Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds
Abstract
1. Introduction
2. Plant-Derived Sulfur Compounds—GSLs and OSCs
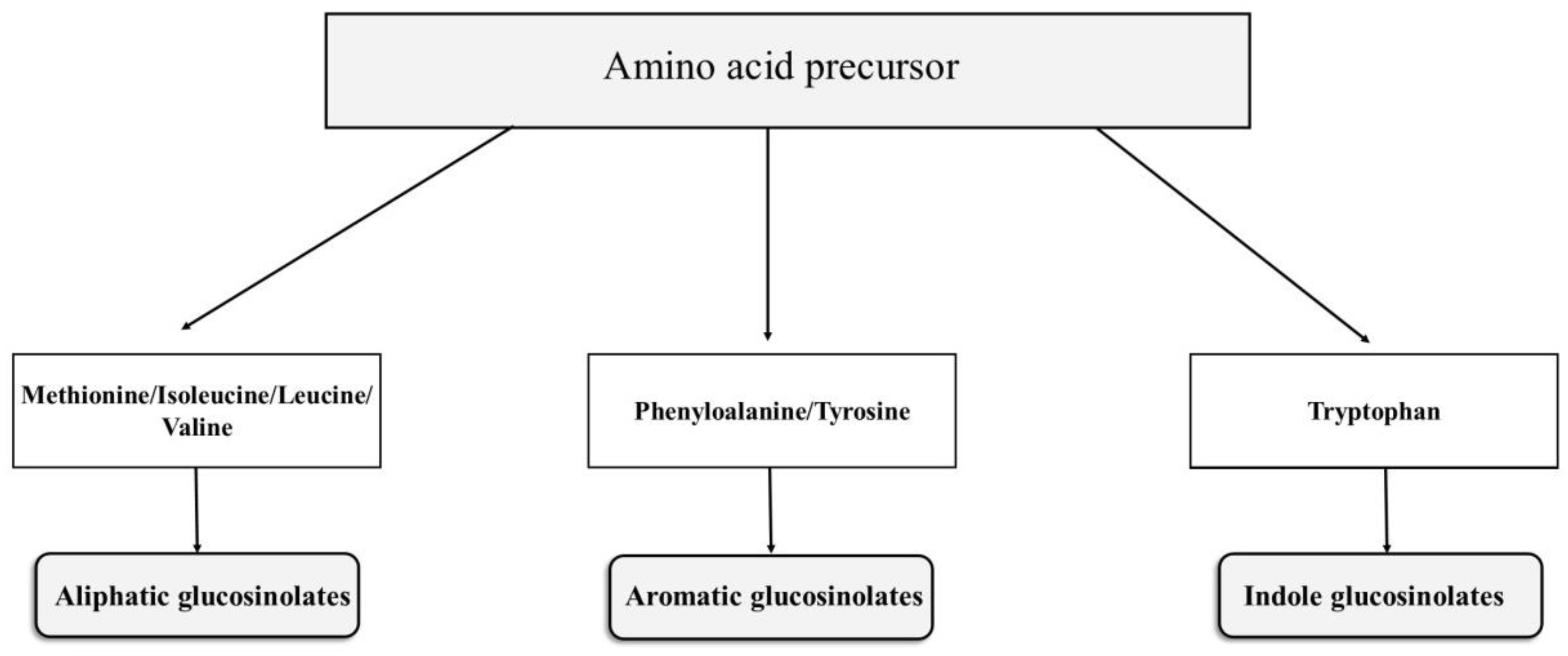
2.1. Glucosinolates (GSLs)
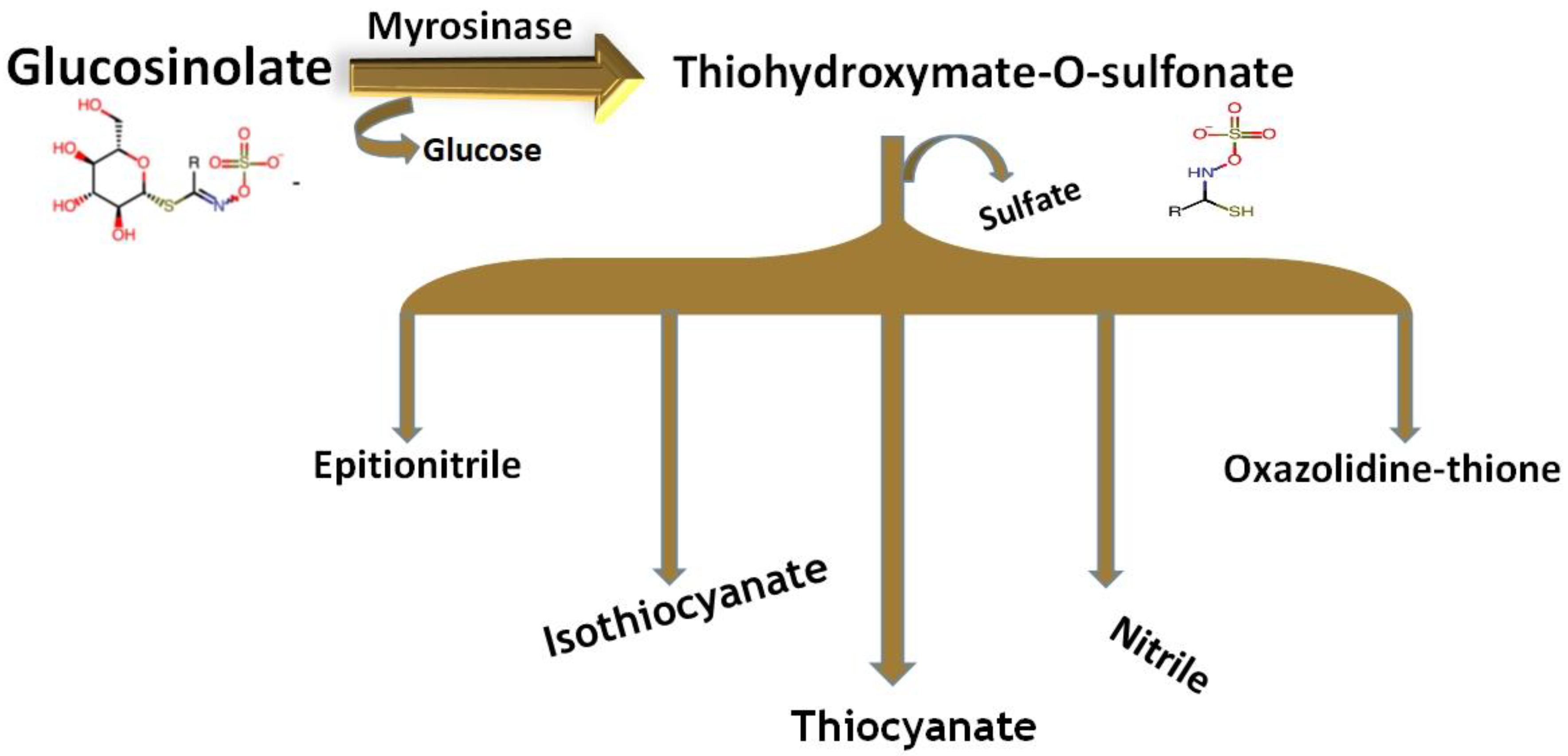
2.1.1. Chemical Structure and Biodegradation
2.1.2. Metabolism Pathway of Glucosinolates in the Body
2.1.3. Health Benefits of Glucosinolates
Protection against Carcinogenesis
Cardiovascular Protection
Protection of the Central Nervous System
Protection against Neuropathy
Significance of Glucosinolates in Response to Fungi, Bacteria, and Microorganisms
Benefits for Diabetic Patients
Benefits in the Skin Problems
2.2. Organosulfur Compounds (OSCs)
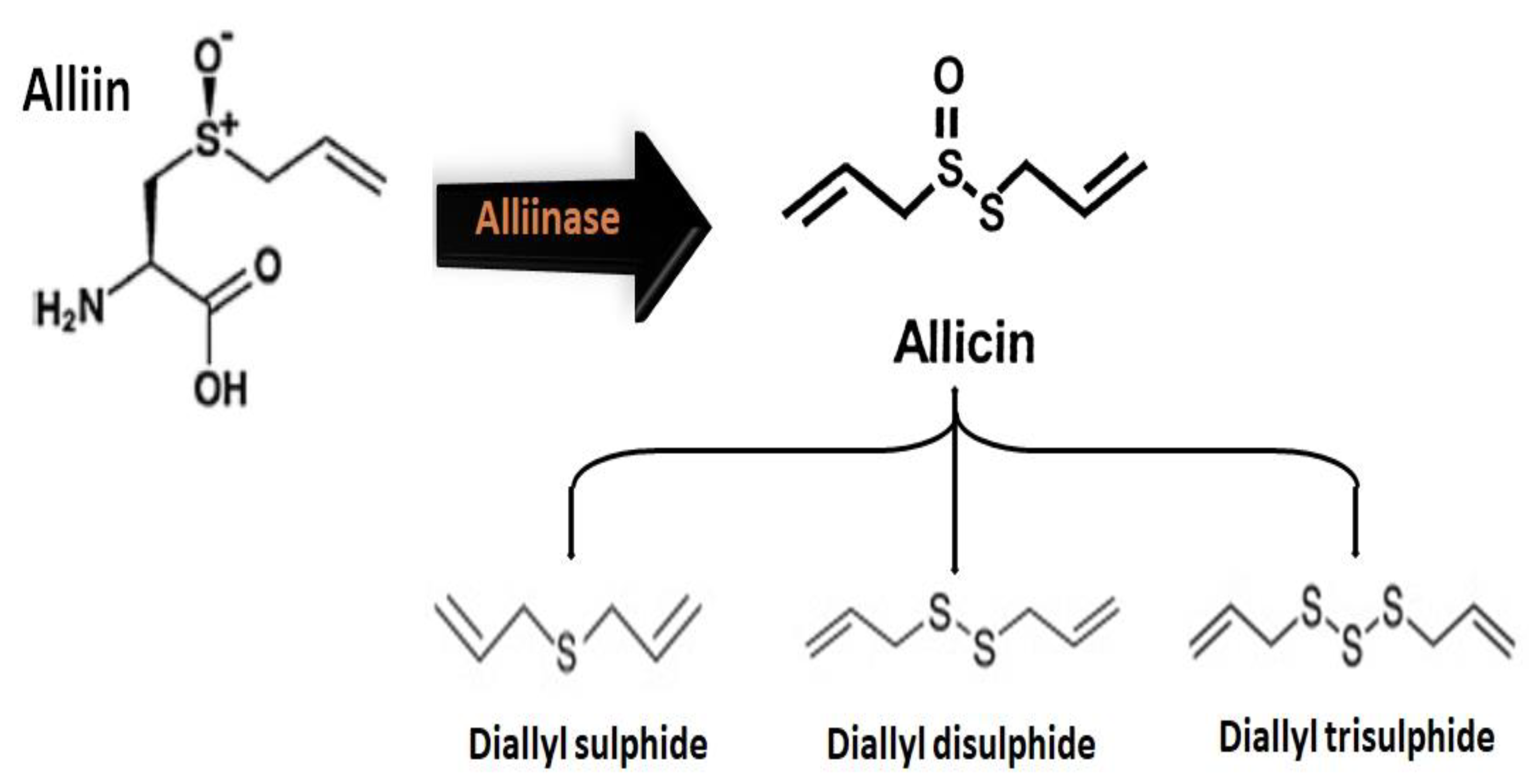
2.2.1. Chemical Structure and Biodegradation
2.2.2. Health Benefits of Organosulfur Compounds
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nauts, H.C. The Bibliography of Reports Concerning the Experimental Clinical Use of Coley Toxins; Cancer Research Institute: New York, NY, USA, 1985. [Google Scholar]
- Hadden, J.W. Immunostimulants. Trends Pharm. Sci. 1993, 14, 169–174. [Google Scholar] [CrossRef]
- Upadhayay, U.P.P.D.D.; Ewam, P.C.V.V.; Ewam, U.P.C.V.V.; Sansthan, G.-A. Immunomodulatory and Therapeutic Potentials of Herbal, Traditional/Indigenous and Ethnoveterinary Medicines” Mahima,“Anu Rahal,” Rajib Deb,“Shyma, K. Latheef,” Hari Abdul Samad. Pak. J. Biol. Sci. 2012, 15, 754–774. [Google Scholar]
- Quintero-Fabián, S.; Ortuño-Sahagún, D.; Vázquez-Carrera, M.; López-Roa, R.I. Alliin, a garlic (Allium sativum) compound, prevents LPS-induced inflammation in 3T3-L1 adipocytes. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Tanaka, S.; Haruma, K.; Yoshihara, M.; Kajiyama, G.; Kira, K.; Amagase, H.; Chayama, K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J. Nutr. 2006, 136, 821S–826S. [Google Scholar] [CrossRef] [PubMed]
- Fund, W.C.R.; Research, A.I. for C. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; Amer Inst for Cancer Research: Washington, DC, USA, 2007; Volume 1, ISBN 0972252223. [Google Scholar]
- Munn, L.L. Cancer and inflammation. Wires Syst. Biol. Med. 2017, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting inflammation in cancer prevention and therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef]
- Grimble, R.F. Interaction between nutrients, pro-inflammatory cytokines and inflammation. Clin. Sci. 1996, 91, 121–130. [Google Scholar] [CrossRef]
- Grimble, R.F. Nutritional modulation of cytokine biology. Nutrition 1996, 14, 634–640. [Google Scholar] [CrossRef]
- Grigore, A. Targeting Tumor-Associated Macrophages by Plant Compounds. In Macrophages; IntechOpen: London, UK, 2020. [Google Scholar]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Antioxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharm. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef]
- Melino, S.; Sabelli, R.; Paci, M. Allyl sulfur compounds and cellular detoxification system: Effects and perspectives in cancer therapy. Amino Acids 2011, 41, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.L.; Singh, S.V. Molecular mechanisms and targets of cancer chemoprevention by garlic-derived bioactive compound diallyl trisulfide. Indian J. Exp. Biol. 2011, 49, 805. [Google Scholar] [PubMed]
- Ohkubo, S.; Dalla Via, L.; Grancara, S.; Kanamori, Y.; García-Argáez, A.N.; Canettieri, G.; Arcari, P.; Toninello, A.; Agostinelli, E. The antioxidant, aged garlic extract, exerts cytotoxic effects on wild-type and multidrug-resistant human cancer cells by altering mitochondrial permeability. Int. J. Oncol. 2018, 53, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Ko, M.; Ishizuka, M.; Fujita, S.; Yabuki, A.; Hossain, M.A.; Yamato, O. Sodium 2-propenyl thiosulfate derived from garlic induces phase II detoxification enzymes in rat hepatoma H4IIE cells. Nutr. Res. 2010, 30, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Modem, S.; DiCarlo, S.E.; Reddy, T.R. Fresh garlic extract induces growth arrest and morphological differentiation of MCF7 breast cancer cells. Genes Cancer 2012, 3, 177–186. [Google Scholar] [CrossRef]
- Desai, G.; Schelske-Santos, M.; Nazario, C.M.; Rosario-Rosado, R.V.; Mansilla-Rivera, I.; Ramírez-Marrero, F.; Nie, J.; Myneni, A.A.; Zhang, Z.-F.; Freudenheim, J.L.; et al. Onion and Garlic Intake and Breast Cancer, a Case-Control Study in Puerto Rico. Nutr. Cancer 2020, 72, 791–800. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Franco, P.; Spinozzi, S.; Pagnotta, E.; Lazzeri, L.; Ugolini, L.; Camborata, C.; Roda, A. Development of a liquid chromatography—electrospray ionization—tandem mass spectrometry method for the simultaneous analysis of intact glucosinolates and isothiocyanates in Brassicaceae seeds and functional foods. J. Chromatogr. A 2016, 1428, 154–161. [Google Scholar] [CrossRef]
- Hirani, A.H.; Li, G.; Zelmer, C.D.; McVetty, P.B.E.; Asif, M.; Goyal, A. Molecular genetics of glucosinolate biosynthesis in Brassicas: Genetic manipulation and application aspect. Crop. Plant. 2012, 189–216. [Google Scholar]
- Bischoff, K.L. Glucosinolates. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 551–554. [Google Scholar]
- Mikkelsen, M.D.; Naur, P.; Halkier, B.A. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant. J. 2004, 37, 770–777. [Google Scholar] [CrossRef]
- Ishida, M.; Nagata, M.; Ohara, T.; Kakizaki, T.; Hatakeyama, K.; Nishio, T. Small variation of glucosinolate composition in Japanese cultivars of radish (Raphanus sativus L.) requires simple quantitative analysis for breeding of glucosinolate component. Breed. Sci. 2012, 62, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Chun, J.-H.; Byeon, D.H.; Chung, S.-O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.-P.; Kim, S.-J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. Lwt-Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Li, G.; Quiros, C.F. In planta side-chain glucosinolate modification in Arabidopsis by introduction of dioxygenase Brassica homolog BoGSL-ALK. Appl. Genet. 2003, 106, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant. Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Collett, M.G.; Stegelmeier, B.L.; Tapper, B.A. Could nitrile derivatives of turnip (Brassica rapa) glucosinolates be hepato-or cholangiotoxic in cattle? J. Agric. Food Chem. 2014, 62, 7370–7375. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; Rohloff, J.; Bones, A.M. Defence mechanisms of Brassicaceae: Implications for plant-insect interactions and potential for integrated pest management. A review. Agron. Sustain. Dev. 2010, 30, 311–348. [Google Scholar] [CrossRef]
- Nair, A.; Chattopadhyay, D.; Saha, B. Plant-derived immunomodulators. In New Look to Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 435–499. [Google Scholar]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant. Sci. 2015, 6, 655. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Skąpska, S.; Mitek, M. A Comparative Study of the Quality of Strawberry Purée Preserved by Continuous Microwave Heating and Conventional Thermal Pasteurization during Long-Term Cold Storage. Food Bioprocess. Technol. 2016, 9, 1100–1112. [Google Scholar] [CrossRef]
- Hallmann, E.; Kazimierczak, R.; Marszałek, K.; Drela, N.; Kiernozek, E.; Toomik, P.; Matt, D.; Luik, A.; Rembiałkowska, E. The Nutritive Value of Organic and Conventional White Cabbage (Brassica Oleracea, L. Var. Capitata) and Anti-Apoptotic Activity in Gastric Adenocarcinoma Cells of Sauerkraut Juice Produced Therof. J. Agric. Food Chem. 2017, 65, 8171–8183. [Google Scholar] [CrossRef]
- Shin, I.-S.; Hong, J.; Jeon, C.-M.; Shin, N.-R.; Kwon, O.-K.; Kim, H.-S.; Kim, J.-C.; Oh, S.-R.; Ahn, K.-S. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem. Toxicol. 2013, 62, 506–513. [Google Scholar] [CrossRef]
- Colín-González, A.L.; Santamaría, A. Garlic, gastrointestinal protection and oxidative stress. In Gastrointestinal Tissue; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–288. [Google Scholar]
- Florkiewicz, A.; Ciska, E.; Filipiak-Florkiewicz, A.; Topolska, K. Comparison of Sous-vide methods and traditional hydrothermal treatment on GLS content in Brassica vegetables. Eur. Food Res. Technol. 2017, 243, 1507–1517. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kusznierewicz, B.; Leszczyńska, T.; Borczak, B. Effect of cooking on the contents of glucosinolates and their degradation products in selected Brassica vegetables. J. Funct. Foods 2016, 23, 412–422. [Google Scholar] [CrossRef]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef] [PubMed]
- Westphal, A.; Riedl, K.M.; Cooperstone, J.L.; Kamat, S.; Balasubramaniam, V.M.; Schwartz, S.J.; Böhm, V. High-pressure processing of broccoli sprouts: Influence on bioactivation of glucosinolates to isothiocyanates. J. Agric. Food Chem. 2017, 65, 8578–8585. [Google Scholar] [CrossRef]
- Fu, E.; Tsai, M.; Chin, Y.; Tu, H.; Fu, M.M.; Chiang, C.; Chiu, H. The effects of diallyl sulfide upon P orphyromonas gingivalis lipopolysaccharide stimulated proinflammatory cytokine expressions and nuclear factor-kappa B activation in human gingival fibroblasts. J. Periodontal Res. 2015, 50, 380–388. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of postharvest UV-B and UV-C radiation treatments to revalorize broccoli byproducts and edible florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhuang, W.; Hu, W.; Liu, G.; Wu, T.; Wu, X. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011, 141, 80–89. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Yuan, J.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the power of plants to people. Mol. Nutr. Food Res. 2018, 62, 1700965. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol. Prev. Biomark. 2001, 10, 501–508. [Google Scholar]
- Zhang, Y.S.; Kolm, R.H.; Mannervik, B.; Talalay, P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem. Biophys. Res. Commun. 1995, 206, 748–755. [Google Scholar] [CrossRef]
- Combourieu, B.; Elfoul, L.; Delort, A.-M.; Rabot, S. Identification of new derivatives of sinigrin and glucotropaeolin produced by the human digestive microflora using 1H NMR spectroscopy analysis of in vitro incubations. Drug Metab. Dispos. 2001, 29, 1440–1445. [Google Scholar] [PubMed]
- Navarro, J.L.; Tárrega, A.; Sentandreu, M.A.; Sentandreu, E. Partial purification and characterization of polyphenol oxidase from persimmon. Food Chem. 2014, 157, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health promoting effects of brassica-derived phytochemicals: From chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Thejass, P.; Kuttan, G. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by diallyl disulfide (DADS). Life Sci. 2007, 80, 515–521. [Google Scholar] [CrossRef]
- Abdull Razis, A.F.; De Nicola, G.R.; Pagnotta, E.; Iori, R.; Ioannides, C. A glucosinolate-rich extract of Japanese Daikon perturbs carcinogen-metabolizing enzyme systems in rat, being a potent inducer of hepatic glutathione S-transferase. Eur. J. Nutr. 2013, 52, 1279–1285. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef]
- Negi, G.; Kumar, A.; S Sharma, S. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr. Neurovasc. Res. 2011, 8, 294–304. [Google Scholar] [CrossRef]
- Gamet-Payrastre, L. Signaling pathways and intracellular targets of sulforaphane mediating cell cycle arrest and apoptosis. Curr. Cancer Drug Targets 2006, 6, 135–145. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T. Chemoprotection against cancer by isothiocyanates: A focus on the animal models and the protective mechanisms. In Natural Products in Cancer Prevention and Therapy; Springer: Berlin, Germany, 2012; pp. 179–201. [Google Scholar]
- Sturm, C.; Wagner, A.E. Brassica-derived plant bioactives as modulators of chemopreventive and inflammatory signaling pathways. Int. J. Mol. Sci. 2017, 18, 1890. [Google Scholar] [CrossRef]
- Senanayake, G.V.K.; Banigesh, A.; Wu, L.; Lee, P.; Juurlink, B.H.J. The dietary phase 2 protein inducer sulforaphane can normalize the kidney epigenome and improve blood pressure in hypertensive rats. Am. J. Hypertens. 2012, 25, 229–235. [Google Scholar] [CrossRef]
- Tanito, M.; Masutani, H.; Kim, Y.-C.; Nishikawa, M.; Ohira, A.; Yodoi, J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.; Diggins, E.L.; Connors, S.L.; Zimmerman, A.W.; Singh, K.; Liu, H.; Talalay, P.; Fahey, J.W. Sulforaphane from broccoli reduces symptoms of autism: A follow-up case series from a randomized double-blind study. Glob. Adv. Heal. Med. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, X.; Wang, Q.; Jiang, Y.; Shang, H.; Cui, L.; Cao, Y. Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar. J. 2012, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Nok, A.J.; Williams, S.; Onyenekwe, P.C. Allium sativum-induced death of African trypanosomes. Parasitol. Res. 1996, 82, 634–637. [Google Scholar] [CrossRef]
- Coppi, A.; Cabinian, M.; Mirelman, D.; Sinnis, P. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob. Agents Chemother. 2006, 50, 1731–1737. [Google Scholar] [CrossRef]
- Drobnica, Ľ.; Zemanova, M.; Nemec, P.; Antoš, K.; Kristian, P.; Štullerová, A.; Knoppova, V. Antifungal Activity of Isothiocyanates and Related Compounds: I. Naturally Occurring Isothiocyanates and Their Analogues. Appl. Microbiol. 1967, 15, 701–709. [Google Scholar] [CrossRef]
- Tsao, R.; Peterson, C.J.; Coats, J.R. Glucosinolate breakdown products as insect fumigants and their effect on carbon dioxide emission of insects. Bmc Ecol. 2002, 2, 5. [Google Scholar]
- Graham, S.; Dayal, H.; Swanson, M.; Mittelman, A.; Wilkinson, G. Diet in the epidemiology of cancer of the colon and rectum. J. Natl. Cancer Inst. 1978, 61, 709–714. [Google Scholar]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Singh, S.V.; Warin, R.; Xiao, D.; Powolny, A.A.; Stan, S.D.; Arlotti, J.A.; Zeng, Y.; Hahm, E.-R.; Marynowski, S.W.; Bommareddy, A. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009, 69, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; Putluri, V.; Arnold, J.M.; Tsouko, E.; Maity, S.; Roberts, J.M.; Coarfa, C.; Frigo, D.E.; Putluri, N.; Sreekumar, A. Differential regulation of metabolic pathways by androgen receptor (AR) and its constitutively active splice variant, AR-V7, in prostate cancer cells. Oncotarget 2015, 6, 31997. [Google Scholar] [CrossRef]
- Gibbs, A.; Schwartzman, J.; Deng, V.; Alumkal, J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc. Natl. Acad. Sci. USA 2009, 106, 16663–16668. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.M.P.; Rodrigues, L.A.R.L.; de Alencar, C.; Lunna, P.; de Lima, S.; Víctor, P.; Nolasco Lugo, L.M.; Nunes, N.M.F.; do Nascimento Silva, J.; da Silva Araûjo, L. Cruciferous vegetables as antioxidative, chemopreventive and antineoplasic functional foods: Preclinical and clinical evidences of sulforaphane against prostate cancers. Curr. Pharm. Des. 2018, 24, 4779–4793. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mao, Q.; Cao, M.; Xie, L. Cruciferous vegetables intake and risk of prostate cancer: A meta-analysis. Int. J. Urol. 2012, 19, 134–141. [Google Scholar] [CrossRef]
- Livingstone, T.L.; Beasy, G.; Mills, R.D.; Plumb, J.; Needs, P.W.; Mithen, R.; Traka, M.H. Plant Bioactives and the Prevention of Prostate Cancer: Evidence from Human Studies. Nutrients 2019, 11, 2245. [Google Scholar] [CrossRef]
- Gerhauser, C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 405–410. [Google Scholar] [CrossRef]
- Myzak, M.C.; Karplus, P.A.; Chung, F.-L.; Dashwood, R.H. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004, 64, 5767–5774. [Google Scholar] [CrossRef]
- Mukherjee, S.; Gangopadhyay, H.; Das, D.K. Broccoli: A unique vegetable that protects mammalian hearts through the redox cycling of the thioredoxin superfamily. J. Agric. Food Chem. 2008, 56, 609–617. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Golzarand, M.; Zojaji, H.; Azizi, F. A comparative study of broccoli sprouts powder and standard triple therapy on cardiovascular risk factors following H. pylori eradication: A randomized clinical trial in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2014, 13, 64. [Google Scholar] [CrossRef]
- Yan, J.Y.; Tian, F.M.; Hu, W.N.; Zhang, J.H.; Cai, H.F.; Li, N. Apoptosis of human gastric cancer cells line SGC 7901 induced by garlic-derived compound S-allylmercaptocysteine (SAMC). Eur. Rev. Med. Pharm. Sci. 2013, 17, 745–751. [Google Scholar]
- Haristoy, X.; Angioi-Duprez, K.; Duprez, A.; Lozniewski, A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob. Agents Chemother. 2003, 47, 3982–3984. [Google Scholar] [CrossRef] [PubMed]
- Via, D. Aged garlic extract and its constituent, SallylLcysteine, induce the apoptosis of neuroblastoma cancer cells due to mitochondrial membrane depolarization. Exp. Med. 2020, 19, 1511–1521. [Google Scholar]
- Hahm, E.-R.; Singh, S.V. Diallyl trisulfide inhibits estrogen receptor-α activity in human breast cancer cells. Breast Cancer Res. Treat. 2014, 144, 47–57. [Google Scholar] [CrossRef]
- Poojary, M.M.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food Compos. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef]
- Guercio, V.; Galeone, C.; Turati, F.; La Vecchia, C. Gastric cancer and allium vegetable intake: A critical review of the experimental and epidemiologic evidence. Nutr. Cancer 2014, 66, 757–773. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, G.; Tian, H.; Hu, Y.; Wu, S.; Geng, Y.; Lin, K.; Wu, W. Sulforaphane metabolites cause apoptosis via microtubule disruption in cancer. Endocr. Relat. Cancer 2018, 25, 255–268. [Google Scholar] [CrossRef]
- Lawson, L.D.; Wang, Z.J. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: Use in measuring allicin bioavailability. J. Agric. Food Chem. 2005, 53, 1974–1983. [Google Scholar] [CrossRef]
- Wang, Y.; Raghavan, S.; Ho, C.-T. Process flavors of Allium vegetables. In Fruit and Vegetable Flavour; Elsevier: Amsterdam, The Netherlands, 2008; pp. 200–226. [Google Scholar]
- Iqbal, A.; Murtaza, A.; Marszałek, K.; Iqbal, M.A.; Chughtai, M.F.J.; Hu, W.; Barba, F.J.; Bi, J.; Liu, X.; Xu, X. Inactivation and structural changes of polyphenol oxidase in quince (Cydonia oblonga Miller) juice subjected to ultrasonic treatment. J. Sci. Food Agric. 2020, 100, 2065–2073. [Google Scholar] [CrossRef]
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res. Int. 2015, 77, 725–742. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Locatelli, D.A.; Gonzalez, R.E.; Cavagnaro, P.F.; Camargo, A.B. Analytical methods for bioactive sulfur compounds in Allium: An integrated review and future directions. J. Food Compos. Anal. 2017, 61, 4–19. [Google Scholar] [CrossRef]
- Ugwu, C.E.; Suru, S.M. The Functional Role of Garlic and Bioactive Components in Cardiovascular and Cerebrovascular Health: What We Do Know. J. Biosci. Med. 2016, 4, 28–42. [Google Scholar] [CrossRef][Green Version]
- Santhosha, S.G.; Jamuna, P.; Prabhavathi, S.N. Bioactive components of garlic and their physiological role in health maintenance: A review. Food Biosci. 2013, 3, 59–74. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Milner, J.A. The influence of heating on the anticancer properties of garlic. J. Nutr. 2001, 131, 1054S–1057S. [Google Scholar] [CrossRef]
- Tomatis, I. Allium sativum bulb absolutes and therapeutic or cosmetic uses. U.S. Patent No. 7,192,613, 20 March 2007. [Google Scholar]
- Murtaza, A.; Iqbal, A.; Marszałek, K.; Iqbal, A.M.; Waseem Ali, S.; Xu, X.; Pan, S.; Hu, W. Enzymatic, Phyto-, and Physicochemical Evaluation of Apple Juice under High-Pressure Carbon Dioxide and Thermal Processing. Foods 2020, 9, 243. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, Y.-T.; Kim, M.; Noh, B.-S.; Choi, W.-S. Effect of high hydrostatic pressure (HHP) treatment on flavor, physicochemical properties and biological functionalities of garlic. Lwt-Food Sci. Technol. 2014, 55, 347–354. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Anthon, G.E.; Barrett, D.M. Onion cells after high pressure and thermal processing: Comparison of membrane integrity changes using different analytical methods and impact on tissue texture. J. Food Sci. 2010, 75, E426–E432. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, X.; Bi, J.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Drying of garlic slices (Allium sativum L.) and its effect on thiosulfinates, total phenolic compounds and antioxidant activity during infrared drying. J. Food Process. Preserv. 2017, 41, e12734. [Google Scholar] [CrossRef]
- Barba, F.J.; Orlien, V. Processing, bioaccessibility and bioavailability of bioactive sulfur compounds: Facts and gaps. J. Food Compos. Anal. 2017, 61, 1–3. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Hu, W.; Ahmad, I.; Ahmed, A.; Xu, X. Food and Bioproducts Processing Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioprod. Process. 2019, 117, 170–182. [Google Scholar] [CrossRef]
- Murtaza, A.; Muhammad, Z.; Iqbal, A.; Ramzan, R.; Liu, Y.; Hu, W.; Pan, S. Aggregation and Conformational Changes in Native and Thermally Treated Polyphenol Oxidase from Apple Juice (Malus domestica). Front. Chem. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Muhammad, Z.; Elkhedir, A.E.; Tao, M. Inactivation, aggregation and conformational changes of polyphenol oxidase from quince (Cydonia oblonga Miller) juice subjected to thermal and high- pressure carbon dioxide treatment. Molecules 2018, 23, 1743. [Google Scholar] [CrossRef]
- Deng, J.; Ren, P.; Deng, D.; Bao, X. Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 2100–2104. [Google Scholar] [CrossRef]
- Murtaza, A.; Iqbal, A.; Linhu, Z.; Liu, Y.; Xu, X.; Pan, S.; Hu, W. Effect of high-pressure carbon dioxide on the aggregation and conformational changes of polyphenol oxidase from apple (Malus domestica) juice. Innov. Food Sci. Emerg. Technol. 2019. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kumagai, H. Characteristics, biosynthesis, decomposition, metabolism and functions of the garlic odour precursor, S-allyl-l-cysteine sulfoxide. Exp. Med. 2020, 9, 1528–1535. [Google Scholar] [CrossRef]
- Bat-Chen, W.; Golan, T.; Peri, I.; Ludmer, Z.; Schwartz, B. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr. Cancer 2010, 62, 947–957. [Google Scholar] [CrossRef]
- Lawson, L.D.; Hunsaker, S.M. Allicin bioavailability and bioequivalence from garlic supplements and garlic foods. Nutrients 2018, 10, 812. [Google Scholar] [CrossRef]
- Pinto, J.T.; Krasnikov, B.F.; Cooper, A.J.L. Redox-sensitive proteins are potential targets of garlic-derived mercaptocysteine derivatives. J. Nutr. 2006, 136, 835S–841S. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Pao, J.; Lin, S.Y.; Sheen, L.Y. Molecular mechanisms of garlic-derived allyl sulfides in the inhibition of skin cancer progression. Ann. N. Y. Acad. Sci. 2012, 1271, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, A.M.; Puupponen-Pimiä, R.; Aarni, M.; Oksman-Caldentey, K.-M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer 2007, 110, 1083–1095. [Google Scholar] [CrossRef]
- 114. Bommareddy, A.; VanWert, A.L.; McCune, D.F.; Brozena, S.L.; Witczak, Z.; Singh, S.V. The role of organosulfur compounds derived from Allium vegetables in cancer prevention and therapy. In Critical Dietary Factors in Cancer Chemoprevention; Springer: Berlin, Germany, 2016; pp. 111–152. [Google Scholar]
- Hsing, A.W.; Chokkalingam, A.P.; Gao, Y.-T.; Madigan, M.P.; Deng, J.; Gridley, G.; Fraumeni, J.F., Jr. Allium vegetables and risk of prostate cancer: A population-based study. J. Natl. Cancer Inst. 2002, 94, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-F.; Ding, Z.-S.; Liu, N.-B. Allium vegetables and risk of prostate cancer: Evidence from 132,192 subjects. Asian Pac. J. Cancer Prev. 2013, 14, 4131–4134. [Google Scholar] [CrossRef] [PubMed]
- Challier, B.; Perarnau, J.-M.; Viel, J.-F. Garlic, onion and cereal fibre as protective factors for breast cancer: A French case–control study. Eur. J. Epidemiol. 1998, 14, 737–747. [Google Scholar] [CrossRef]
- Torres-Sánchez, L.; López--Carrillo, L.; Ló--Cervantes, M.; Rueda-Neria, C.; Wolff, M.S. Food sources of phytoestrogens and breast cancer risk in Mexican women. Nutr. Cancer 2000, 37, 134–139. [Google Scholar] [CrossRef]
- Zou, X.; Liang, J.; Sun, J.; Hu, X.; Lei, L.; Wu, D.; Liu, L. Allicin sensitizes hepatocellular cancer cells to anti-tumor activity of 5-fluorouracil through ROS-mediated mitochondrial pathway. J. Pharm. Sci. 2016, 131, 233–240. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, D. Allicin suppresses the migration and invasion in cervical cancer cells mainly by inhibiting NRF2. Exp. Med. 2018, 1523–1528. [Google Scholar] [CrossRef]
- You, S.; Nakanishi, E.; Kuwata, H.; Chen, J.; Nakasone, Y.; He, X.; He, J.; Liu, X.; Zhang, S.; Zhang, B. Inhibitory effects and molecular mechanisms of garlic organosulfur compounds on the production of inflammatory mediators. Mol. Nutr. Food Res. 2013, 57, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Feng, F.; Xiang, Z.; Ge, L. The effects of allitridin on the expression of transcription factors T-bet and GATA-3 in mice infected by murine cytomegalovirus. J. Med. Food 2005, 8, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, Y.; Peng, T.; Guo, Y.; Chen, F. Phenolic composition and effects on allergic contact dermatitis of phenolic extracts Sapium sebiferum (L.) Roxb. leaves. J. Ethnopharmacol. 2015, 162, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Weng, C.; Jhang, J.; Cheng, Y.; Huang, S.; Yen, G. Diallyl sulfide as a potential dietary agent to reduce TNF-α-and histamine-induced proinflammatory responses in A 7r5 cells. Mol. Nutr. Food Res. 2014, 58, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, F.; Liu, X.; Shu, S.; Huang, Y.; Cheng, H.; Fang, F. Allium sativum-derived allitridin inhibits treg amplification in cytomegalovirus infection. J. Med. Virol. 2013, 85, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.C.; Haar, C.P.; Vandergrift, W.A.; Giglio, P.; Dixon-Mah, Y.N.; Varma, A.K.; Ray, S.K.; Patel, S.J.; Banik, N.L.; Das, A. Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J. Neurooncol. 2013, 114, 43–50. [Google Scholar] [CrossRef]
- Chandra-Kuntal, K.; Lee, J.; Singh, S.V. Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res. Treat. 2013, 138, 69–79. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kaschula, C.H.; Priedigkeit, N.; Lee, A.V.; Singh, S.V. Forkhead box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J. Biol. Chem. 2016, 291, 13495–13508. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, P.; Wang, Y.; Wei, Z.; Tao, L.; Zhu, Z.; Sheng, X.; Wang, S.; Ruan, J.; Liu, Z. Antimetastatic therapies of the polysulfide diallyl trisulfide against triple-negative breast cancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK signaling pathways. PLoS ONE 2015, 10, e0123781. [Google Scholar] [CrossRef]
- Kim, S.-H.; Hahm, E.-R.; Singh, K.B.; Singh, S.V. Diallyl Trisulfide Inhibits Leptin-induced Oncogenic Signaling in Human Breast Cancer Cells but Fails to Prevent Chemically-induced Luminal-type Cancer in Rats. J. Cancer Prev. 2020, 25, 1. [Google Scholar] [CrossRef]
- Kiesel, V.A.; Stan, S.D. Diallyl trisulfide, a chemopreventive agent from Allium vegetables, inhibits alpha-secretases in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 484, 833–838. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Li, L.; Li, Y.; Si, M.; Yin, H. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of osteosarcoma cells by switching on suppressor microRNAs and inactivating of Notch-1 signaling. Carcinogenesis 2013, 34, 1601–1610. [Google Scholar]
- Kalra, N.; Arora, A.; Shukla, Y. Involvement of multiple signaling pathways in diallyl sulfide mediated apoptosis in mouse skin tumors. Asian Pacific J. Cancer Prev. 2006, 7, 556–562. [Google Scholar]
- Shrotriya, S.; Kundu, J.K.; Na, H.-K.; Surh, Y.-J. Diallyl Trisulfide Inhibits Phorbol Ester–Induced Tumor Promotion, Activation of AP-1, and Expression of COX-2 in Mouse Skin by Blocking JNK and Akt Signaling. Cancer Res. 2010, 70, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Yang, J.-H.; Hsieh, S.-C.; Sheen, L.-Y. Allyl sulfides inhibit cell growth of skin cancer cells through induction of DNA damage mediated G2/M arrest and apoptosis. J. Agric. Food Chem. 2010, 58, 7096–7103. [Google Scholar] [CrossRef]
- Colín-González, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Santamaría, A.; Maldonado, P.D. The Antioxidant Mechanisms Underlying the Aged Garlic Extract- and S-Allylcysteine-Induced Protection. Oxid. Med. Cell. Longev. 2012, 2012, 907162. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Chen, K.; Yang, J.; Chen, R.; Wang, T.; Liu, J.; Yang, W.; Ye, Z. S-allylcysteine induces cell cycle arrest and apoptosis in androgen-independent human prostate cancer cells. Mol. Med. Rep. 2012, 5, 439–443. [Google Scholar]
- Ng, K.T.P.; Guo, D.Y.; Cheng, Q.; Geng, W.; Ling, C.C.; Li, C.X.; Liu, X.B.; Ma, Y.Y.; Lo, C.M.; Poon, R.T.P. A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma. PLoS ONE 2012, 7, e31655. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.C.; Chen, Y.; Fu, J.; Cheng, S.; Lin, F. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int. J. Cancer 2009, 125, 181–188. [Google Scholar] [CrossRef]
- Dorant, E.; van den Brandt, P.A.; Goldbohm, R.A. Allium vegetable consumption, garlic supplement intake, and female breast carcinoma incidence. Breast Cancer Res. Treat. 1995, 33, 163–170. [Google Scholar] [CrossRef]
| Glucosinolate | Type of Compound | Health Promoting Roles and Plant Protection | Reference |
|---|---|---|---|
| Sulforaphane | Isothiocyanates | Inhibition of transcription regulator (NF-κB), which is relevant against inflammation and for minimizing diabetes-related complications such as diabetic neuropathy. | [13,53] |
| Mediation of cell cycle arrest and apoptosis; inhibition of the activity of histone deacetylase; and increasing histone acetylation, which leads to the enhancement of protection against carcinogenesis. Induction of cytotoxicity. | [54,55,56] | ||
| Normalization of kidney genome and blood pressure owing to the decrease in oxidative stress in cardiovascular and kidney tissues. | [57] | ||
| Decreasing infarct size, brain edema, and cortical apoptosis, reducing the inflammation and tissue damage of the central nervous system due to the activation of the transcription factor Nrf2, and the upregulation of different target genes. | [58] | ||
| Reduction in the damage induced by high concentrations of substances that mimic the pathomechanism of autism spectrum disorders in mice models. | [59] | ||
| Treatment of Helicobacter pylori. | [60] | ||
| Reduces the risk of skin lesions caused by UV radiation, especially in high-risk patients. | [44] | ||
| Glucoiberin, Sinigrin, and Progoitrin | Suppressing agents, protection of human and animal cells against carcinogenesis owing to the induction of Phase II detoxification enzymes or the inhibition of Phase I enzymes. | [60,61,62,63] | |
| Indole-3-Carbinol | Chemopreventive agent. | [13] | |
| Benzyl Isothiocyanate | Bactericidal and fungicidal properties and has proven effective in combating respiratory and urinary tract infections. | [64] | |
| Chemopreventive agent. | [13] | ||
| Allyl Isothiocyanate, Allyl Thiocyanate, and Allyl Isocyanate | Effective natural insecticides, efficiency in eliminating nematodes or flying insects. Possible mechanisms: the inhibition of the activity of the thiol groups of key enzymes, or the blocking of electron transport and ATP synthesis. | [65] |
| Organosulfur Compound | Type of Compound | Health Promoting Role | Reference |
|---|---|---|---|
| Alliin (S-Allylcysteine Sulfoxide) | Natural bioactive constituent, with general formula C6H11NO3S. | It modulates the generation of proinflammatory cytokines by increasing the expression of cytokine genes like IL-6, MCP-1, and EGR-1. It also shows strong antioxidant and radical-scavenging properties. Alliin has also been found to boost the immune response in blood. | [4,31] |
| Allicin | Thiosulfinate and also the precursor of various sulfur-containing compounds, with the general formula C6H10OS2. | Allicin exhibits anti-cancer, anti-bacterial, anti-fungal, and anti-tumor activities. Allicin can inhibit the proliferation of tumor cells and can induce apoptosis in gastric cells by activating both the intrinsic and extrinsic pathways. Allicin shows anti-bacterial effects against a wide range of Gram-negative and Gram-positive bacteria (Staphylococcus, Escherichia, Klebsiella, Salmonella, Bacillus, Streptococcus, Proteus, and Clostridium). Moreover, allicin can also stimulate cytokine release, enhance immune resistance, and has anti-parasitic activity against several parasites. | [61,62,63] |
| Sulfenic Acid | First member of the family of organosulfur oxoacids, with the general formula RSOH. | Upon the chopping, damage, chewing, or crushing of Allium plants, the enzyme alliinase catalyzes the decomposition of alliin into short-lived and unstable sulfenic acid. It is thought to be responsible for antioxidant activities. | [32] |
| Diallyl Sulfide (DAS) | Derived bioactive compound which is a lipophilic thioether, with the general formula C6H10S. | Diallyl sulfide can boost the detoxification functions of liver cells, preventing symptoms of inflammation. It significantly enhances the production of the enzyme glutathione S-transferase (GST), which binds the electrophilic toxins inside the cell. DAS can inhibit the activation of nicotine-derived nitrosamine ketone (NNK), which is related to carcinogenesis. The preventive treatment with DAS also decreases the acetaminophen-induced hepatotoxicity and nephrotoxicity, indicating that it can decrease liver damage induced by drugs. It is also found to be effective against cardiovascular disease and colon cancer. | [35,36] |
| Diallyl Disulfide (DADS) | Derived organosulfur compound with the general formula C6H10S2. | Diallyl disulfide has multitargeted anti-carcinogenic activities, by (1) promoting carcinogen metabolism, (2) retarding the progression of the cell cycle, (3) inhibiting the proliferation of cells and inducing apoptosis. Moreover, diallyl disulfide also inhibit histone deacetylase activities, which have a therapeutic effect to stop cancer, since it can modulate histone hyper-acetylation and can reactivate tumor suppressor genes involved in cancer progression. Besides these benefits, it can induce allergens in Allium plants. | [35,36] |
| Allitridin or Diallyl tri-Sulfide (DATS) | Derived organosulfur compound with the formula C6H10S3. | Diallyl trisulfide have several health-promoting benefits, including having anti-cancer properties, being an antiviral immune booster, causing an increase in the reactive oxygen species (ROS) level, causing platelet aggregation, causing a decrease in blood pressure, causing cholesterol reduction, and being helpful in the treatment of cardiac arrhythmias. It has been found to selectively kill the cancerous cells in the breast and prostate, leaving the healthy cells unharmed. | [39,41,121,122,123,124,125] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. https://doi.org/10.3390/molecules25173804
Miękus N, Marszałek K, Podlacha M, Iqbal A, Puchalski C, Świergiel AH. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules. 2020; 25(17):3804. https://doi.org/10.3390/molecules25173804
Chicago/Turabian StyleMiękus, Natalia, Krystian Marszałek, Magdalena Podlacha, Aamir Iqbal, Czesław Puchalski, and Artur H. Świergiel. 2020. "Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds" Molecules 25, no. 17: 3804. https://doi.org/10.3390/molecules25173804
APA StyleMiękus, N., Marszałek, K., Podlacha, M., Iqbal, A., Puchalski, C., & Świergiel, A. H. (2020). Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules, 25(17), 3804. https://doi.org/10.3390/molecules25173804








