Colorimetry of Luminescent Lanthanide Complexes
Abstract
1. Introduction
Colour Theory
2. Materials and Methods
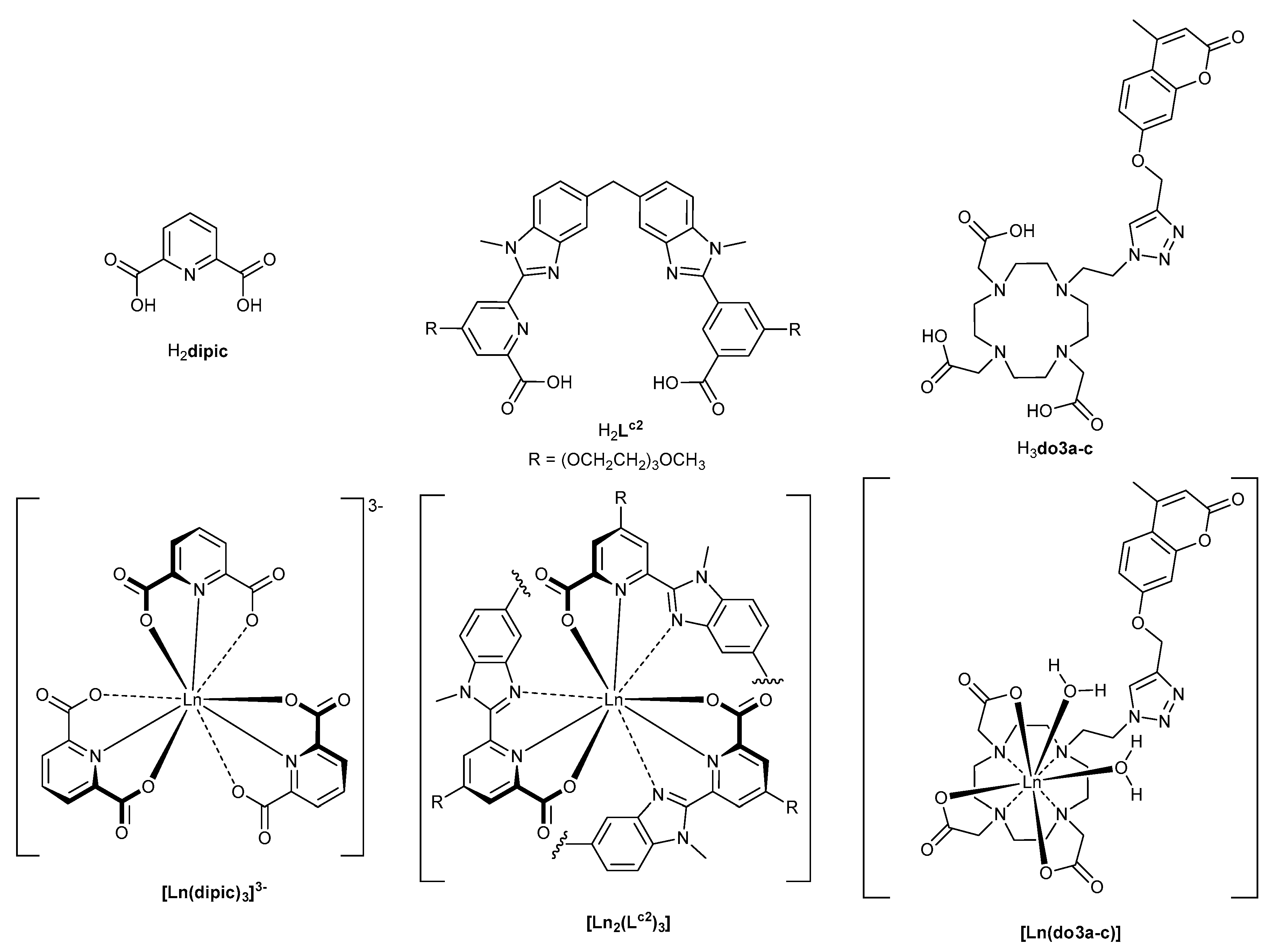
2.1. Preparation of the Ln Complexes
2.2. Measurement of the Emission Spectra
2.3. Data Treatment
Extraction of the Ln Fingerprints and Conversion to Relative Spectral Irradiances
2.4. Broadening Simulation
2.5. Wavelength Shifts
2.6. Colorimetric Analysis
3. Results and Discussion
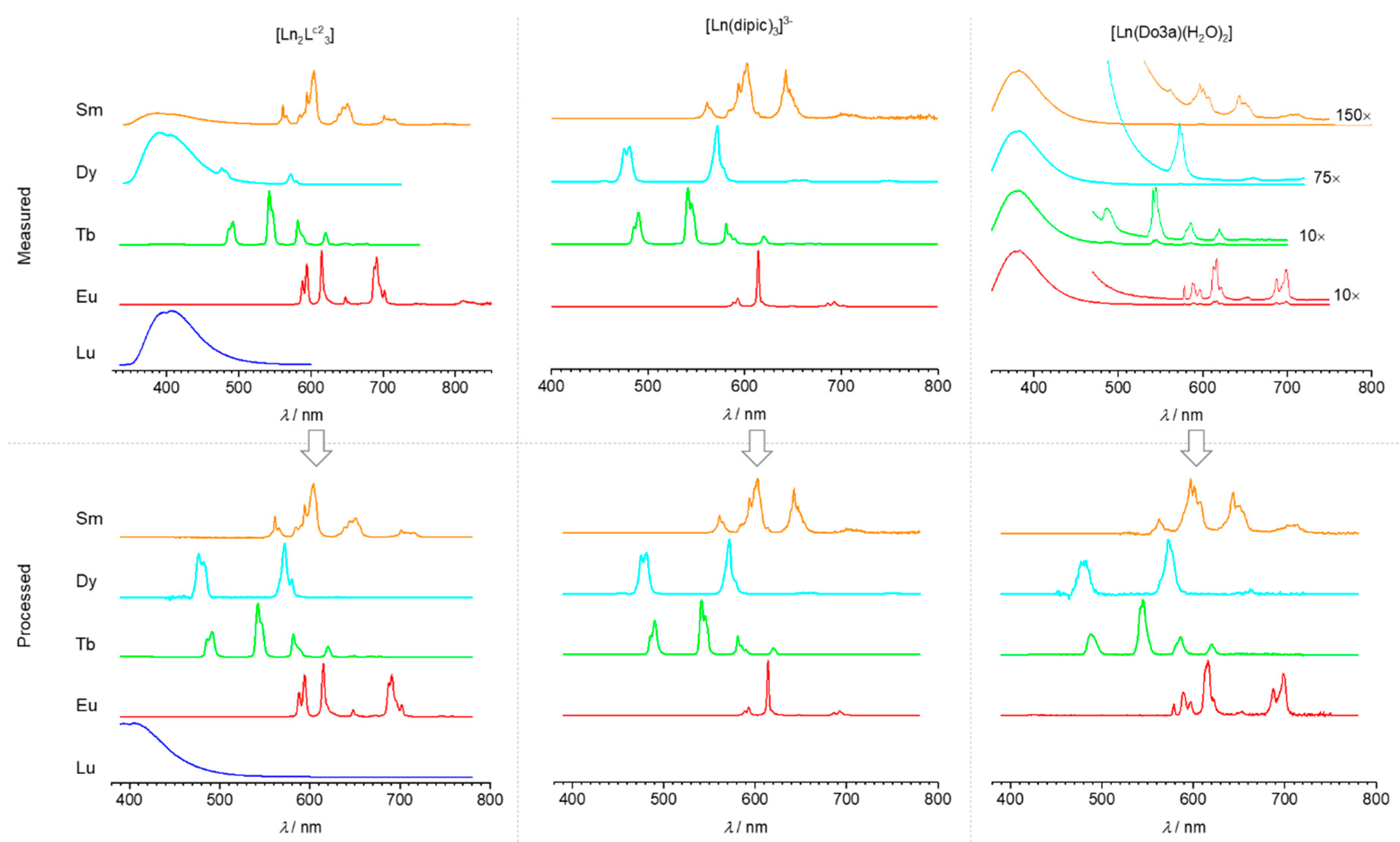
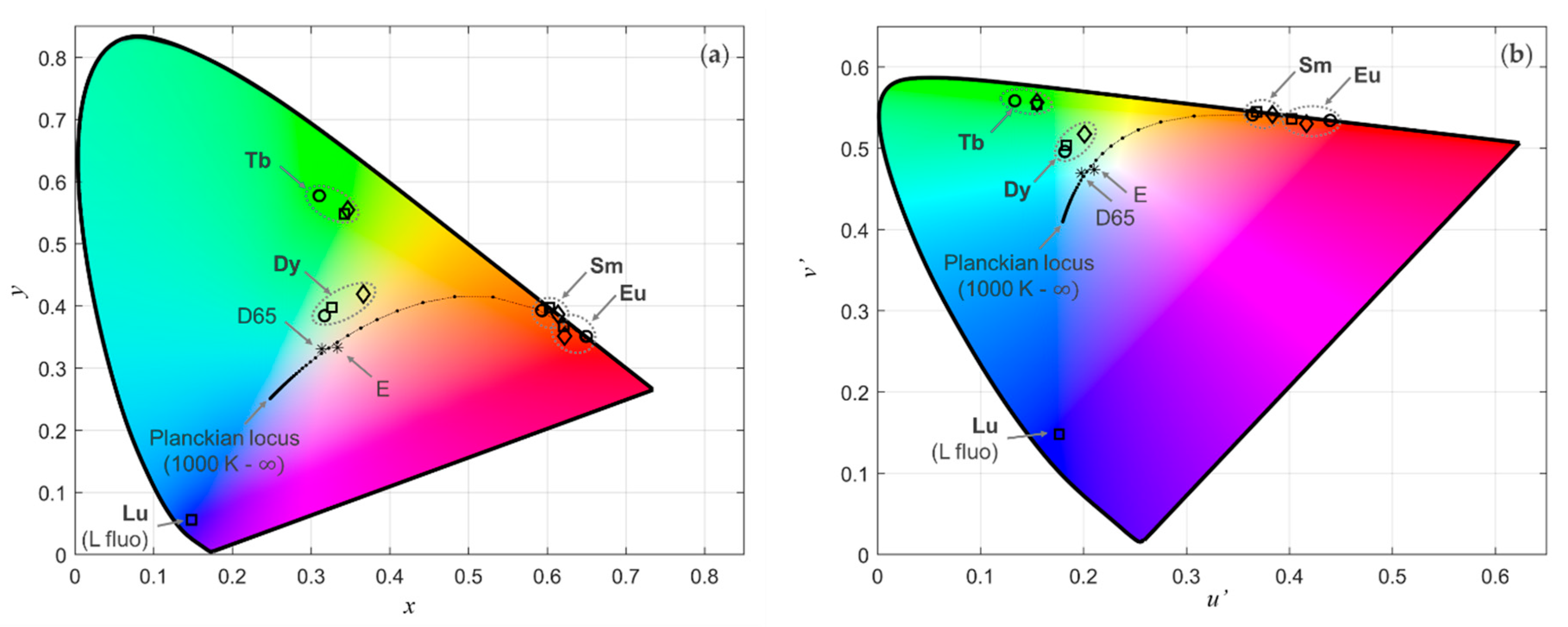
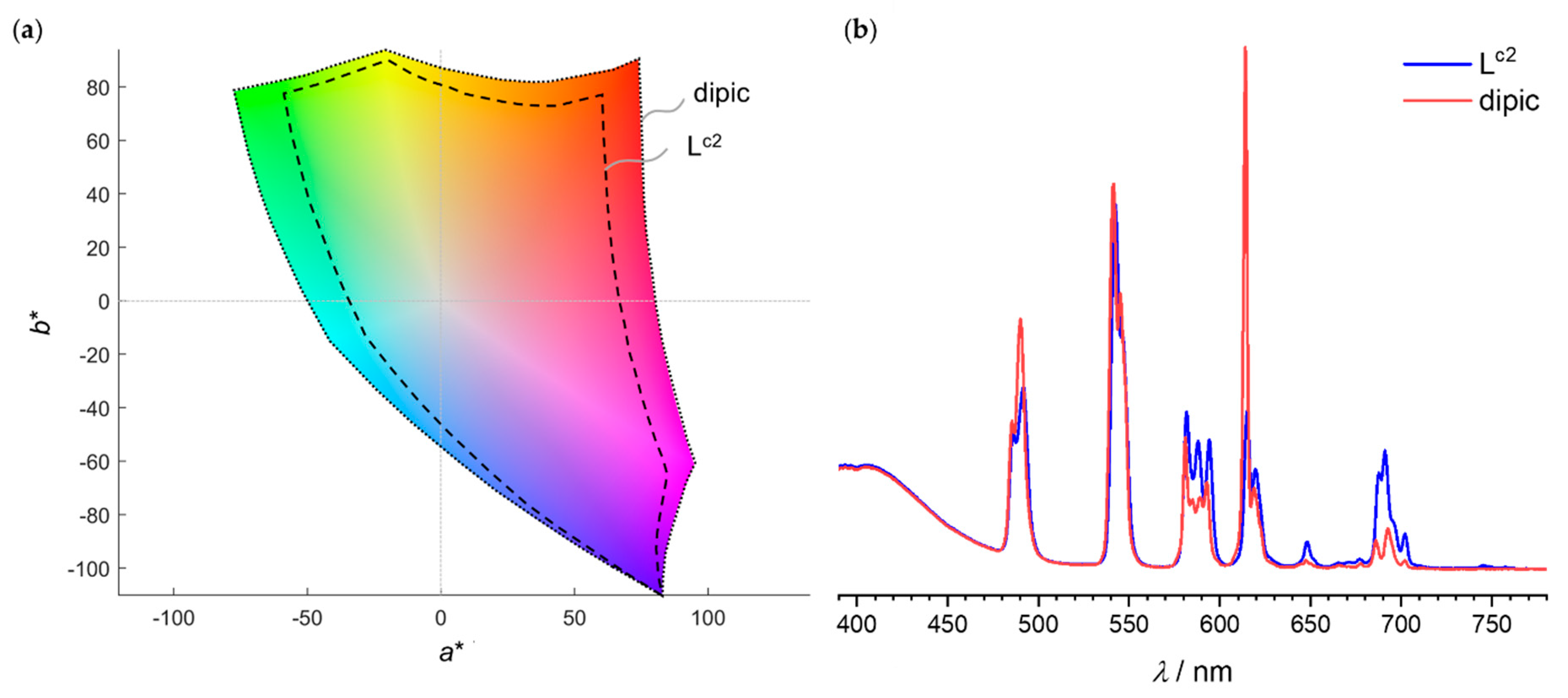
3.1. Colours of the Visible Ln(III) Ions and Variations Induced by the Ligand Field
3.2. Effect of the Bandwidth on the Calculated Ln Luminescent Colours
3.3. Effect of the Spectrometer Correction Function and Spectral Radiometric Conversion on the Calculated Ln Luminescent Colours
3.4. Combinations and Gamut
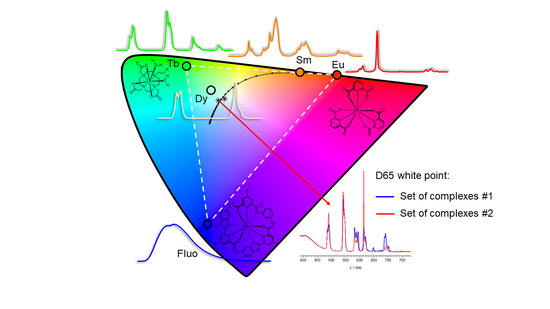
3.4.1. Combining Eu, Tb, Sm, and Dy for Colour Reproduction
3.4.2. Tuning of Red, Green, and Blue Primaries: Matching Intensities and White Points
3.4.3. Combining Red, Green, and Blue: Full 3-D Colour Gamuts
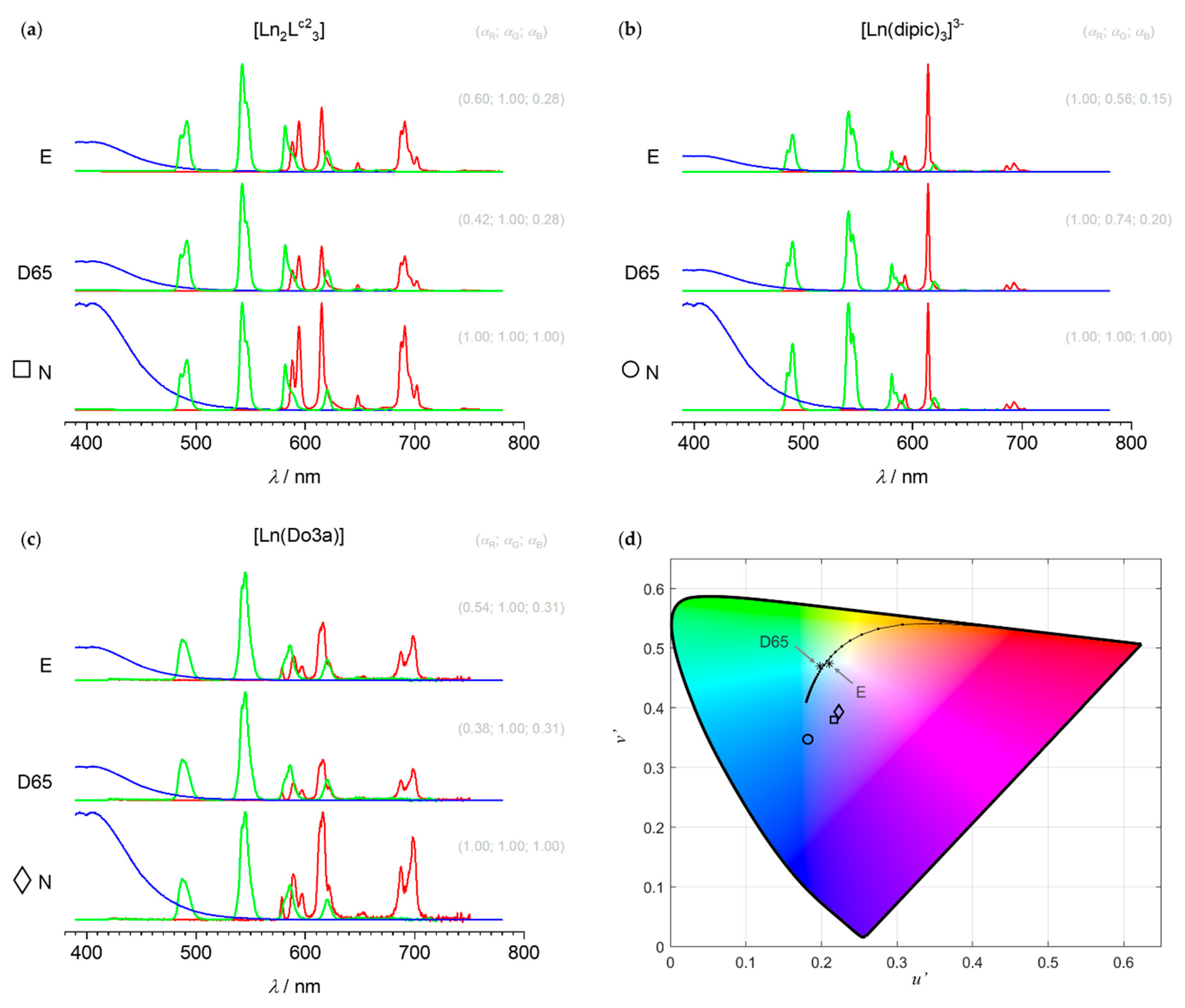
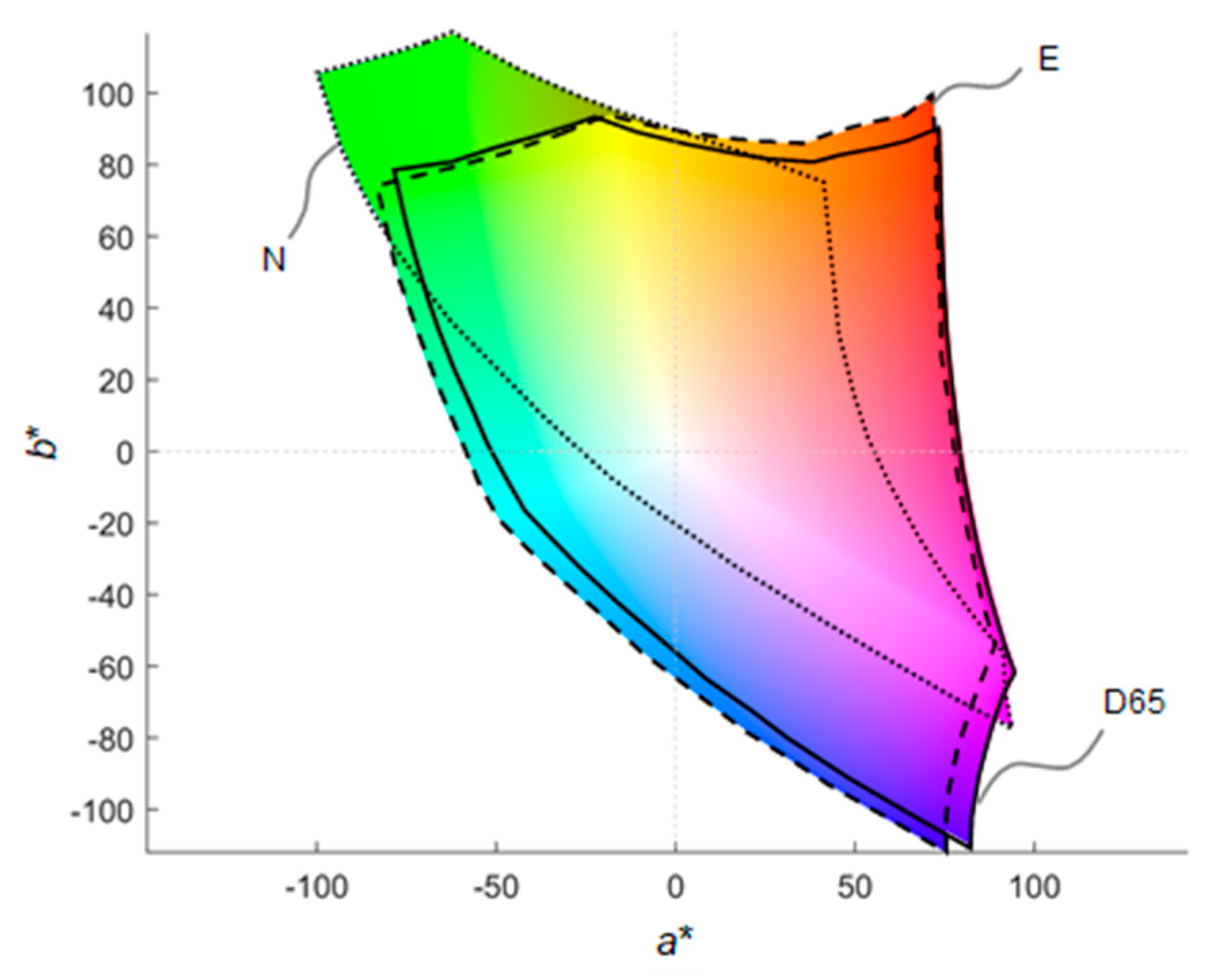
3.4.4. Metamerism and Intensity Matching between Sets of Primaries
3.4.5. Discussion: From Computer Simulations to Real Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-infrared (NIR) lanthanide molecular probes for bioimaging and biosensing. Coord. Chem. Rev. 2019, 399, 213028. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Wu, K.; Jiang, J.-J.; Hsu, C.-W.; Pan, M.; Lehn, J.-M.; Su, C.-Y. Pure white-light and yellow-to-blue emission tuning in single crystals of Dy(iii) metal–organic frameworks. Chem. Commun. 2014, 50, 7702–7704. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.H.S.K.; de Bettencourt-Dias, A. 3-Lanthanide ion emission in multicolor OLEDs (Ce3+, Pr3+, Tb3+, Dy3+, Tm3+, and white light Eu3+/Tb3+ hybrid systems) and device characterization. In Lanthanide-Based Multifunctional Materials; Martín-Ramos, P., Ramos Silva, M., Eds.; Elsevier: London, UK, 2018; pp. 99–131. [Google Scholar]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G. Rising Stars in Science and Technology: Luminescent Lanthanide Materials. Eur. J. Inorg. Chem. 2017, 2017, 5058–5063. [Google Scholar] [CrossRef]
- Ramya, A.R.; Varughese, S.; Reddy, M.L.P. Tunable white-light emission from mixed lanthanide (Eu3+, Gd3+, Tb3+) coordination polymers derived from 4-(dipyridin-2-yl)aminobenzoate. Dalton Trans. 2014, 43, 10940–10946. [Google Scholar] [CrossRef]
- Roberts, R.J.; Le, D.; Leznoff, D.B. Color-Tunable and White-Light Luminescence in Lanthanide–Dicyanoaurate Coordination Polymers. Inorg. Chem. 2017, 56, 7948–7959. [Google Scholar] [CrossRef]
- Andres, J.; Hersch, R.D.; Moser, J.-E.; Chauvin, A.-S. A New Anti-Counterfeiting Feature Relying on Invisible Luminescent Full Color Images Printed with Lanthanide-Based Inks. Adv. Funct. Mater. 2014, 24, 5029–5036. [Google Scholar] [CrossRef]
- Gai, Y.; Guo, Q.; Xiong, K.; Jiang, F.; Li, C.; Li, X.; Chen, Y.; Zhu, C.; Huang, Q.; Yao, R.; et al. Mixed-Lanthanide Metal–Organic Frameworks with Tunable Color and White Light Emission. Cryst. Growth Des. 2017, 17, 940–944. [Google Scholar] [CrossRef]
- Meyer, L.V.; Schönfeld, F.; Müller-Buschbaum, K. Lanthanide based tuning of luminescence in MOFs and dense frameworks–from mono- and multimetal systems to sensors and films. Chem. Commun. 2014, 50, 8093–8108. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Law, G.-L.; Wong, K.-L.; Tam, H.-L.; Cheah, K.-W.; Wong, W.-T. White OLED with a Single-Component Europium Complex. Inorg. Chem. 2009, 48, 10492–10494. [Google Scholar] [CrossRef] [PubMed]
- Zinna, F.; Pasini, M.; Galeotti, F.; Botta, C.; Di Bari, L.; Giovanella, U. Design of Lanthanide-Based OLEDs with Remarkable Circularly Polarized Electroluminescence. Adv. Funct. Mater. 2017, 27, 1603719. [Google Scholar] [CrossRef]
- Jenks, T.C.; Bailey, M.D.; Corbin, B.A.; Kuda-Wedagedara, A.N.W.; Martin, P.D.; Schlegel, H.B.; Rabuffetti, F.A.; Allen, M.J. Photophysical characterization of a highly luminescent divalent-europium-containing azacryptate. Chem. Commun. 2018, 54, 4545–4548. [Google Scholar] [CrossRef] [PubMed]
- Ekanger, L.A.; Mills, D.R.; Ali, M.M.; Polin, L.A.; Shen, Y.; Haacke, E.M.; Allen, M.J. Spectroscopic Characterization of the 3+ and 2+ Oxidation States of Europium in a Macrocyclic Tetraglycinate Complex. Inorg. Chem. 2016, 55, 9981–9988. [Google Scholar] [CrossRef] [PubMed]
- Basal, L.A.; Allen, M.J. Synthesis, Characterization, and Handling of EuII-Containing Complexes for Molecular Imaging Applications. Front. Chem. 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Lu, X.; Mészáros, L.S.; Ott, M.; Andres, J.; Borbas, K.E. Photophysics of Coumarin and Carbostyril-Sensitized Luminescent Lanthanide Complexes: Implications for Complex Design in Multiplex Detection. J. Am. Chem. Soc. 2017, 139, 5756–5767. [Google Scholar] [CrossRef]
- Kovacs, D.; Borbas, K.E. The role of photoinduced electron transfer in the quenching of sensitized Europium emission. Coord. Chem. Rev. 2018, 364, 1–9. [Google Scholar] [CrossRef]
- Ćirić, A.; Stojadinović, S.; Dramićanin, M.D. Approximate prediction of the CIE coordinates of lanthanide-doped materials from the Judd-Ofelt intensity parameters. J. Lumin. 2019, 213, 395–400. [Google Scholar] [CrossRef]
- Alsaadi, B.M.; Rossotti, F.J.C.; Williams, R.J.P. Studies of lanthanide(III) pyridine-2,6-dicarboxylate complexes in aqueous solution. Part 1. Structures and 1H nuclear magnetic resonance spectra. J. Chem. Soc. Dalton Trans. 1980, 597–602. [Google Scholar] [CrossRef]
- Alsaadi, B.M.; Rossotti, F.J.C.; Williams, R.J.P. Studies of lanthanide (III) dipicolinate complexes in aqueous solution. Part 2. Hydration. J. Chem. Soc. Dalton Trans. 1980, 1, 813–816. [Google Scholar] [CrossRef]
- Sveshnikova, E.B.; Shakhverdov, P.A.; Shakhverdov, T.A.; Lanin, V.E.; Safina, R.U.; Bolotin, B.M.; Ermolaev, V.L. Luminescence of dipicolinic complexes of lanthanide ions. Opt. Spectrosc. 2003, 95, 898–907. [Google Scholar] [CrossRef]
- Chauvin, A.; Gumy, F.; Imbert, D.; Bünzli, J.G. Europium and Terbium tris(Dipicolinates) as Secondary Standards for Quantum Yield Determination. Spectrosc. Lett. 2004, 37, 517–532. [Google Scholar] [CrossRef]
- Gassner, A.-L.; Duhot, C.; Bünzli, J.-C.; Chauvin, A.-S. Remarkable Tuning of the Photophysical Properties of Bifunctional Lanthanide tris(Dipicolinates) and its Consequence on the Design of Bioprobes. Inorg. Chem. 2008, 47, 7802–7812. [Google Scholar] [CrossRef]
- D’Aléo, A.; Picot, A.; Beeby, A.; Gareth Williams, J.A.; Le Guennic, B.; Andraud, C.; Maury, O. Efficient Sensitization of Europium, Ytterbium, and Neodymium Functionalized Tris-Dipicolinate Lanthanide Complexes through Tunable Charge-Transfer Excited States. Inorg. Chem. 2008, 47, 10258–10268. [Google Scholar] [CrossRef]
- Aebischer, A.; Gumy, F.; Bünzli, J.-C.G. Intrinsic quantum yields and radiative lifetimes of lanthanide tris(dipicolinates). Phys. Chem. Chem. Phys. 2009, 11, 1346–1353. [Google Scholar] [CrossRef]
- Andres, J.; Chauvin, A.-S. Energy transfer in coumarin-sensitised lanthanide luminescence: investigation of the nature of the sensitiser and its distance to the lanthanide ion. Phys. Chem. Chem. Phys. 2013, 15, 15981–15994. [Google Scholar] [CrossRef]
- Dumont, E.; Pompidor, G.; D’Aléo, A.; Vicat, J.; Toupet, L.; Kahn, R.; Girard, E.; Maury, O.; Giraud, N. Exploration of the supramolecular interactions involving tris-dipicolinate lanthanide complexes in protein crystals by a combined biostructural, computational and NMR study. Phys. Chem. Chem. Phys. 2013, 15, 18235–18242. [Google Scholar] [CrossRef]
- Andres, J.; Borbas, K.E. Expanding the Versatility of Dipicolinate-Based Luminescent Lanthanide Complexes: A Fast Method for Antenna Testing. Inorg. Chem. 2015, 54, 8174–8176. [Google Scholar] [CrossRef]
- Balogh, C.M.; Veyre, L.; Pilet, G.; Charles, C.; Viriot, L.; Andraud, C.; Thieuleux, C.; Riobé, F.; Maury, O. Two-Color Three-State Luminescent Lanthanide Core–Shell Crystals. Chem. Eur. J. 2017, 23, 1784–1788. [Google Scholar] [CrossRef]
- Vithanarachchi, S.M.; Kovacs, D.; Borbas, K.E. Synthesis and photophysical characterization of luminescent lanthanide complexes of nucleotide-functionalized cyclen- and dipicolinate-based ligands. Inorg. Chim. Acta 2017, 460, 148–158. [Google Scholar] [CrossRef]
- Sharma, G.; Bala, R. Digital Color Imaging Handbook; CRC press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Westland, S. UCS Diagrams; Uniform Chromaticity Scales. In Encyclopedia of Color Science and Technology; Luo, M.R., Ed.; Springer: New York, NY, USA, 2016; pp. 1243–1245. [Google Scholar]
- International Electrotechnical Commission. Multimedia Systems and Equipment-Colour Measurement and Management-Part 2-1: Colour Management-Default RGB Colour Space-sRGB; International Electrotechnical Commission: Geneva, Switzerland, 1999. [Google Scholar]
- Sharma, A. Understanding RGB Color Spaces for Monitors, Projectors, and Televisions. Inf. Disp. 2019, 35, 17–43. [Google Scholar] [CrossRef]
- Chauvin, A.-S.; Comby, S.; Song, B.; Vandevyver, C.D.B.; Bünzli, J.-C.G. A Versatile Ditopic Ligand System for Sensitizing the Luminescence of Bimetallic Lanthanide Bio-Imaging Probes. Chem. Eur. J. 2008, 14, 1726–1739. [Google Scholar] [CrossRef] [PubMed]
- Comby, S. Luminescent Lanthanides Bioprobes Emitting in the Visible and/or Near-Infrared Ranges. Ph.D. Thesis, EPFL, Lausanne, Switzerland, 2008. [Google Scholar]
- Bernardini, F.; Mittleman, J.; Rushmeier, H.; Silva, C.; Taubin, G. The ball-pivoting algorithm for surface reconstruction. IEEE Trans. Vis. Comput. Graph. 1999, 5, 349–359. [Google Scholar] [CrossRef]
- Nielsen, L.G.; Sørensen, T.J. Including and Declaring Structural Fluctuations in the Study of Lanthanide(III) Coordination Chemistry in Solution. Inorg. Chem. 2020, 59, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, P.R.; Kofod, N.; Juelsholt, M.; Jensen, K.M.Ø.; Sørensen, T.J. The effect of weighted averages when determining the speciation and structure–property relationships of europium(iii) dipicolinate complexes. Phys. Chem. Chem. Phys. 2020, 22, 12794–12805. [Google Scholar] [CrossRef] [PubMed]
- Pershagen, E.; Borbas, K.E. Designing reactivity-based responsive lanthanide probes for multicolor detection in biological systems. Appl. Lanthan. Compd. Mater. Sci. Biol. 2014, 273–274, 30–46. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Na3[Ln(dipic)3] (Ln = Eu, Tb, Sm, Dy) are available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andres, J.; Chauvin, A.-S. Colorimetry of Luminescent Lanthanide Complexes. Molecules 2020, 25, 4022. https://doi.org/10.3390/molecules25174022
Andres J, Chauvin A-S. Colorimetry of Luminescent Lanthanide Complexes. Molecules. 2020; 25(17):4022. https://doi.org/10.3390/molecules25174022
Chicago/Turabian StyleAndres, Julien, and Anne-Sophie Chauvin. 2020. "Colorimetry of Luminescent Lanthanide Complexes" Molecules 25, no. 17: 4022. https://doi.org/10.3390/molecules25174022
APA StyleAndres, J., & Chauvin, A.-S. (2020). Colorimetry of Luminescent Lanthanide Complexes. Molecules, 25(17), 4022. https://doi.org/10.3390/molecules25174022