Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications
Abstract
1. Introduction
2. Results
2.1. Fatty Acid Profiles and Lipid Quality Indices
2.2. Antioxidant Activity
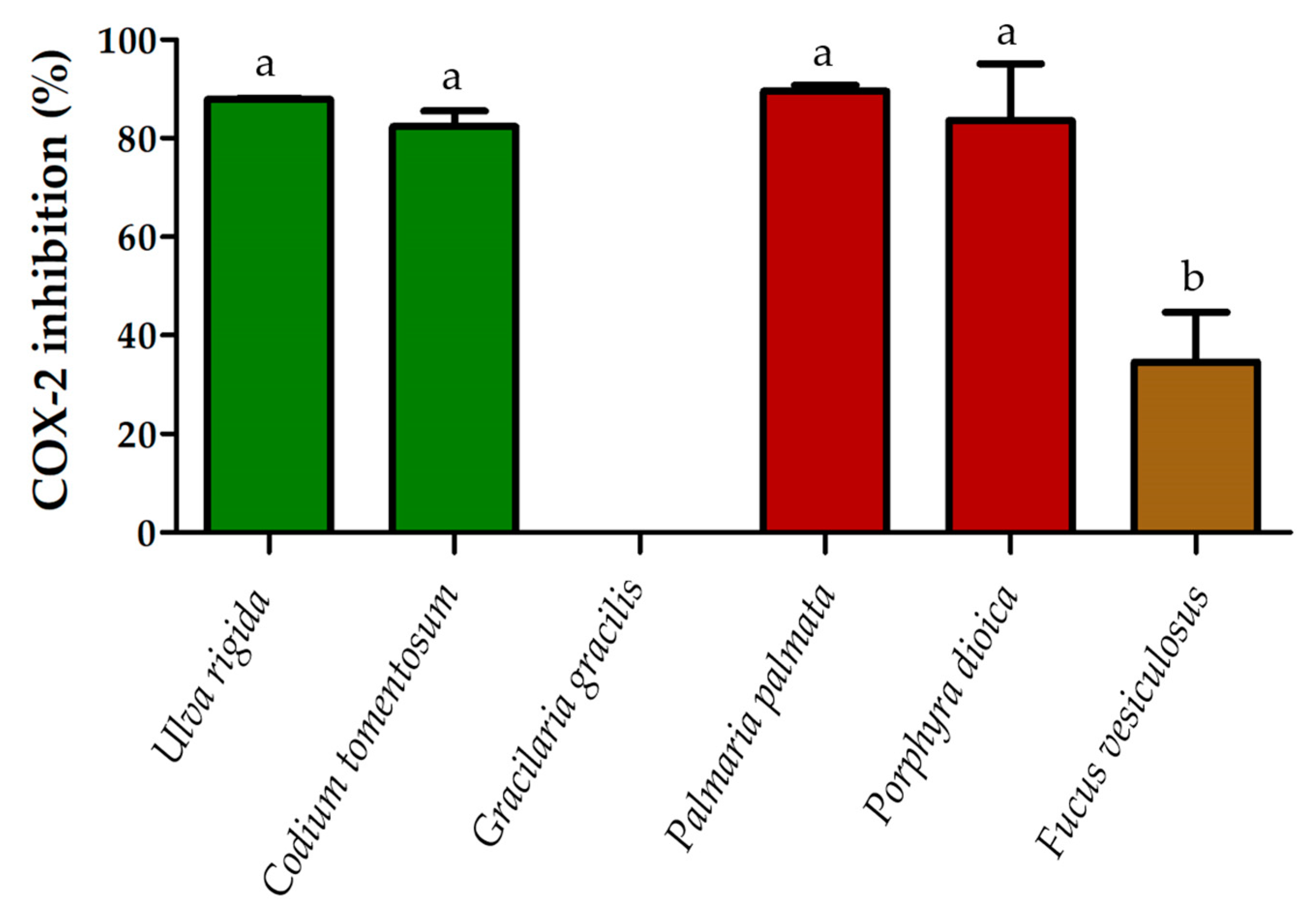
2.3. Anti-Inflammatory Activity
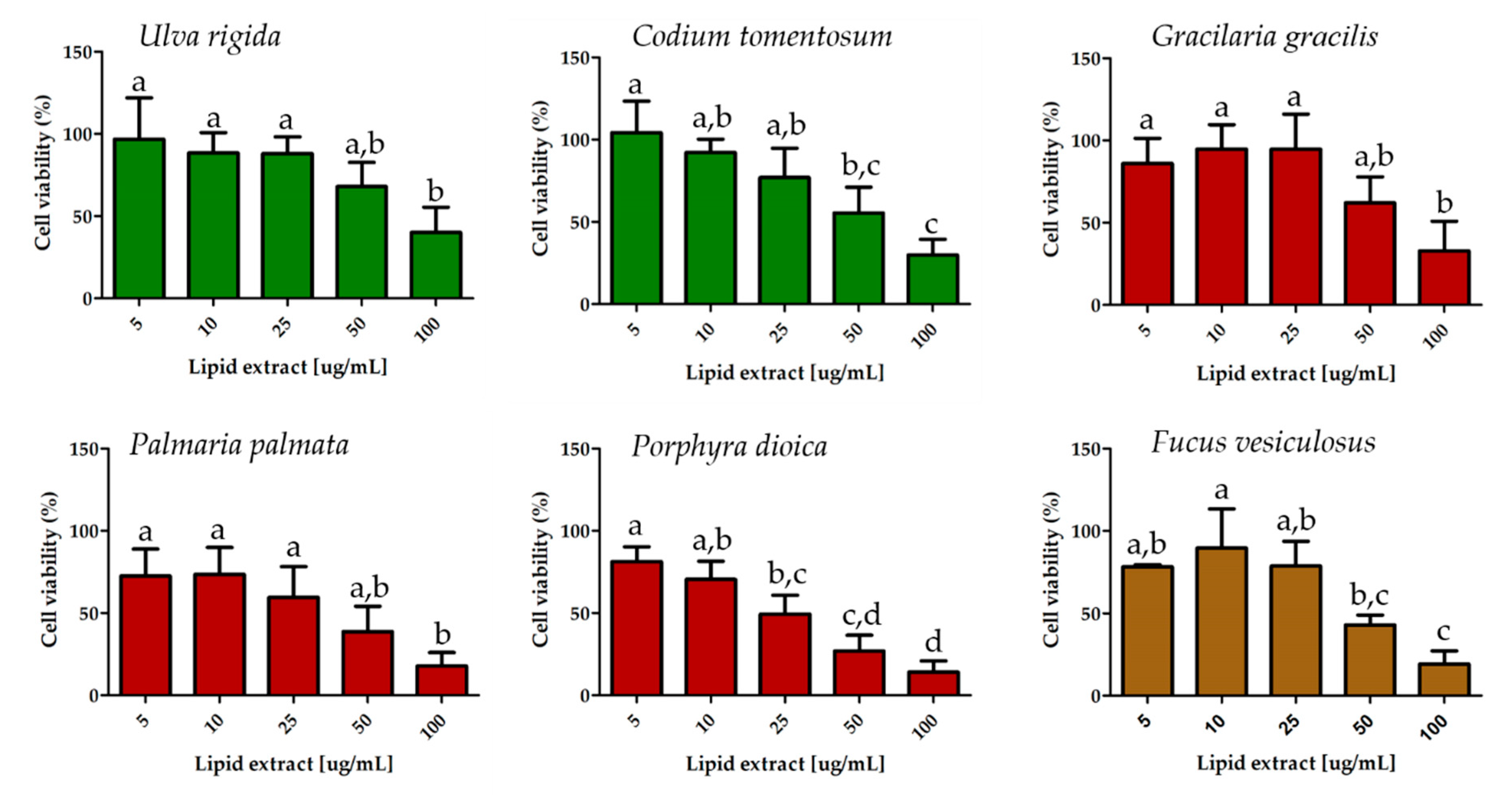
2.4. Effect on Breast Cancer Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Macroalgae Biomass
4.2. Lipid Extraction
4.3. Fatty Acid Analysis
4.4. 2,2′-Azino-bis-3-Ethylbenzothiazoline-6-Sulfonic Acid Radical Cation Assay—ABTS Radical Scavenging Activity
4.5. 2,2-Diphenyl-1-Picrylhydrazyl Radical Assay—DPPH Radical Scavenging Activity
4.6. In Vitro Cyclooxygenase Inhibition Assay
4.7. Cell Viability Assay on MDA-MB-231 Breast Cell Line
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Dillehay, T.D.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, Food, Medicine, and the Peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Mizoue, T.; Shimazu, T.; Ishihara, J.; Takachi, R.; Noda, M.; Iso, H.; Sasazuki, S.; Sawada, N.; Tsugane, S. Dietary patterns and all-cause, cancer, and cardiovascular disease mortality in Japanese men and women: The Japan public health center-based prospective study. PLoS ONE 2017, 12, e0174848. [Google Scholar] [CrossRef]
- Maruyama, K.; Iso, H.; Date, C.; Kikuchi, S.; Watanabe, Y.; Wada, Y.; Inaba, Y.; Tamakoshi, A.; Group, J.S. Dietary patterns and risk of cardiovascular deaths among middle-aged Japanese: JACC Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 519–527. [Google Scholar] [CrossRef]
- Chu, S.-M.; Shih, W.-T.; Yang, Y.-H.; Chen, P.-C.; Chu, Y.-H. Use of traditional Chinese medicine in patients with hyperlipidemia: A population-based study in Taiwan. J. Ethnopharmacol. 2015, 168, 129–135. [Google Scholar] [CrossRef]
- Shimazu, T.; Kuriyama, S.; Hozawa, A.; Ohmori, K.; Sato, Y.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Dietary patterns and cardiovascular disease mortality in Japan: A prospective cohort study. Int. J. Epidemiol. 2007, 36, 600–609. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yoon, S.-J.; Kim, A.; Seo, H.; Ko, S. Health performance and challenges in Korea: A Review of the Global Burden of Disease Study 2013. J. Korean Med. Sci. 2016, 31, S114–S120. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustain. Action 2020. Available online: http://www.fao.org/3/ca9229en/ca9229en.pdf (accessed on 19 August 2020). [CrossRef]
- Grote, B. Recent developments in aquaculture of Palmaria palmata (Linnaeus) (Weber & Mohr 1805): Cultivation and uses. Rev. Aquac. 2019, 11, 25–41. [Google Scholar]
- Ashkenazi, D.Y.; Israel, A.; Abelson, A. A novel two-stage seaweed integrated multi-trophic aquaculture. Rev. Aquac. 2019, 11, 246–262. [Google Scholar] [CrossRef]
- Food and Agriculture Organization and World Health Organization. Sustainable Healthy Diets—Guiding Principles; FAO: Rome, Italy, 2019; p. 37. [Google Scholar]
- Palmieri, N.; Forleo, M.B. The potential of edible seaweed within the western diet. A segmentation of Italian consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Bouga, M.; Combet, E. Emergence of Seaweed and Seaweed-Containing Foods in the UK: Focus on Labeling, Iodine Content, Toxicity and Nutrition. Foods 2015, 4, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The Protective Role of Sulfated Polysaccharides from Green Seaweed Udotea flabellum. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra. J. Funct. Foods 2017, 28, 64–75. [Google Scholar] [CrossRef]
- Rey, F.; Cartaxana, P.; Melo, T.; Calado, R.; Pereira, R.; Abreu, H.; Domingues, P.; Cruz, S.; Domingues, R.M. Domesticated Populations of Codium tomentosum Display Lipid Extracts with Lower Seasonal Shifts than Conspecifics from the Wild—Relevance for Biotechnological Applications of this Green Seaweed. Mar. Drugs 2020, 18, 188. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, H.M.; Pereira, R.; Domingues, P.; Lillebø, I.A.; Calado, R.; Domingues, R.M. A New Look for the Red Macroalga Palmaria palmata: A Seafood with Polar Lipids Rich in EPA and with Antioxidant Properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef]
- Rey, F.; Lopes, D.; Maciel, E.; Monteiro, J.; Skjermo, J.; Funderud, J.; Raposo, D.; Domingues, P.; Calado, R.; Domingues, M.R. Polar lipid profile of Saccharina latissima, a functional food from the sea. Algal Res. 2019, 39, 101473. [Google Scholar] [CrossRef]
- da Costa, E.; Domingues, P.; Melo, T.; Coelho, E.; Pereira, R.; Calado, R.; Abreu, H.M.; Domingues, R.M. Lipidomic Signatures Reveal Seasonal Shifts on the Relative Abundance of High-Valued Lipids from the Brown Algae Fucus vesiculosus. Mar. Drugs 2019, 17, 335. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Miraliakbari, H. Omega-3 (n-3) Fatty Acids in Health and Disease: Part 1—Cardiovascular Disease and Cancer. J. Med. Food 2004, 7, 387–401. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Moreira, A.S.P.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Domingues, P.; Calado, R.; et al. Valorization of Lipids from Gracilaria sp. through Lipidomics and Decoding of Antiproliferative and Anti-Inflammatory Activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- da Costa, E.; Azevedo, V.; Melo, T.; Rego, A.M.; Evtuguin, D.V.; Domingues, P.; Calado, R.; Pereira, R.; Abreu, M.H.; Domingues, M.R. High-Resolution Lipidomics of the Early Life Stages of the Red Seaweed Porphyra dioica. Molecules 2018, 23, 1–20. [Google Scholar] [CrossRef]
- Kindleysides, S.; Quek, S.-Y.; Miller, M.R. Inhibition of fish oil oxidation and the radical scavenging activity of New Zealand seaweed extracts. Food Chem. 2012, 133, 1624–1631. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Lopes, G.; Daletos, G.; Proksch, P.; Andrade, P.B.; Valentão, P. Anti-inflammatory potential of monogalactosyl diacylglycerols and a monoacylglycerol from the edible brown seaweed Fucus spiralis linnaeus. Mar. Drugs 2014, 12, 1406–1418. [Google Scholar] [CrossRef]
- Tsai, C.J.; Sun Pan, B. Identification of sulfoglycolipid bioactivities and characteristic fatty acids of marine macroalgae. J. Agric. Food Chem. 2012, 60, 8404–8410. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Nuñez, M.; Picon, A. Seaweeds in yogurt and quark supplementation: Influence of five dehydrated edible seaweeds on sensory characteristics. Int. J. Food Sci. Technol. 2017, 52, 431–438. [Google Scholar] [CrossRef]
- Vilar, E.G.; Ouyang, H.; O’Sullivan, M.G.; Kerry, J.P.; Hamill, R.M.; O’Grady, M.N.; Mohammed, H.O.; Kilcawley, K.N. Effect of salt reduction and inclusion of 1% edible seaweeds on the chemical, sensory and volatile component profile of reformulated frankfurters. Meat Sci. 2020, 161, 108001. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Černá, M. Chapter 24-Seaweed Proteins and Amino Acids as Nutraceuticals. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 297–312. ISBN 1043-4526. [Google Scholar]
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Chapter 3-Health Benefits of Algal Polysaccharides in Human Nutrition. In Advances in Food and Nutrition Research; Henry, J., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 66, pp. 75–145. ISBN 1043-4526. [Google Scholar]
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, M.L.A.; Pinto, C.G.A.D.; Michalak, I.; Trincone, A.; Mishra, P.A.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Plasticity and remodelling of lipids support acclimation potential in two species of low-intertidal macroalgae, Fucus serratus (Phaeophyceae) and Palmaria palmata (Rhodophyta). Algal Res. 2017, 26, 104–114. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Maccarrone, M. Bioactive lipids as modulators of immunity, inflammation and emotions. Curr. Opin. Pharmacol. 2016, 29, 54–62. [Google Scholar] [CrossRef] [PubMed]
- da Costa, E.; Melo, T.; Moreira, A.S.P.; Alves, E.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Decoding bioactive polar lipid profile of the macroalgae Codium tomentosum from a sustainable IMTA system using a lipidomic approach. Algal Res. 2015, 12, 388–397. [Google Scholar] [CrossRef]
- Maciel, E.; Leal, M.C.; Lillebø, A.I.; Domingues, P.; Domingues, M.R.; Calado, R. Bioprospecting of marine macrophytes using MS-based lipidomics as a new approach. Mar. Drugs 2016, 14, 49. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty acids profile, atherogenic (IA) and thrombogenic (IT) health lipid indices, of raw roe of blue fin tuna (Thunnus thynnus L.) and their salted product” Bottarga”. Food Nutr. Sci. 2011, 2, 736. [Google Scholar]
- Telahigue, K.; Hajji, T.; Rabeh, I.; Cafsi, M. El The changes of fatty acid composition in sun dried, oven dried and frozen hake (Merluccius merluccius) and sardinella (Sardinella aurita). Afr. J. Biochem. Res. 2013, 7, 158–164. [Google Scholar]
- Stancheva, M.; Merdzhanova, A.; Dobreva, D.A.; Makedonski, L. Common carp (Cyprinus caprio) and European catfish (Sillurus glanis) from the Danube River as sources of fat soluble vitamins and fatty acids. Czech J. Food Sci. 2014, 32, 16–24. [Google Scholar] [CrossRef]
- Ouraji, H.; Shabanpour, B.; Kenari, A.A.; Shabani, A.; Nezami, S.; Sudagar, M.; Faghani, S. Total lipid, fatty acid composition and lipid oxidation of Indian white shrimp (Fenneropenaeus indicus) fed diets containing different lipid sources. J. Sci. Food Agric. 2009, 89, 993–997. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M.J. Mercury, Fatty Acids Content and Lipid Quality Indexes in Muscles of Freshwater and Marine Fish on the Polish Market. Risk Assessment of Fish Consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef]
- Marques, B.; Lillebø, A.I.; Domingues, M.d.R.M.; Saraiva, J.A.; Calado, R. Effect of High-Pressure Processing (HPP) on the Fatty Acid Profile of Different Sized Ragworms (Hediste diversicolor) Cultured in an Integrated Multi-Trophic Aquaculture (IMTA) System. Molecules 2019, 24, 4503. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics-From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Ananya; Kamal, A. Fatty Acid Profiling and Antioxidant Potential of Total Lipid Content of Cyanobacterium nostoc muscurum. Int. J. Pharm. Pharm. Sci. 2016, 8, 159–163. [Google Scholar]
- Puchau, B.; Ochoa, M.C.; Zulet, M.Á.; Marti, A.; Martínez, J.A.; Members, G. Dietary total antioxidant capacity and obesity in children and adolescents. Int. J. Food Sci. Nutr. 2010, 61, 713–721. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Zhang, L.; Wang, H.; Ho, C.-T.; Li, S.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Pang, J.R.; Goh, V.M.J.; Tan, C.Y.; Phang, S.M.; Wong, K.H.; Yow, Y.Y. Neuritogenic and in vitro antioxidant activities of Malaysian Gracilaria manilaensis Yamamoto & Trono. J. Appl. Phycol. 2018, 30, 3253–3260. [Google Scholar]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Raphael, W.; Sordillo, L.M. Dietary polyunsaturated fatty acids and inflammation: The role of phospholipid biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167–21188. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution? Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Mozaffarian, D.; Chiuve, S.E.; Rimm, E.B. Fish consumption and risk of major chronic disease in men. Am. J. Clin. Nutr. 2008, 88, 1618–1625. [Google Scholar] [CrossRef]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Rose, D.P.; Connolly, J.M. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol. Ther. 1999, 83, 217–244. [Google Scholar] [CrossRef]
- Merluzzi, V.J.; Adams, J. The Search for Anti-Inflammatory Drugs: Case Histories from Concept to Clinic; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 146159846X. [Google Scholar]
- Hla, T.; Neilson, K. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. USA 1992, 89, 7384–7388. [Google Scholar] [CrossRef] [PubMed]
- Meade, E.A.; Smith, W.L.; Dewitt, D.L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 1993, 268, 6610–6614. [Google Scholar] [PubMed]
- Szczepanski, A.; Moatter, T.; Carley, W.W.; Gerritsen, M.E. Induction of cyclooxygenase ii in human synovial microvessel endothelial cells by interleukin-1. Arthritis Rheum. 1994, 37, 495–503. [Google Scholar] [CrossRef]
- Mun, O.-J.; Kwon, M.S.; Karadeniz, F.; Kim, M.; Lee, S.-H.; Kim, Y.-Y.; Seo, Y.; Jang, M.-S.; Nam, K.-H.; Kong, C.-S. Fermentation of Sargassum thunbergii by Kimchi-Derived Lactobacillus sp. SH-1 Attenuates LPS-Stimulated Inflammatory Response Via Downregulation of JNK. J. Food Biochem. 2017, 41, e12306. [Google Scholar] [CrossRef]
- Kim, K.-N.; Kim, J.; Yoon, W.-J.; Yang, H.-M.; Heo, S.Y.; Ko, J.-Y.; Woon Roh, S.; Jeon, Y.-J.; Kang, S.-M.; Heo, S.-J. Inhibitory effect of Sargassum patens on inflammation and melanogenesis. Int. J. Pharmacol. 2013, 9, 524–532. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Ahn, E.M.; Park, C.-I.; Jang, J.-H. Sargassum fulvellum Protects HaCaT Cells and BALB/c Mice from UVB-Induced Proinflammatory Responses. Evid. Based Complement. Alternat. Med. 2013, 2013, 747846. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J.-W.; Lee, D.-S.; Yim, M.-J.; Lee, J.M.; Han, M.H.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; Park, E.K.; et al. Sargassum serratifolium Extract Attenuates Interleukin-1β-Induced Oxidative Stress and Inflammatory Response in Chondrocytes by Suppressing the Activation of NF-κB, p38 MAPK, and PI3K/Akt. Int. J. Mol. Sci. 2018, 19, 2308. [Google Scholar] [CrossRef]
- George, A.; Chinnappan, S.; Chintamaneni, M.; Kotak, C.V.; Choudhary, Y.; Kueper, T.; Radhakrishnan, A.K. Anti-inflammatory effects of Polygonum minus (Huds) extract (LineminusTM) in in-vitro enzyme assays and carrageenan induced paw edema. BMC Complement. Altern. Med. 2014, 14, 355. [Google Scholar] [CrossRef]
- Cardoso, C.; Pereira, H.; Franca, J.; Matos, J.; Monteiro, I.; Pousão-Ferreira, P.; Gomes, A.; Barreira, L.; Varela, J.; Neng, N.; et al. Lipid composition and some bioactivities of 3 newly isolated microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.). Aquac. Int. 2020, 28, 711–727. [Google Scholar] [CrossRef]
- Gullo, V.P.; McAlpine, J.; Lam, K.S.; Baker, D.; Petersen, F. Drug discovery from natural products. J. Ind. Microbiol. Biotechnol. 2006, 33, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Walsh, N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006, 44, 1144–1150. [Google Scholar] [CrossRef]
- Schley, P.D.; Jijon, H.B.; Robinson, L.E.; Field, C.J. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2005, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Jurkowski, J.J.; Cave Jr, W.T. Dietary effects of menhaden oil on the growth and membrane lipid composition of rat mammary tumors. J. Natl. Cancer Inst. 1985, 74, 1145–1150. [Google Scholar] [PubMed]
- Karmali, R.A.; Donner, A.; Gobel, S.; Shimamura, T. Effect of n-3 and n-6 fatty acids on 7, 12 dimethylbenz (a) anthracene-induced mammary tumorigenesis. Anticancer Res. 1989, 9, 1161–1167. [Google Scholar] [PubMed]
- Rose, D.P.; Connolly, J.M.; Rayburn, J.; Coleman, M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J. Natl. Cancer Inst. 1995, 87, 587–592. [Google Scholar] [CrossRef]
- Eitsuka, T.; Nakagawa, K.; Igarashi, M.; Miyazawa, T. Telomerase inhibition by sulfoquinovosyldiacylglycerol from edible purple laver (Porphyra yezoensis). Cancer Lett. 2004, 212, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Mizushina, Y.; Hirata, N.; Takemura, M.; Sugawara, F.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Sulfoquinovosyldiacylglycerol, KM043, a new potent inhibitor of eukaryotic DNA polymerases and HIV-reverse transcriptase type 1 from a marine red alga, Gigartina tenella. Chem. Pharm. Bull. (Tokyo) 1998, 46, 684–686. [Google Scholar] [CrossRef]
- Ohta, K.; Mizushima, Y.; Hirata, N.; Takemura, M.; Sugawara, F.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Action of a New Mammalian DNA Polymerase Inhibitor, Sulfoquinovosyldiacylglycerol. Biol. Pharm. Bull. 1999, 22, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Sugiyama, Y.; Yoshida, H.; Hanashima, S.; Yamazaki, T.; Kamisuki, S.; Ohta, K.; Takemura, M.; Yamaguchi, T.; Matsukage, A.; et al. Galactosyldiacylglycerol, a Mammalian DNA Polymerase Alpha-Specific Inhibitor from a Sea Alga, Petalonia bingbamiae. Biol. Pharm. Bull. 2001, 24, 982–987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thiès, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001, 16, 201–204. [Google Scholar] [CrossRef]
- Picq, M.; Chen, P.; Perez, M.; Michaud, M.; Véricel, E.; Guichardant, M.; Lagarde, M. DHA Metabolism: Targeting the Brain and Lipoxygenation. Mol. Neurobiol. 2010, 42, 48–51. [Google Scholar] [CrossRef]
- Kostetsky, E.; Chopenko, N.; Barkina, M.; Velansky, P.; Sanina, N. Fatty Acid Composition and Thermotropic Behavior of Glycolipids and Other Membrane Lipids of Ulva lactuca (Chlorophyta) Inhabiting Different Climatic Zones. Mar. Drugs 2018, 16, 494. [Google Scholar] [CrossRef]
- Melo, T.; Alves, E.; Azevedo, V.; Martins, A.S.; Neves, B.; Domingues, P.; Calado, R.; Abreu, H.; Domingues, M.R. Lipidomics as a new approach for the bioprospecting of marine macroalgae-unraveling the polar lipid and fatty acid composition of Chondrus crispus. Algal Res. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Melo, T.; Marques, S.S.; Ferreira, I.; Cruz, M.T.; Domingues, P.; Segundo, M.A.; Domingues, M.R.M. New Insights into the Anti-Inflammatory and Antioxidant Properties of Nitrated Phospholipids. Lipids 2018, 53, 117–131. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Automatic method for determination of total antioxidant capacity using 2,2-diphenyl-1-picrylhydrazyl assay. Anal. Chim. Acta 2006, 558, 310–318. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016, pdb–prot087379. [Google Scholar] [CrossRef] [PubMed]
- RCore, Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Team, R.S. RStudio: Integrated development environment for R. RStudio, PBC: Boston, MA, USA, 2016. [Google Scholar]
Sample Availability: Samples of the seaweeds and lipid extracts are available from the authors. |
| FA | Ulva rigida | Codium tomentosum | Gracilaria gracilis | Palmaria palmata | Porphyra dioica | Fucus vesiculosus |
|---|---|---|---|---|---|---|
| 14:0 | 3.7 ± 0.4 | 3.2 ± 0.3 | 5.3 ± 0.4 | 9.3 ± 0.6 | 9.3 ± 0.5 | |
| 16:0 | 20.2 ± 0.4 | 22.3 ± 1.2 | 27.1 ± 1.2 | 24.4 ± 1.1 | 23.3 ± 1.1 | 11.9 ± 0.5 |
| 16:1n-7 | 1.3 ± 0.1 | 4.9 ± 0.2 | 2.8 ± 0.8 | 2 ± 0.4 | 18.3 ± 0.7 | 1 ± 0.0 |
| 16:1n-9 | 2.1 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.1 | |||
| 16:2n-4 | 0.9 ± 0.1 | |||||
| 16:2n-6 | 0.8 ± 0.1 | 1.8 ± 0.1 | ||||
| 16:3n-4 | 1.7 ± 0.1 | 1.3 ± 0.1 | ||||
| 16:3n-6 | 1.4 ± 0.1 | |||||
| 16:3n-3 | 10.3 ± 0.4 | |||||
| 16:4n-1 | 1.5 ± 0.1 | 3.5 ± 0.3 | ||||
| 16:4n-3 | 19 ± 0.6 | |||||
| 18:0 | 2.9 ± 1 | 2.6 ± 0.6 | 4.6 ± 0.8 | 12.5 ± 6.8 | 4.9 ± 1 | 3.6 ± 1.1 |
| 18:1 * | 9.5 ± 0.3 | 11.1 ± 0.4 | 9.7 ± 0.4 | 2.8 ± 0.5 | 3.3 ± 0.2 | 22.2 ± 1.3 |
| 18:2n-6 | 1.5 ± 0.1 | 3.4 ± 0.1 | 2 ± 0.4 | 1.7 ± 0.1 | 8.5 ± 0.2 | |
| 18:2n-3 | 3.6 ± 0.1 | |||||
| 18:3n-6 | 0.4 ± 0.1 | 2 ± 0.1 | 0.8 ± 0.0 | |||
| 18:3n-3 | 10.9 ± 0.4 | 14 ± 0.6 | 2.7 ± 0.2 | 6.7 ± 0.3 | ||
| 18:4n-3 | 24.4 ± 0.4 | 4.4 ± 0.1 | 7 ± 0.2 | 3.4 ± 0.2 | 6.2 ± 0.3 | |
| 20:3n-6 | 2.4 ± 0.5 | 0.9 ± 0.1 | ||||
| 20:4n-6 | 4.5 ± 0.4 | 35.4 ± 1.5 | 0.9 ± 0.2 | 2.7 ± 0.3 | 16.7 ± 0.7 | |
| 20:4n-3 | 1.2 ± 0.1 | 0.6 ± 0.1 | ||||
| 20:5-n-3 | 1.4 ± 0.1 | 7.9 ± 0.8 | 5.5 ± 0.2 | 51.9 ± 6.5 | 20.5 ± 2.3 | 10.3 ± 0.5 |
| 22:0 | 1 ± 0.1 | 1.7 ± 0.4 | 0.3 ± 0.0 | |||
| 22:5n-3 | 4.1 ± 0.1 |
| Indicators | Ulva rigida | Codium tomentosum | Gracilaria gracilis | Palmaria palmata | Porphyra dioica | Fucus vesiculosus |
|---|---|---|---|---|---|---|
| SFA | 24.1 ± 1.4 | 30.2 ± 1.6 | 34.9 ± 0.9 | 42.3 ± 7.3 | 37.5 ± 2.4 | 25.2 ± 2 |
| MUFA | 13 ± 0.3 | 16.8 ± 0.3 | 12.5 ± 0.7 | 4.9 ± 0.9 | 22.5 ± 0.7 | 23.3 ± 1.2 |
| PUFA | 62.9 ± 1.1 | 53 ± 1.4 | 52.6 ± 1.4 | 52.8 ± 6.7 | 40 ± 3 | 51.6 ± 1.5 |
| PUFA omega-6 | 2 ± 0.1 | 8.7 ± 0.4 | 37.4 ± 1.3 | 0.9 ± 0.2 | 10.7 ± 0.8 | 28.3 ± 0.7 |
| PUFA omega-3 | 60.9 ± 1.1 | 40.2 ± 1.3 | 15.1 ± 0.3 | 51.9 ± 6.5 | 24.5 ± 2.5 | 23.3 ± 0.9 |
| AI | 0.3 ± 0.0 a | 0.6 ± 0.1 a,b | 0.6 ± 0.0 a,c,d | 0.8 ± 0.1 c,e | 1.1 ± 0.1 e | 0.7 ± 0.1 b,d,e |
| TI | 0.1 ± 0.0 a | 0.2 ± 0.0 a,b | 0.5 ± 0.0 c | 0.2 ± 0.1 a,d | 0.4 ± 0.1 b,c | 0.3 ± 0.0 b,c,d |
| Ulva rigida | Codium tomentosum | Gracilaria gracilis | Palmaria palmata | Porphyra dioica | Fucus vesiculosus | ||
|---|---|---|---|---|---|---|---|
| ABTS●+ | IC50 | 30.7 ± 0.1 a,b | 48.1 ± 0.0 a,c | 86.4 ± 3.4 a | 23.7 ± 0.6 b,d | 41.1 ± 2.5 a,d | 27.3 ± 0.2 b,c,d |
| TE | 500.5 ± 1.7 a,b | 327.9 ± 0.2 a,b | 183.0 ± 7.1 a | 606.1 ± 14.6 b | 338.8 ± 20.5 a,b | 507.1 ± 3.5 b | |
| DPPH● | IC20 | 120.8 ± 3.8 a,b | 249.9 ± 66.7 a | 119.5 ± 1.8 a,b | 119.6 ± 8.0 a,b | 212.5 ± 7.0 a | 106.0 ± 5.6 b |
| TE | 88.0 ± 2.8 | 249.9 ± 66.7 | 89.2 ± 1.3 | 89.5 ± 6.3 | 44.9 ± 1.5 | 89.7 ± 4.6 |
| Macroalgae | IC50 |
|---|---|
| Ulva rigida | 82.7 ± 19.1% |
| Codium tomentosum | 66.4 ± 12.0% |
| Gracilaria gracilis | 74.7 ± 19.1% |
| Palmaria palmata | 40.4 ± 19.2% |
| Pporphyra dioica | 35.5 ± 10.5% |
| Fucus vesiculosus | 52.5 ± 10.9% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules 2020, 25, 3883. https://doi.org/10.3390/molecules25173883
Lopes D, Melo T, Rey F, Meneses J, Monteiro FL, Helguero LA, Abreu MH, Lillebø AI, Calado R, Domingues MR. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules. 2020; 25(17):3883. https://doi.org/10.3390/molecules25173883
Chicago/Turabian StyleLopes, Diana, Tânia Melo, Felisa Rey, Joana Meneses, Fátima Liliana Monteiro, Luisa A. Helguero, Maria Helena Abreu, Ana Isabel Lillebø, Ricardo Calado, and Maria Rosário Domingues. 2020. "Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications" Molecules 25, no. 17: 3883. https://doi.org/10.3390/molecules25173883
APA StyleLopes, D., Melo, T., Rey, F., Meneses, J., Monteiro, F. L., Helguero, L. A., Abreu, M. H., Lillebø, A. I., Calado, R., & Domingues, M. R. (2020). Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules, 25(17), 3883. https://doi.org/10.3390/molecules25173883











