Lanthanide Luminescence in Visible-Light-Promoted Photochemical Reactions
Abstract
1. Introduction
2. Samarium
2.1. Luminescence of Samarium
2.2. Effect of Additives on Luminscence of Samarium
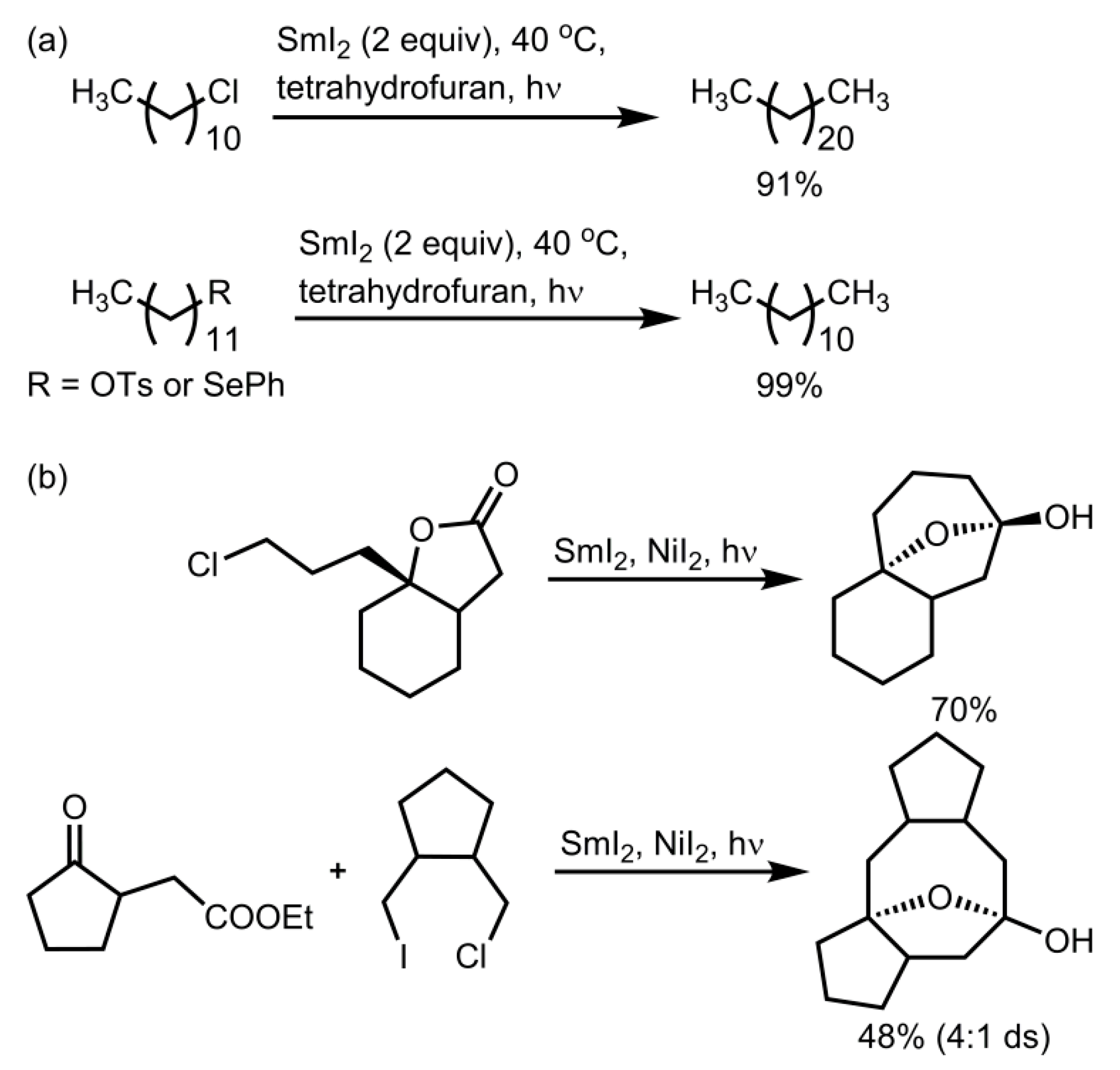
2.3. Photochemical Applications of Samarium
3. Europium
3.1. Luminescence of Europium
Ligand Effects on Luminescence of Europium
3.2. Photochemical Applications of Europium
4. Cerium
4.1. Luminescence of Cerium
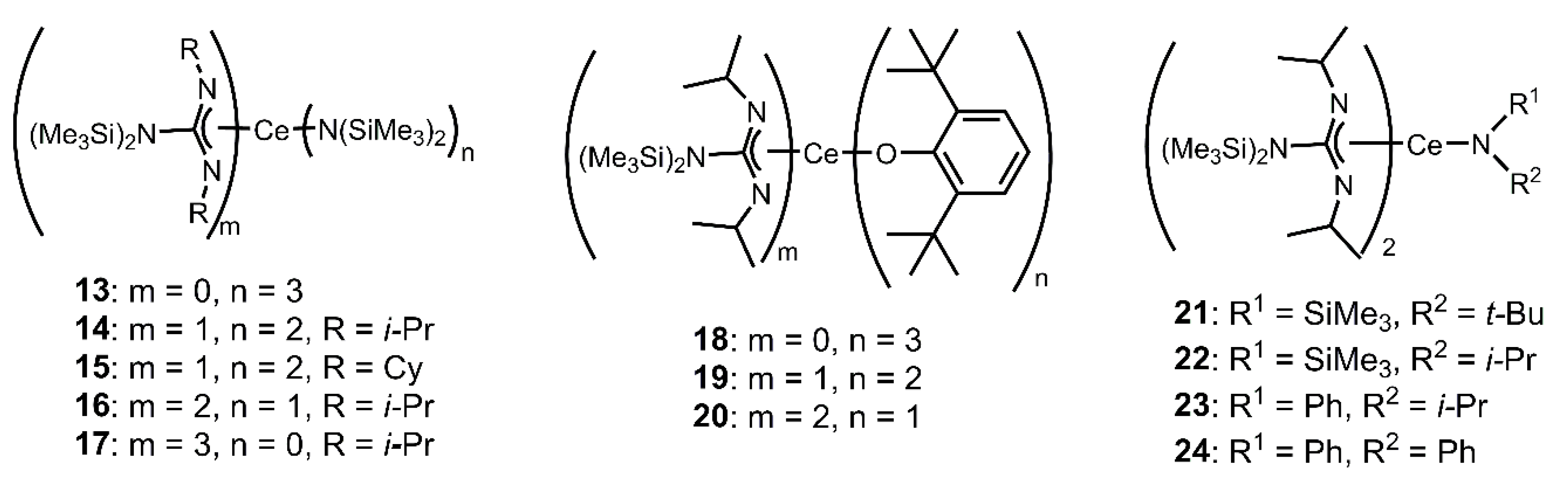
Ligand Effects on Luminescence of Cerium
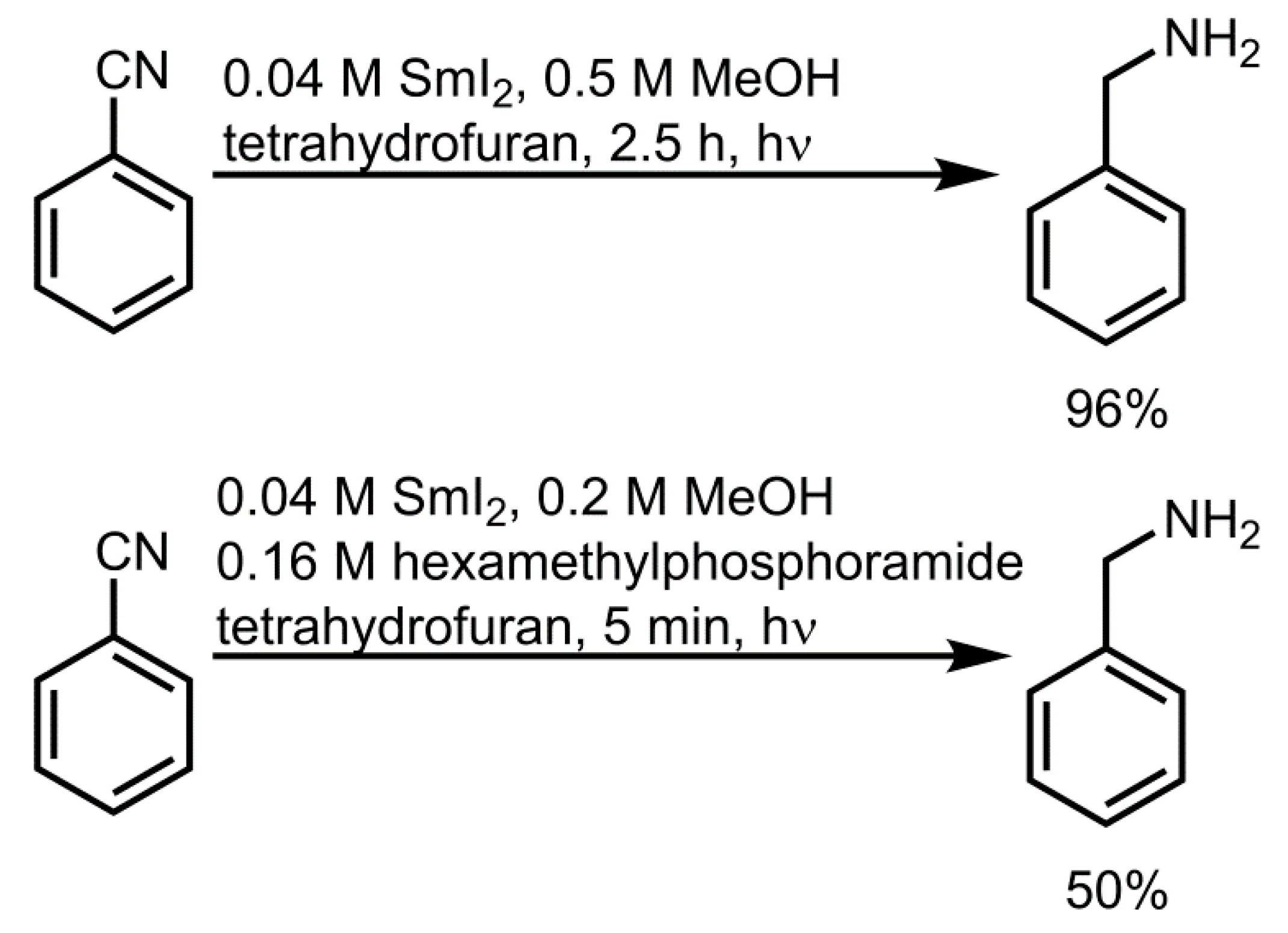
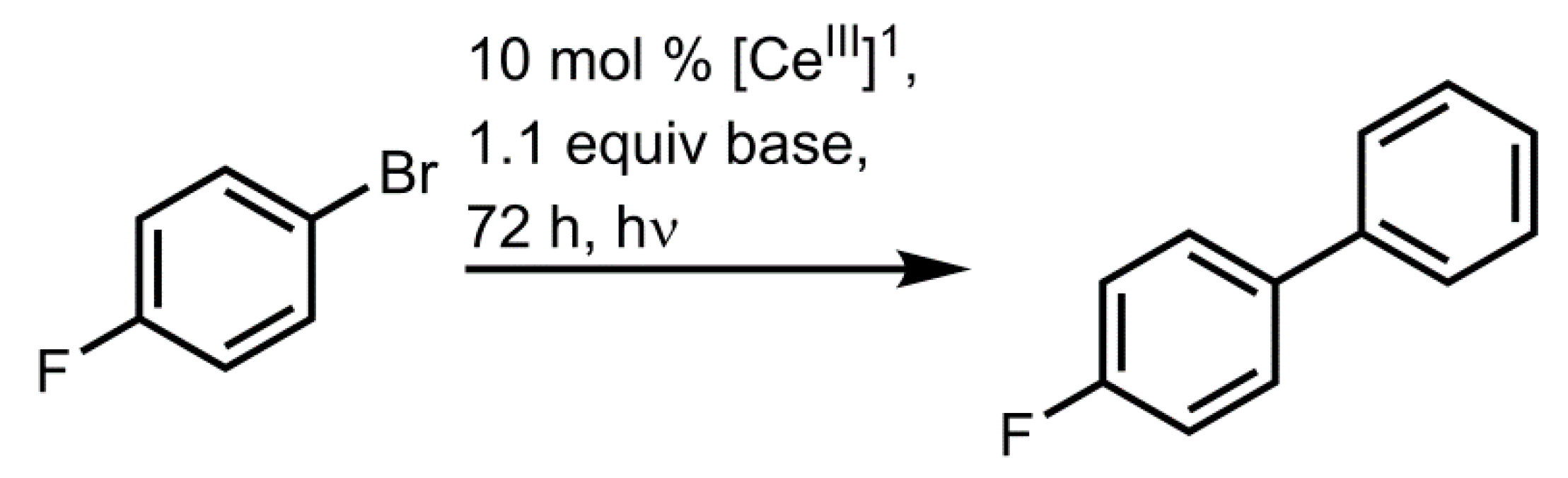
4.2. Photochemical Applications of Cerium
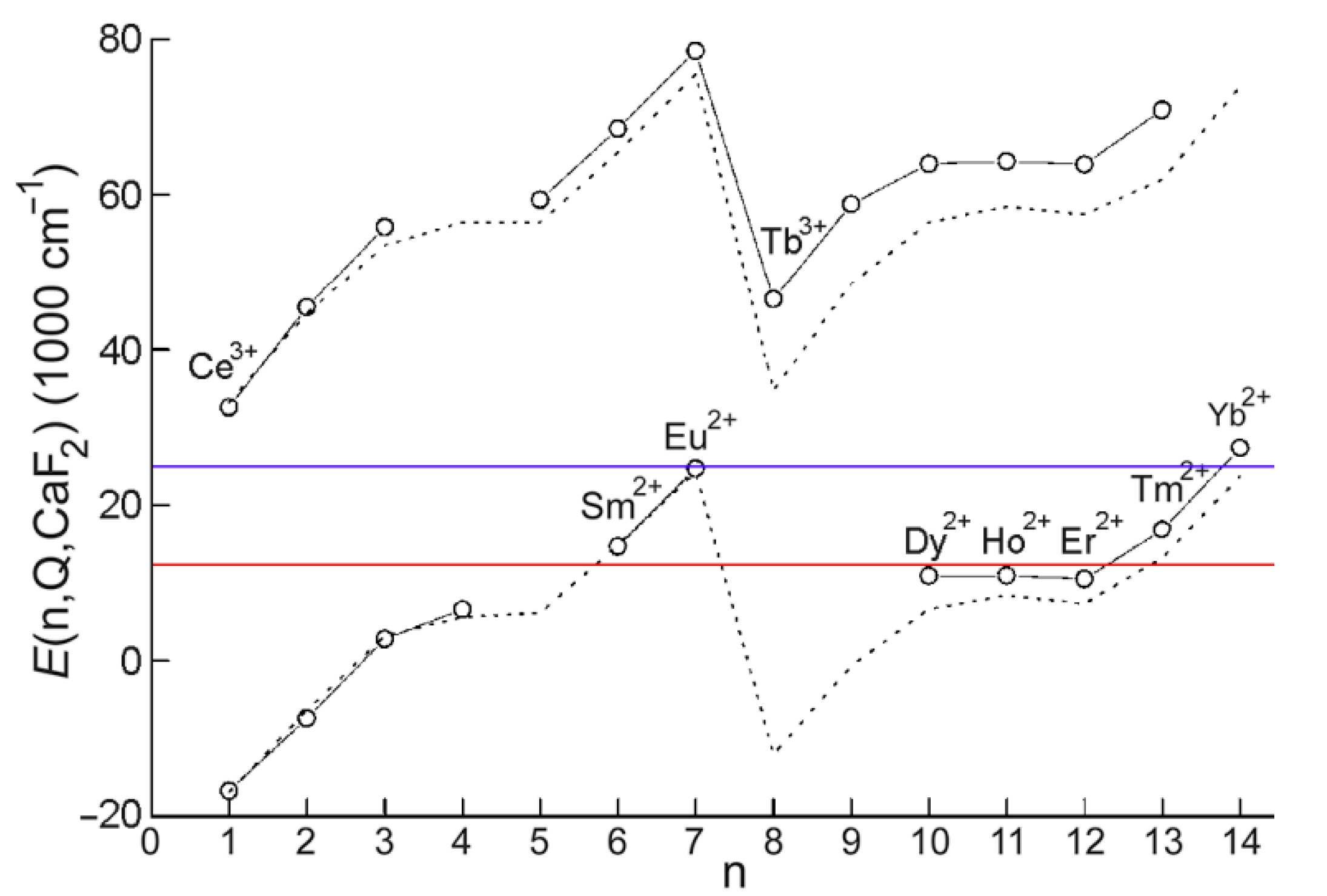
5. Other Lanthanides
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Zhang, Y.; Jamison, T.F.; Stephenson, C.R.J. Visible-light photoredox catalysis in flow. Angew. Chem. Int. Ed. 2012, 51, 4144–4147. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef]
- Bou-Hamdan, F.R.; Seeberger, P.H. Visible-light-mediated photochemistry: Accelerating Ru(bpy)32+-catalyzed reactions in continuous flow. Chem. Sci. 2012, 3, 1612–1616. [Google Scholar] [CrossRef]
- Qiao, Y.; Schelter, E.J. Lanthanide Photocatalysis. Acc. Chem. Res. 2018, 51, 2926–2936. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photophysics, photochemistry and solar energy conversion with tris(bipyridyl)ruthenium(II) and its analogues. Coord. Chem. Rev. 1982, 46, 159–244. [Google Scholar] [CrossRef]
- Larsen, C.B.; Wenger, O.S. Photoredox catalysis with metal complexes made from earth-abundant elements. Chem. Eur. J. 2018, 24, 2039–2058. [Google Scholar] [CrossRef]
- Ochola, J.R.; Wolf, M.O. The effect of photocatalyst excited state lifetime on the rate of photoredox catalysis. Org. Biomol. Chem. 2016, 14, 9088–9092. [Google Scholar] [CrossRef]
- Lammer, A.D.; Thiabaud, G.; Brewster, J.T., II; Alaniz, J.; Bender, J.A.; Sessler, J.L. Lanthanide texaphyrins as photocatalysts. Inorg. Chem. 2018, 57, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, R.L.; Gao, S. Composition on the continental crust. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–51. [Google Scholar]
- Suta, M.; Wickleder, C. Synthesis, spectroscopic properties and applications of divalent lanthanides apart from Eu2+. J. Lumin. 2019, 210, 210–238. [Google Scholar] [CrossRef]
- Vogler, A.; Kunkely, H. Excited state properties of lanthanide complexes: Beyond ff states. Inorg. Chim. Acta 2006, 359, 4130–4138. [Google Scholar] [CrossRef]
- Dorenbos, P. f→d transition energies of divalent lanthanides in organic compounds. J Phys.Condens. Matter 2003, 15, 575–594. [Google Scholar] [CrossRef]
- Sumino, Y.; Harato, N.; Tomisaka, Y.; Ogawa, A. A novel photoinduced reduction system of low-valent samarium species: Reduction of organic halides and chalcogenides, and its application to carbonylation with carbon monoxide. Tetrahedron 2003, 59, 10499–10508. [Google Scholar] [CrossRef]
- Douglas, D.L.; Yost, D.M. Photo-chemical reduction of water by europium(II) ion, and the magnetic susceptibilities of europium(II) and (III) ions. J. Chem. Phys. 1949, 17, 1345–1346. [Google Scholar] [CrossRef]
- Davis, D.D.; Stevenson, K.L.; King, G.K. Photolysis of europium(II) perchlorate in aqueous acidic solution. Inorg. Chem. 1997, 16, 670–673. [Google Scholar] [CrossRef]
- Ishida, A.; Toki, S.; Takamuku, S. Photochemical Reactions of α-methylstyrene induced by Eu(III)/Eu(II) photoredox system in methanol. Chem. Lett. 1985, 14, 893–896. [Google Scholar] [CrossRef]
- Kondo, T.; Akazome, M.; Watanabe, Y. Lanthanide(II) iodide catalysed photochemical allylation of aldehydes with allylic halides. J. Chem. Soc. Chem. Commun. 1991, 757–758. [Google Scholar] [CrossRef]
- de Bettencourt-Dias, A. Editorial for the virtual issue on photochemistry and photophysics of lanthanide compounds. Inorg. Chem. 2016, 55, 3199–3202. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.; Huang, W.; Bettinelli, M.; Liu, X. Lanthanide-activated phosphors based on 4f-5d optical transitions: Theoretical and experimental aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef]
- Lin, Y.C.; Karlsson, M.; Bettinelli, M. Inorganic phosphor materials for lighting. Top. Curr. Chem. 2016, 374, 21. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, D.J.; Johnston, D.; Procter, D.J. Samarium(II)-iodide-mediated cyclizations in natural product synthesis. Chem. Rev. 2004, 104, 3371–3403. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Fazakerley, N.J.; Parmar, D.; Procter, D.J. Cross-coupling reactions using samarium(II) iodide. Chem. Rev. 2014, 114, 5959–6039. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, A.; Hilmersson, G. Samarium(II) iodide mediated reductions – influence of various additives. Eur. J. Inorg. Chem. 2004, 3393–3403. [Google Scholar] [CrossRef]
- Yin, H.; Jin, Y.; Hertzog, J.E.; Mullane, K.C.; Carroll, P.J.; Manor, B.C.; Anna, J.M.; Schelter, E.J. The hexachlorocerate(III) anion: A potent, benchtop stable, and readily available ultraviolet A photosensitizer for aryl chlorides. J. Am. Chem. Soc. 2016, 138, 16266–16273. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, Q.; Schelter, E.J. Photoinduced Miyaura borylation by a rare-earth-metal photoreductant: The hexachlorocerate(III) anion. Angew. Chem. Int. Ed. 2018, 57, 10999–11003. [Google Scholar] [CrossRef]
- Elbanowski, M.; Mąkowska, B.; Staninski, K.; Kaczmarek, M. Chemiluminescence of systems containing lanthanide ions. J. Photochem. 2000, 130, 75–81. [Google Scholar]
- Bulgakov, R.G.; Eliseeva, S.M.; Galimov, D.I. The first registration of a green liquid-phase chemiluminescence of the divalent Eu2+* ion in interaction of β-diketonate complexes Eu(acac)3·H2O, Eu(dpm)3, Eu(fod)3 and Eu(CH3COO)3·6H2O with Bui2AlH in THF with the participation of oxygen. RSC Adv. 2015, 5, 52132–52140. [Google Scholar] [CrossRef]
- Bulgakov, R.G.; Eliseeva, S.M.; Galimov, D.I. Peculiarities of bright blue liquid-phase chemiluminescence of the Eu2+* ion generated at interactions in the systems of EuX3·6H2O-THF-R3–nAlHn–O2 (X = Cl, NO3; R = Bui, Et and Me; n = 0, 1). J. Lumin. 2016, 172, 71–82. [Google Scholar] [CrossRef]
- Bulgakov, R.G.; Eliseeva, S.M.; Galimov, D.I. The first observation of emission of electronically-excited states of divalent Eu2+* ion in the new chemiluminescent system of EuCl3·6H2O-Bui2AlH–O2 and the energy transfer from Eu2+* ion to the trivalent ion, Tb3+. J. Lumin. 2013, 136, 95–99. [Google Scholar] [CrossRef]
- Kaczmarek, M. Lanthanide-sensitized luminescence and chemiluminescence in the systems containing most often used medicines; a review. J. Lumin. 2020, 222, 117174. [Google Scholar] [CrossRef]
- Cowie, B.E.; Purkis, J.M.; Austin, J.; Love, J.B.; Arnold, P.L. Thermal and photochemical reduction and functionalization chemistry of the uranyl dictation, [UVIO2]2+. Chem. Rev. 2019, 119, 10595–10637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, G.; Eugen Schwarz, W.H.; Li, J. Excited-state chemistry: Photocatalytic methanol oxidation by uranyl@zeolite through oxygen-centered radicals. Inorg. Chem. 2020, 59, 6287–6300. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, L.S. Developments in the photophysics and photochemistry of actinide ions and their coordination compounds. Coord. Chem. Rev. 2012, 256, 1583–1603. [Google Scholar] [CrossRef]
- Yusov, A.B.; Shilov, V.P. Photochemistry of f-element ions. Russ. Chem. Bull. Int. Ed. 2000, 49, 1925–1953. [Google Scholar] [CrossRef]
- Ogawa, A.; Sumino, Y.; Nanke, T.; Ohya, S.; Sonoda, N.; Hirao, T. Photoinduced reduction and carbonylation of organic chlorides with samarium diiode. J. Am. Chem. Soc. 1997, 119, 2745–2746. [Google Scholar] [CrossRef]
- Prasad, E.; Knettle, B.W.; Flowers, R.A., II. Photoinduced electron transfer reactions by SmI2 in THF: Luminescence quenching studies and mechanistic investigations. Chem. Eur. J. 2005, 11, 3105–3112. [Google Scholar] [CrossRef]
- Rao, C.N.; Hoz, S. Synergism and inhibition in the combination of visible light and HMPA in SmI2 reductions. J. Org. Chem. 2012, 77, 9199–9204. [Google Scholar] [CrossRef]
- Teprovich, J.A., Jr.; Prasad, E.; Flowers, R.A., II. Solvation-controlled luminescence of SmII complexes. Angew. Chem. Int. Ed. 2007, 46, 1145–1148. [Google Scholar] [CrossRef]
- Molander, G.A.; Alonso-Alija, C. Sequenced reactions with samarium(II) iodide. Sequential intermolecular carbonyl addition/intramolecular nucleophilic acyl substitution for the preparation of seven-, eight-, and nine-membered carbocycles. J. Org. Chem. 1998, 63, 4366–4373. [Google Scholar] [CrossRef]
- Rao, C.N.; Hoz, S. Photostimulated reduction of nitriles by SmI2. J. Org. Chem. 2012, 77, 4029–4034. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Choquette, K.A.; Flowers, R.A., II; Prasad, E. Effect of crown ethers on the ground and excited state reactivity of samarium diiodide in acetonitrile. J. Phys. Chem. A 2012, 116, 2154–2160. [Google Scholar] [CrossRef]
- Nimkar, A.; Maity, S.; Flowers, R.A., II; Hoz, S. Contrasting effect of additives on photoinduced reactions of SmI2. Chem. Eur. J. 2019, 25, 10499–10504. [Google Scholar] [CrossRef] [PubMed]
- de Bettencourt-Dias, A. (Ed.) Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials, 1st ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 1–384. [Google Scholar]
- Jiang, J.; Higashiyama, N.; Machida, K.; Adachi, G. The luminescent properties of divalent europium complexes of crown ethers and cryptands. Coord. Chem. Rev. 1998, 170, 1–29. [Google Scholar] [CrossRef]
- Kelly, R.P.; Bell, T.D.M.; Cox, R.P.; Daniels, D.P.; Deacon, G.B.; Jaroschik, F.; Junk, P.C.; Le Goff, X.F.; Lemercier, G.; Martinez, A.; et al. Divalent tetra- and penta-phenylcyclcopentadienyl europium and samarium sandwich and half-sandwich complexes: Synthesis characterization, and remarkable luminescence properties. Organometallics 2015, 34, 5624–5636. [Google Scholar] [CrossRef]
- Tsuji, T.; Fukazaw, S.; Sugiyama, R.; Kawasaki, K.; Iwasa, T.; Tsunoyama, H.; Tokitoh, N.; Nakajima, A. Physical properties of mononuclear organoeuropium sandwich complexes ligated by cyclooctatetraene and bis(trimethylsilyl)cyclooctatetraene. Chem. Phys. Lett. 2014, 595–596, 144–150. [Google Scholar] [CrossRef]
- Kawasaki, K.; Sugiyama, R.; Tsuji, T.; Iwasa, T.; Tsunoyama, H.; Mizuhata, Y.; Tokitoh, N.; Nakajima, A. A designer ligand field for blue-green luminescence of organoeuropium(II) sandwich complexes with cyclononatetraenyl ligands. Chem. Commun. 2017, 53, 6557–6560. [Google Scholar] [CrossRef]
- Kühling, M.; Wickleder, C.; Ferguson, M.J.; Hrib, C.G.; McDonald, R.; Suta, M.; Hilfert, L.; Takats, J.; Edelmann, F.T. Investigation of the “bent sandwich-like” divalent lanthanide hydro-tris(pyrazolyl)borates Ln(TpiPr2)2 (Ln = Sm, Eu, Tm, Yb). New J. Chem. 2015, 39, 7617–7625. [Google Scholar]
- Goodwin, C.A.P.; Chilton, N.F.; Natrajan, L.S.; Boulon, M.E.; Ziller, J.W.; Evans, W.J.; Mills, D.P. Investigation into the effects of a trigonal-planar ligand field on the electronic properties of lanthanide(II) tris(silylamide)complexes (Ln = Sm, Eu, Tm, Yb). Inorg. Chem. 2017, 56, 5959–5970. [Google Scholar] [CrossRef]
- Kuzyaev, D.M.; Rumyantsev, R.V.; Fukin, G.K.; Bochkarev, M.N. Hexafluoroisopropoxides of divalent and trivalent lanthanides. Structures and luminescence properties. Russ. Chem. Bull. Int. Ed. 2014, 63, 848–853. [Google Scholar] [CrossRef]
- Kuzyaev, D.M.; Balashova, T.V.; Burin, M.E.; Fukin, G.K.; Rumyantcev, R.V.; Pushkarev, A.P.; Ilichev, V.A.; Grishin, I.D.; Vorozhtsov, D.L.; Bochkarev, M.N. Synthesis, structure and luminescent properties of lanthanide fluoroalkoxides. Dalton Trans. 2016, 45, 3464–3472. [Google Scholar] [CrossRef] [PubMed]
- Kuda-Wedagedara, A.N.W.; Wang, C.; Martin, P.D.; Allen, M.J. Aqueous EuII-containing complex with bright yellow luminescence. J. Am. Chem. Soc. 2015, 137, 4960–4963. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-X.; Bailey, M.D.; Allen, M.J. Unique EuII coordination environments with a Janus cryptand. Inorg. Chem. 2016, 55, 9085–9090. [Google Scholar] [CrossRef] [PubMed]
- Jenks, T.C.; Bailey, M.D.; Hovey, J.L.; Fernando, S.; Basnayake, G.; Cross, M.E.; Li, W.; Allen, M.J. First use of a divalent lanthanide for visible-light-promoted photoredox catalysis. Chem Sci. 2018, 9, 1273–1278. [Google Scholar] [CrossRef]
- Corbin, B.A.; Hovey, J.L.; Thapa, B.; Schlegel, H.B.; Allen, M.J. Luminescence differences between two complexes of divalent europium. J. Organomet. Chem. 2018, 857, 88–93. [Google Scholar] [CrossRef]
- Jenks, T.C.; Bailey, M.D.; Corbin, B.A.; Kuda-Wedagedara, A.N.W.; Martin, P.D.; Schlegel, H.B.; Rabuffetti, F.A.; Allen, M.J. Photophysical characterization of a highly luminescent divalent-europium-containing azacryptate. Chem. Commun. 2018, 54, 4545–4548. [Google Scholar] [CrossRef]
- Basal, L.A.; Kajjam, A.B.; Bailey, M.D.; Allen, M.J. Systematic tuning of the optical properties of discrete complexes of EuII in solution using counterions and solvents. Inorg. Chem. 2020, 59, 9476–9480. [Google Scholar] [CrossRef]
- Tucker, J.W.; Stephenson, C.R.J. Shining light on photoredox catalysis: Theory and synthetic applications. J. Org. Chem. 2012, 77, 1617–1622. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Qiao, Y.; Sergentu, D.C.; Yin, H.; Zabula, A.V.; Cheisson, T.; McSkimming, A.; Manor, B.C.; Carroll, P.J.; Anna, J.M.; Autschbach, J.; et al. Understanding and controlling the emission brightness and color of molecular cerium luminophores. J. Am. Chem. Soc. 2018, 140, 4588–4595. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Carroll, P.J.; Anna, J.M.; Schelter, E.J. Luminescent Ce(III) complexes as stoichiometric and catalytic photoreductants for halogen atom abstraction reactions. J. Am. Chem. Soc. 2015, 137, 9234–9237. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Carroll, P.J.; Manor, B.C.; Anna, J.M.; Schelter, E.J. Cerium photosensitizers: Structure–function relationships and applications in photocatalytic aryl coupling reactions. J. Am. Chem. Soc. 2016, 138, 5984–5993. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Cheisson, T.; Manor, B.C.; Carroll, P.J.; Schelter, E.J. A strategy to improve the performance of cerium(III) photocatalysts. Chem. Commun. 2019, 55, 4067–4070. [Google Scholar] [CrossRef]
- Fieser, M.E.; MacDonald, M.R.; Krull, B.T.; Bates, J.E.; Ziller, J.W.; Furche, F.; Evans, W.J. Structural, spectroscopic, and theoretical comparison of traditional vs. recently discovered Ln2+ ions in the [K(2.2.2.-cryptand)][C5H4SiMe3)3Ln] complexes: The variable nature of Dy2+ and Nd2+. J. Am. Chem. Soc. 2015, 137, 369–382. [Google Scholar] [CrossRef]
- Evans, W.J. Tutorial on the role of cyclopentadienyl ligands in the discovery of molecular complexes of the rare-earth and actinide metals in new oxidation states. Organometallics 2016, 35, 3088–3100. [Google Scholar] [CrossRef]
- MacDonald, M.R.; Bates, J.E.; Ziller, J.W.; Furche, F.; Evans, W.J. Completing the series of +2 ions for the lanthanide elements: Synthesis of molecular complexes of Pr2+, Gd2+, Tb2+, and Lu2+. J. Am. Chem. Soc. 2013, 135, 9857–9868. [Google Scholar] [CrossRef]
- Jenks, T.C.; Kuda-Wedagedara, A.N.W.; Bailey, M.D.; Ward, C.L.; Allen, M.J. Spectroscopic and electrochemical trends in divalent lanthanides through modulation of coordination environment. Inorg. Chem. 2020, 59, 2613–2620. [Google Scholar] [CrossRef]

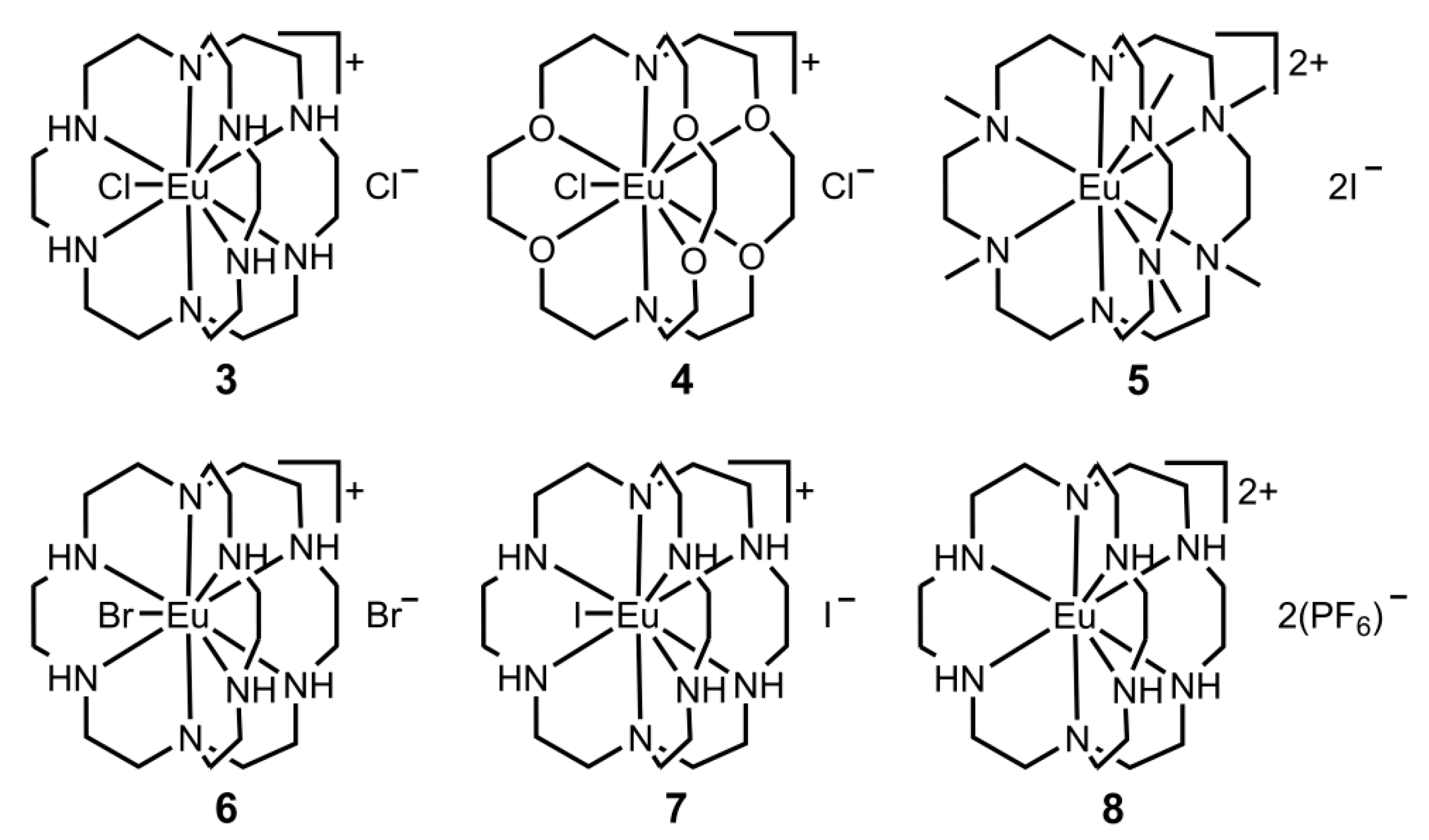
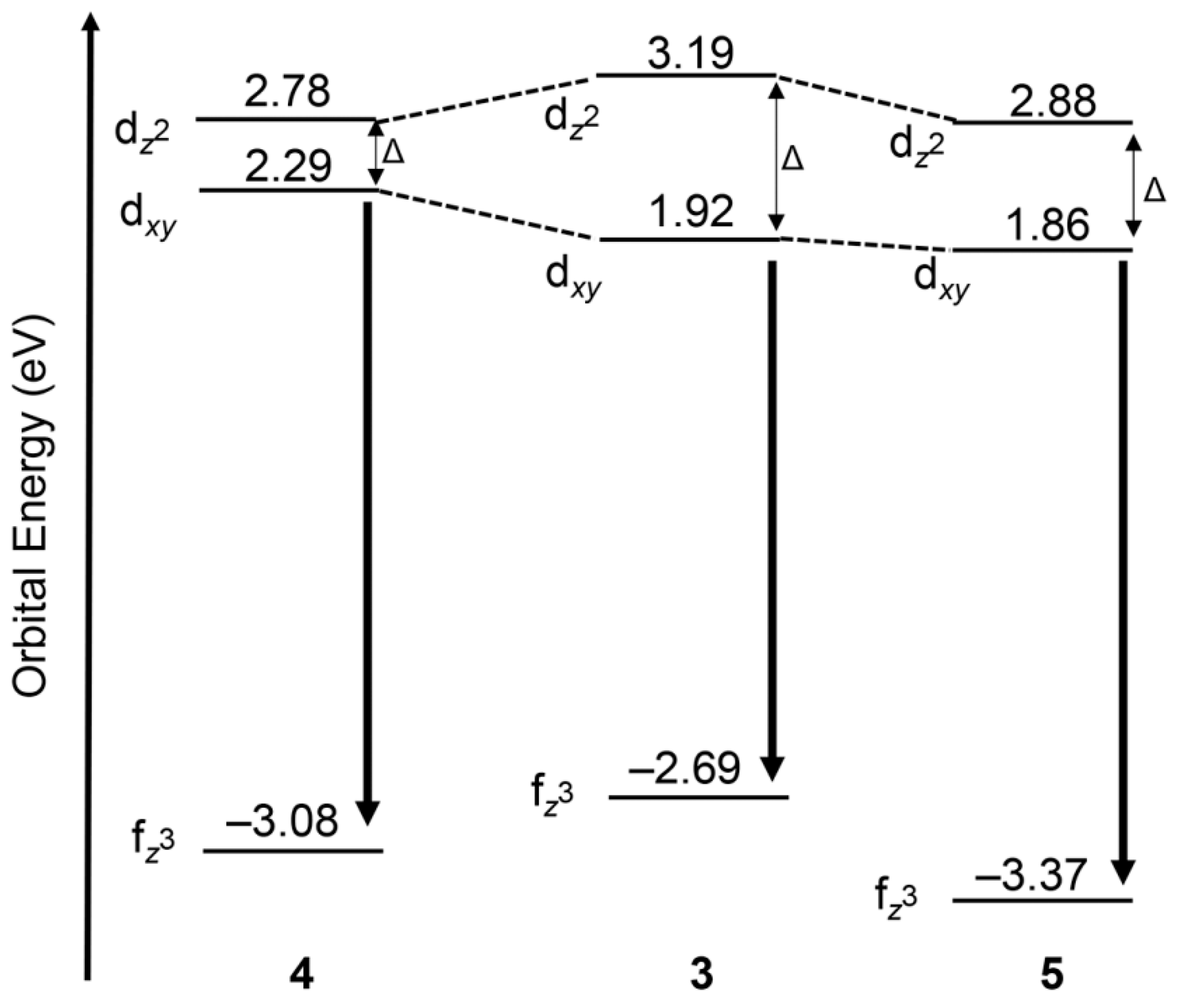
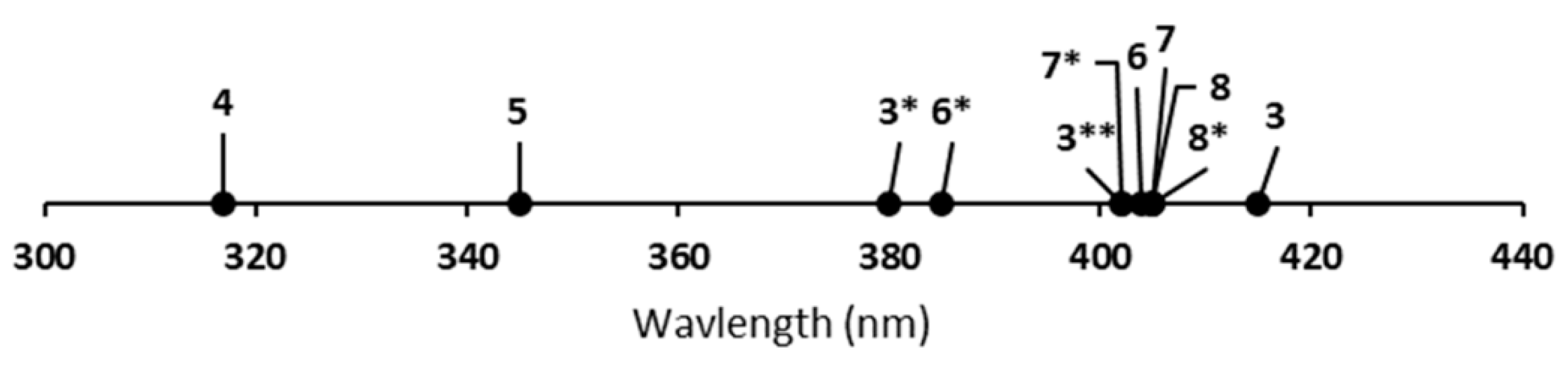

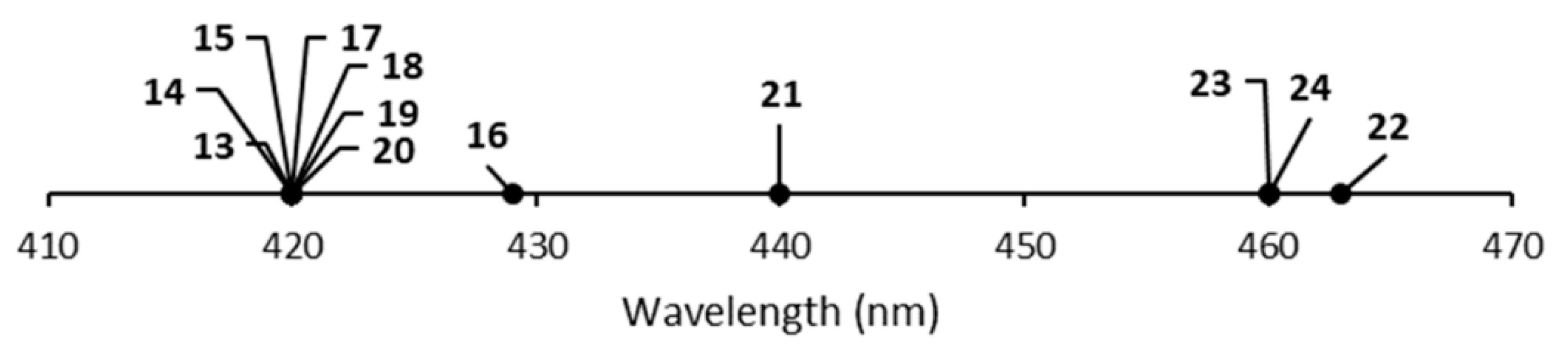
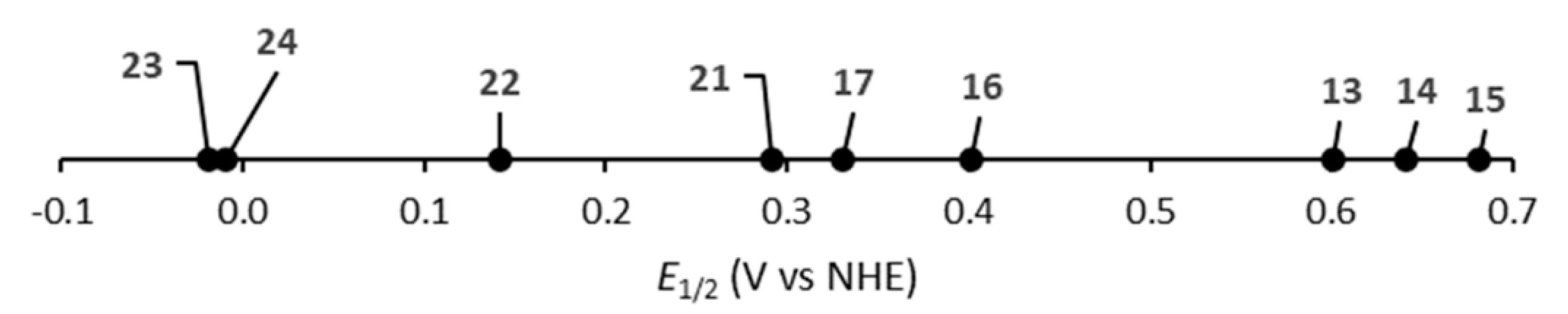

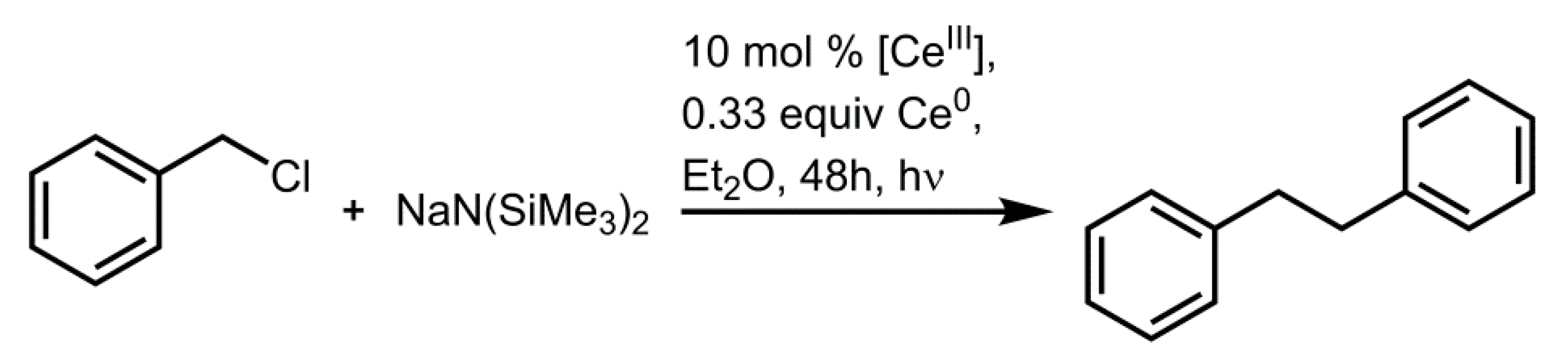

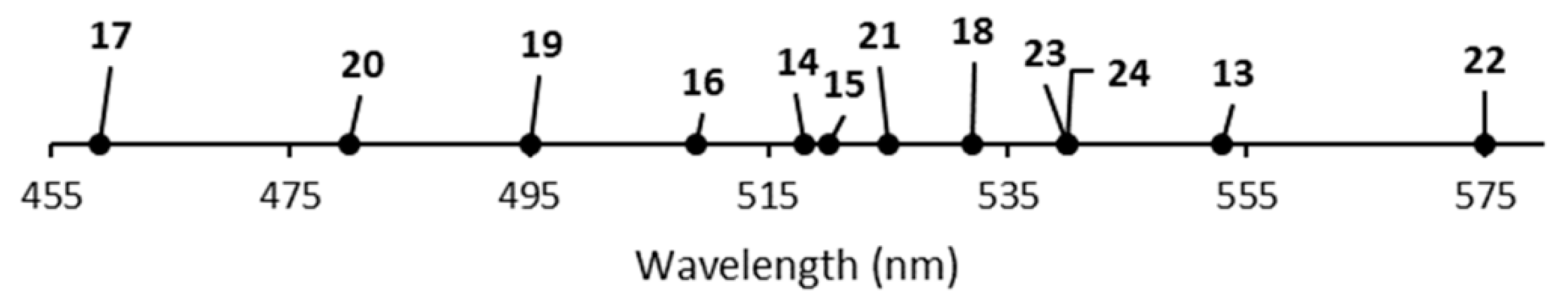
| Complex | Crown | λab (nm) | λem (nm) | E1/2 (V vs. NHE) |
|---|---|---|---|---|
| SmI2 | none | 685 | * | −0.74 ± 0.05 |
| 1 | 15-crown-5 | 540 | 723 | −0.46 ± 0.05 |
| 2 | 18-crown-6 | 606 | 712 | −0.77 ± 0.02 |
| Complex | Solvent | λab (nm) | λem (nm) |
|---|---|---|---|
| 3 | Methanol | 402 | 577 |
| 6 | Methanol | 404 | 577 |
| 7 | Methanol | 405 | 577 |
| 8 | Methanol | 405 | 577 |
| 3 | Acetonitrile | 380 | 508 |
| 6 | Acetonitrile | 385 | 572 |
| 7 | Acetonitrile | 402 | 577 |
| 8 | Acetonitrile | 405 | 577 |
| Compound Number | Substrate | Epc (V vs. NHE) | Yield of Reduction (%) | Product of Reduction |
|---|---|---|---|---|
| 9 | t-BuCl | −2.85 | 1.9 ± 0.1 | (t-Bu)2 |
| 10 | PhCl | −2.73 | 5.4 ± 0.4 | C6H6 |
| 11 | CH2CHCH2Cl | −2.15 | 46 ± 2 | (CH2CHCH2)2 |
| 12 | BnCl | −2.14 | 85 ± 2 | Bn2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barraza, R., Jr.; Allen, M.J. Lanthanide Luminescence in Visible-Light-Promoted Photochemical Reactions. Molecules 2020, 25, 3892. https://doi.org/10.3390/molecules25173892
Barraza R Jr., Allen MJ. Lanthanide Luminescence in Visible-Light-Promoted Photochemical Reactions. Molecules. 2020; 25(17):3892. https://doi.org/10.3390/molecules25173892
Chicago/Turabian StyleBarraza, Ramiro, Jr., and Matthew J. Allen. 2020. "Lanthanide Luminescence in Visible-Light-Promoted Photochemical Reactions" Molecules 25, no. 17: 3892. https://doi.org/10.3390/molecules25173892
APA StyleBarraza, R., Jr., & Allen, M. J. (2020). Lanthanide Luminescence in Visible-Light-Promoted Photochemical Reactions. Molecules, 25(17), 3892. https://doi.org/10.3390/molecules25173892