Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide
Abstract
1. Introduction
2. Results
2.1. MA Decreases Proliferation of B16F10 Cells by a Dose-Dependent Mechanism
2.2. H2O2 Modifies Cell Viability
2.3. Maslinic Acid’s Influence on Mitochondrial-Membrane Potential
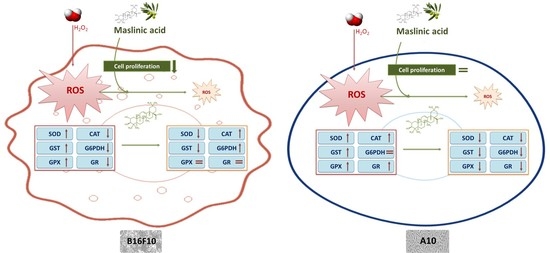
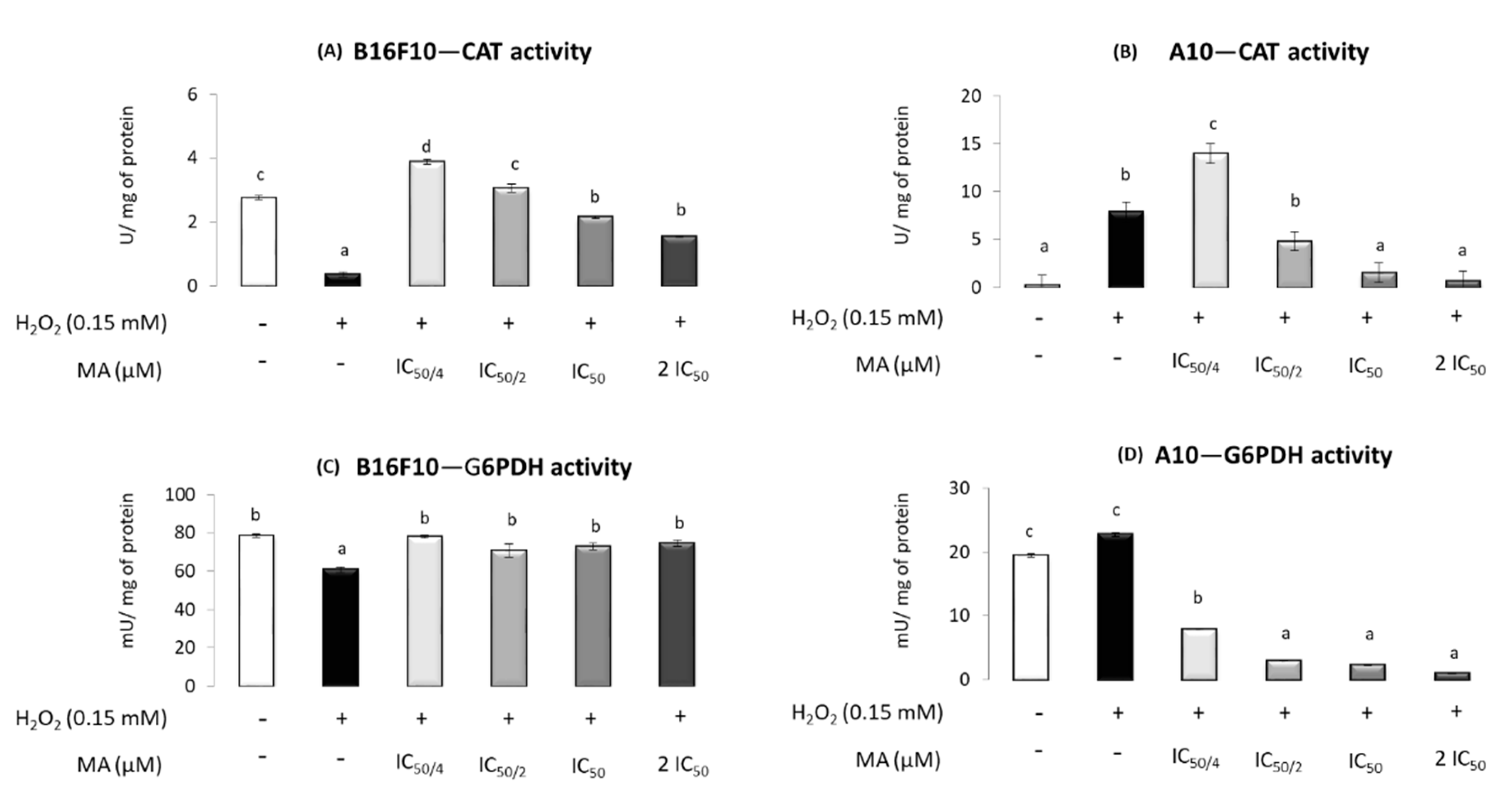
2.4. MA Exerts Antioxidant Activity, Modulating Enzymatic Defense System
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Cell Lines and Cultures
4.3. MTT Assay
4.4. Flow-Cytometry Analysis of the Mitochondrial-Membrane Potential
4.5. Antioxidant Enzyme Assays
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodríguez-Rodríguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Lupiáñez, J.A.; Adroher, F.J.; Vargas, A.M.; Osuna, A. Differential behaviour of glucose 6-phosphate dehydrogenase in two morphological forms of Trypanosoma cruzi. Int. J. Biochem. 1987, 19, 1085–1089. [Google Scholar] [CrossRef]
- Adroher, F.J.; Osuna, A.; Lupiáñez, J.A. Differential energetic metabolism during Trypanosoma cruzi differentiation. I: Citrate synthase, NADP-isocitrate and succinate dehydrogenases. Arch. Biochem. Biophys. 1988, 267, 252–261. [Google Scholar] [CrossRef]
- Adroher, F.J.; Osuna, A.; Lupiáñez, J.A. Differential energetic metabolism during Trypanosoma cruzi differentiation. II. Hexokinase, phosphofructokinase and pyruvate kinase. Mol. Cell. Biochem. 1990, 94, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Peragón, J.; Barroso, J.B.; de la Higuera, M.; Lupiáñez, J.A. Relationship between growth and protein turnover rates and nucleic acids in the liver of rainbow trout (Oncorhynchus mykiss) during development. Can. J. Fish. Aquat. Sci. 1998, 55, 649–657. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; Aranda, F.; de la Higuera, M.; Lupiáñez, J.A. Selective changes in the protein-turnover rates and nature of growth induced in trout liver by long-term starvation followed by re-feeding. Mol. Cell. Biochem. 1999, 201, 1–10. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Dietary alterations in protein, carbohydrates and fat increase liver protein-turnover rate and decrease overall growth rate in the rainbow trout (Oncorhynchus mykiss). Mol. Cell. Biochem. 2000, 209, 97–104. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Dietary-protein effects on growth and fractional protein-synthesis and degradation rates in liver and white muscle of rainbow-trout (Oncorhynchus mykiss). Aquaculture 1994, 124, 35–46. [Google Scholar] [CrossRef]
- Barroso, J.B.; Peragón, J.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Carbohydrate deprivation reduces NADPH-production in fish liver but not in adipose tissue. Int. J. Biochem. Cell Biol. 2001, 33, 785–796. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Growth, protein-turnover rates and nucleic-acid concentrations in the white muscle of rainbow trout during development. Int. J. Biochem. Cell Biol. 2001, 33, 1227–1238. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; García-Rejón, L.; Lupiáñez, J.A.; de la Higuera, M. Long-term nutritional effects on the primary liver and kidney metabolism in rainbow trout (Oncorhynchus mykiss). II. Adaptive response of glucose 6-phosphate dehydrogenase activity to high-carbohydrate/low-protein and high-fat/on-carbohydrate diets. Aquacult. Nutr. 1996, 2, 193–200. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernández-Marcos, P.J.; Brioche, T.; Gómez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Vina, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.P.; Southworth, R.; Medina, R.A.; Davidson, S.M.; Duchen, M.R.; Shattock, M.J. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovas. Res. 2006, 72, 313–321. [Google Scholar] [CrossRef]
- Kurze, A.K.; Buhs, S.; Eggert, D.; Oliveira-Ferrer, L.; Muller, V.; Niendorf, A.; Wagener, C.; Nollau, P. Immature O-glycans recognized by the macrophage glycoreceptor CLEC10A (MGL) are induced by 4-hydroxy-tamoxifen, oxidative stress and DNA-damage in breast cancer cells. Cell Commun. Signal. 2019, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic acid, a triterpene from olive, affects the antioxidant and mitochondrial status of B16F10 melanoma cells grown under stressful conditions. Evid.-Based Complement. Altern. Med. 2015, 2015, 272457. [Google Scholar] [CrossRef]
- Ahmad, K.; Hafeez, Z.B.; Bhat, A.R.; Rizvi, M.A.; Thakur, S.C.; Azam, A.; Athar, F. Antioxidant and apoptotic effects of Callistemon lanceolatus leaves and their compounds against human cancer cells. Biomed. Pharmacother. 2018, 106, 1195–1209. [Google Scholar] [CrossRef]
- De Santiago-Arteche, R. Efecto de la Quimioterapia Antineoplásica en Pacientes con Cancer Colorectal Sobre Biomarcadores del Estrés Oxidativo y del Estado Redox Plasmático. Ph.D. Thesis, University of Burgos, Burgos, Spain, 2010; p. 262. [Google Scholar]
- Zhang, Y.S.; Ning, Z.X.; Yang, S.Z.; Wu, H. Antioxidation properties and mechanism of action of dihydromyricetin from Ampelopsis grossedentata. Acta Pharm. Sin. 2003, 38, 241–244. [Google Scholar]
- Yang, W.F.; Zhao, W.L. Determination of ginsenosides Re, Rb1 in Panax quinquefolius by micellar electrokinetic chromatography. China J. Chin. Mater. Med. 2003, 28, 1135–1137. [Google Scholar]
- Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Ferrer-Martín, R.M.; Rufino-Palomares, E.E.; Martín-Fonseca, S.; Rivas, F.; Martínez, A.; García-Granados, A.; Pérez-Jiménez, A.; García-Salguero, L.; et al. The oleanolic acid derivative, 3-O-succinyl-28-O-benzyl oleanolate, induces apoptosis in B16-F10 melanoma cells via the mitochondrial apoptotic pathway. RSC Adv. 2016, 6, 93590–93601. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.J.; Centelles, J.J.; Lupiáñez, J.A.; Cascante, M. (2Alpha,3beta)-2,3-dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006, 580, 6302–6310. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Herrera-Merchán, A.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Anti-cancer and anti-angiogenic properties of various natural pentacyclic tri-terpenoids and some of their chemical derivatives. Curr. Org. Chem. 2015, 19, 919–947. [Google Scholar] [CrossRef]
- Juan, M.E.; Planas, J.M. Bioavailability and metabolism of maslinic acid, a natural pentacyclic triterpene. In Recent Advances in Pharmaceutical Sciences VI; Publisher: Kerala, India, 2016; pp. 131–145. [Google Scholar]
- Siewert, B.; Csuk, R. Membrane damaging activity of a maslinic acid analog. Eur. J. Med. Chem. 2014, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003, 69, 472–474. [Google Scholar] [PubMed]
- Barroso, J.B.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. The influence of dietary-protein on the kinetics of NADPH production systems in various tissues of rainbow-trout (Oncorhynchus mykiss). Aquaculture 1994, 124, 47–59. [Google Scholar] [CrossRef]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef]
- Chen, J.C.; Zhang, G.H.; Zhang, Z.Q.; Qiu, M.H.; Zheng, Y.T.; Yang, L.M.; Yu, K.B. Octanorcucurbitane and cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity. J. Nat. Prod. 2008, 71, 153–155. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; García-Salguero, L.; Peragón, J.; Medina, P.P.; Parra, A.; Cascante, M.; Lupiáñez, J.A. Maslinic acid, a natural T¡triterpene, induces a death receptor-mediated apoptotic mechanism in Caco-2 p53-deficient colon adenocarcinoma cells. PLoS ONE 2016, 11, e0146178. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A.; Peragón, J. Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. J. Proteom. 2013, 83, 15–25. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoon, S.K.; Ryu, S.Y. Cytotoxic triterpenes from stem bark of Physocarpus intermedius. Planta Med. 2000, 66, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Pachón-Pena, G.; Lizarraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupiáñez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tena, S.; Reyes-Zurita, F.J.; Díaz-Moralli, S.; Vinardell, M.P.; Reed, M.; García-García, F.; Dopazo, J.; Lupiáñez, J.A.; Gunther, U.; Cascante, M. Maslinic acid-enriched diet decreases intestinal tumorigenesis in Apc(Min/+) mice through transcriptomic and metabolomic reprogramming. PLoS ONE 2013, 8, e59392. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Medina, P.P.; García-Salguero, L.; Peragón, J.; Cascante, M.; Lupiáñez, J.A. Antitumour activity on extrinsic apoptotic targets of the triterpenoid maslinic acid in p53-deficient Caco-2 adenocarcinoma cells. Biochimie 2013, 95, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, D.; Zhang, X.; Shan, L.; Liu, Z. Maslinic acid induced apoptosis in bladder cancer cells through activating p38 MAPK signaling pathway. Mol. Cell. Biochem. 2014, 392, 281–287. [Google Scholar] [CrossRef]
- Medina, I.; Lois, S.; Lizarraga, D.; Pazos, M.; Tourino, S.; Cascante, M.; Torres, J.L. Functional fatty fish supplemented with grape procyanidins. Antioxidant and proapoptotic properties on colon cell lines. J. Agric. Food Chem. 2006, 54, 3598–3603. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Choi, B.S.; Kim, H.; Lee, H.J.; Sapkota, K.; Park, S.E.; Kim, S.; Kim, S.J. Celastrol from ‘Thunder God Vine’ protects SH-SY5Y cells through the preservation of mitochondrial function and inhibition of p38 MAPK in a rotenone model of Parkinson’s disease. Neurochem. Res. 2014, 39, 84–96. [Google Scholar] [CrossRef]
- Rafatian, G.; Khodagholi, F.; Farimani, M.M.; Abraki, S.B.; Gardaneh, M. Increase of autophagy and attenuation of apoptosis by Salvigenin promote survival of SH-SY5Y cells following treatment with H2O2. Mol. Cell. Biochem. 2012, 371, 9–22. [Google Scholar] [CrossRef]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Yang, Z.G.; Li, H.R.; Wang, L.Y.; Li, Y.H.; Lu, S.G.; Wen, X.F.; Wang, J.; Daikonya, A.; Kitanaka, S. Triterpenoids from Hippophae rhamnoides L. and their nitric oxide production-inhibitory and DPPH radical-scavenging activities. Chem. Pharm. Bull. 2007, 55, 15–18. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Rolfo, M.; Ferraris, A.M.; Gaetani, G.F. Mechanisms of protection of catalase by NADPH—Kinetics and stoichiometry. J. Biol. Chem. 1999, 274, 13908–13914. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, A.; Abellán, E.; Arizcun, M.; Cardenete, G.; Morales, A.E.; Hidalgo, M.C. Dietary carbohydrates improve oxidative status of common dentex (Dentex dentex) juveniles, a carnivorous fish species. Comp. Biochem. Physiol. A 2017, 203, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Z.; Yang, M.; Liu, R.; Wang, W.; Liu, P.; Guo, D. Structural determination of seven new triterpenoids from Kadsura heteroclita by NMR techniques. Magn. Reson. Chem. 2007, 45, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Subbarao, V. Effect of dexamethasone on ciprofibrate-induced cell proliferation and peroxisome proliferation. Fundam. Appl. Toxicol. 1997, 35, 78–83. [Google Scholar] [CrossRef]
- Rothe, G.; Oser, A.; Valet, G. Dihydrorhodamine 123: A new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Die Nat. 1988, 75, 354–355. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Peragón, J.; Aranda, F.; García-Salguero, L.; Corpas, F.J.; Lupiáñez, J.A. Stimulation of rat-kidney hexose-monophosphate shunt dehydrogenase-activity by chronic metabolic-acidosis. Biochem. Int. 1989, 18, 1041–1050. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Carlberg, I.; Mannervik, B. Purification by affinity chromatography of yeast glutathione reductase, the enzyme responsible for the NADPH-dependent reduction of the mixed disulfide of coenzyme A and glutathione. Biochim. Biophys. Acta 1977, 484, 268–274. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound maslinic acid are available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtari, K.; Pérez-Jiménez, A.; García-Salguero, L.; A. Lupiáñez, J.; Rufino-Palomares, E.E. Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide. Molecules 2020, 25, 4020. https://doi.org/10.3390/molecules25174020
Mokhtari K, Pérez-Jiménez A, García-Salguero L, A. Lupiáñez J, Rufino-Palomares EE. Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide. Molecules. 2020; 25(17):4020. https://doi.org/10.3390/molecules25174020
Chicago/Turabian StyleMokhtari, Khalida, Amalia Pérez-Jiménez, Leticia García-Salguero, José A. Lupiáñez, and Eva E. Rufino-Palomares. 2020. "Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide" Molecules 25, no. 17: 4020. https://doi.org/10.3390/molecules25174020
APA StyleMokhtari, K., Pérez-Jiménez, A., García-Salguero, L., A. Lupiáñez, J., & Rufino-Palomares, E. E. (2020). Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide. Molecules, 25(17), 4020. https://doi.org/10.3390/molecules25174020