical, human hepatocellular carcinoma, MO59J, U343 and human glioblastoma, lung. | HNSCC L-540 HD-MyZ HepG2 MO59J U343 U251 NCI-H187 |
| Cinnamon [216,217] | essential oil water extract aqueous and ethanolic extracts, distillate oil | (E)-cinnamaldehyde, benzaldehyde, (E)-cinnamyl acetate, saponins, tannins, phenols, terpenoids, and phytosterols, flavonoids and amino acids, coumarin, melatonin | decrease the lipid peroxidation via enhancement of the hepatic antioxidant enzyme activities | basal cell carcinoma, cervix carcinomacancer, leukemia, colorectal carcinoma, epidermoid carcinoma, brain cancer, breast cancer | HeLa HL-60 HCT-116 HT-29 SW-480 A431 SiHa SK-N-MC MCF-7, MDA-MB-231, BT-549 |
| Damask rose [218] | essential oil, aqueous and ethanolic extracts, methanolic extracts | flavonoid, citronellol, n-nonadecane, n-heneicosane, 1-nonadecene, geraniol | inhibits acetylcholinesterase and butyrylcholinesterase, radical scavenging and ferric reducing antioxidant | lung | A549 |
| Chamomile [219,220] | water and alcohol extracts, methanol extract, hydroalcoholic Extract | terpenoids α-bisabolol and its oxides and azulenes, including chamazulene, β-farnesene, α-farnesene, α-bisabolol, and its oxide and chamazulene, bisabololoxide A | free radical scavenging, increase SOD, GPXs, and catalase activities, reduce lipid peroxidation | Leukemia, colon | K562 HT29 |
| Primrose [221,222] | water extract, dimethyl sulfoxide Extract, oil, crude aqueous ethanolic extract | ρ-coumaric acid and rutin, decane, campesterol, caryophyllene, sitosterol, flavanol (proanthocyanidins) | reduces H2O2-induced DNA damage, increases malondialdehyde, and TNF-α, decrease NF-kB, cyclooxygenase-2, and MMP -9 | lung, liver, breast, and prostate and cervix cancer cells, colon cancer | A549 HepG2 MCF-7 PC-3 HeLa SW-480 |
| Hibiscus sabdariffa L. [223,224] | methanol extract, aqueous extract, ethanolic extract, n-hexane extract, ethyl acetate extract | alkaloids, tannins, saponins, glycosides, flavonoids (anthocyanin), alkaloids, phenolic acid, ethanimidic acid and ethyl ester | scavenge ROS and free radicals, potent metal-reducing activity, inhibits tumor Ras, NF-κB, CD31, and VEGF/VEGF-R-induced angiogenesis, JNK/p38 signaling cascade -induced apoptosis, increase activation of p21, p53, and caspase-3 | adenocarcinoma, breast cancer, estrogen receptor-expressing breast cancer, human gastric carcinoma, lung cancer | MCF-7 MDA-MB-231 T-47-D A549 |
| Pomegranate [225,226] | alcoholic, aqueous, chloroform extracts, juice | anthocyanins, triterpenoids, steroids, glycosides, saponins, alkaloids, flavonoids, tannins, carbohydrates, and vitamin C, naphthalene, decahydro-1-pentadactyl, 5 hydroxymethyl furfurals, and 1, 3-cyclohexadiene, ellagic acid and luteolin, polyphenols | scavenger for free radical and significant reducing power of the Fe3+/ferricyanide complex, down-regulate various signaling pathways like NF-κB, P13K/AKT/mTOR, and Wnt, reduces MMPs, VEGF, c-met, pro-inflammatory cytokines, cyclines, and Cdks, induces the expression of caspase-3 and -8, reduce phosphorylation levels of Akt, S6K1, inhibit IGF-I/Akt/mTOR pahway | prostate cancer, Ehrlich-ascites-carcinoma and ovarian cancer, thyroid cancer | PC-3 LNCaP A2780 ES-2 DU145 BCPAP |
| Anise seeds (Pimpinella anisum L.) [227,228] | water extract, alcohol extract, ethanolic extract, aqueous-n-butanolic extract, essential oils | flavonoids, phenols, and anthocyanins, lignin-carbohydrate protein, fatty acids (linoleic, oleic, and palmitic acids), triterpenoids (lupeol, β-amyrin and betulinic acids), and sterols (β-sitosterol and stigmasterol), anethole, gallic Acid, catechins, estragole, naringin, chloroginic acid, rosmarinic acid | scavenge DPPH free radicals, reduce oxidant potency, down-regulate of caspase 3 | oral squamous cell carcinoma, gastric cancer, human prostate cancer, breast cancer | AG HUVEC PC-3 MCF-7 |
| Cumin (Cuminum cyminum L.) [163,229] | essential oil, alcoholic and aqueous extracts | alkaloids, anthraquinones, coumarins, flavonoids, glycosides, resins, saponins, tannins, steroids, 3-caren-10-al and cuminal | reducing ROS, diminish the expressions of mTOR and survivin and elevate BECN1 expression | cervical, colon cancer, neuroblastoma, breast cancer | Hela 502713 IMR32 MCF-7 AU565 |
| Mellissa officinalis L. [182,230] | essential oil, aqueous extract, infusion extracts, hydromethanolic, hydroethanolic, methanolic and alcoholic extracts, dichloromethane extract | geranial and neral, luteolin-7-O-glucoside, caffeic acid, 3,4-dihydroxyphenyl lactic acid, 3,4-dihydroxybenzoic acid, lithospermic acid, luteolin-7-O-glucoside, methyl caffeate and rosmarinic acid. | inducing antioxidant enzymes and alleviated liver damage, free radical scavenging activities, increasing GSH concentration and inhibiting lipid peroxidation in the liver tissues, reduce pro-caspase 3 levels | liver, non-small cell lung cancer, breast and gastric cancer | HCC MDA-MB-231 MCF-7 NCI-H460 AGS LNCAP, PC3 MDA-MB-468 |
| Rosemary [191,202] | oil-soluble extracts, aqueous extract, essintial oils, crude extract | caffeic acid, rosmarinic acid, ursolic acid, carnosic acid, and carnosol, α-pinene, camphene, eucalyptol, p-cymene, camphor bornyl acetate, berbonone, 1,8-cineole | scavenge DPPH, prevent Akt and mTOR, reduce cyclin D1, suppress expression of Bcl-2, Bcl-x, cIAP-1, HIF-, and HO-1, Bax, Fas and FADD | breast, cervical, colon, ovarian, lung, esophageal and prostate cancer | MCF7 HeLa A2780 A2780CP7 A549 MDA-MB-231 KYSE30 184-B5/HER HT-29 HCT116 HDAC2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talib, W.H.; AL-ataby, I.A.; Mahmod, A.I.; Jawarneh, S.; Al Kury, L.T.; AL-Yasari, I.H. The Impact of Herbal Infusion Consumption on Oxidative Stress and Cancer: The Good, the Bad, the Misunderstood. Molecules 2020, 25, 4207. https://doi.org/10.3390/molecules25184207
Talib WH, AL-ataby IA, Mahmod AI, Jawarneh S, Al Kury LT, AL-Yasari IH. The Impact of Herbal Infusion Consumption on Oxidative Stress and Cancer: The Good, the Bad, the Misunderstood. Molecules. 2020; 25(18):4207. https://doi.org/10.3390/molecules25184207
Chicago/Turabian StyleTalib, Wamidh H., Israa A. AL-ataby, Asma Ismail Mahmod, Sajidah Jawarneh, Lina T. Al Kury, and Intisar Hadi AL-Yasari. 2020. "The Impact of Herbal Infusion Consumption on Oxidative Stress and Cancer: The Good, the Bad, the Misunderstood" Molecules 25, no. 18: 4207. https://doi.org/10.3390/molecules25184207
APA StyleTalib, W. H., AL-ataby, I. A., Mahmod, A. I., Jawarneh, S., Al Kury, L. T., & AL-Yasari, I. H. (2020). The Impact of Herbal Infusion Consumption on Oxidative Stress and Cancer: The Good, the Bad, the Misunderstood. Molecules, 25(18), 4207. https://doi.org/10.3390/molecules25184207







