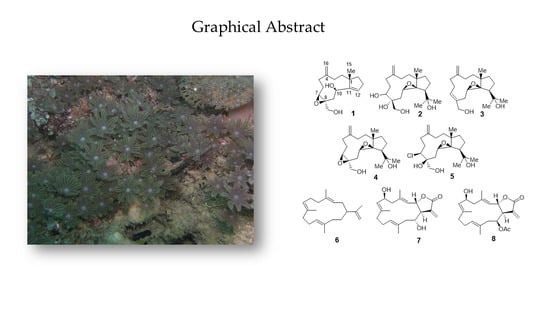
Sangiangols A and B, Two New Dolabellanes from an Indonesian Marine Soft Coral, Anthelia sp.
Abstract
1. Introduction
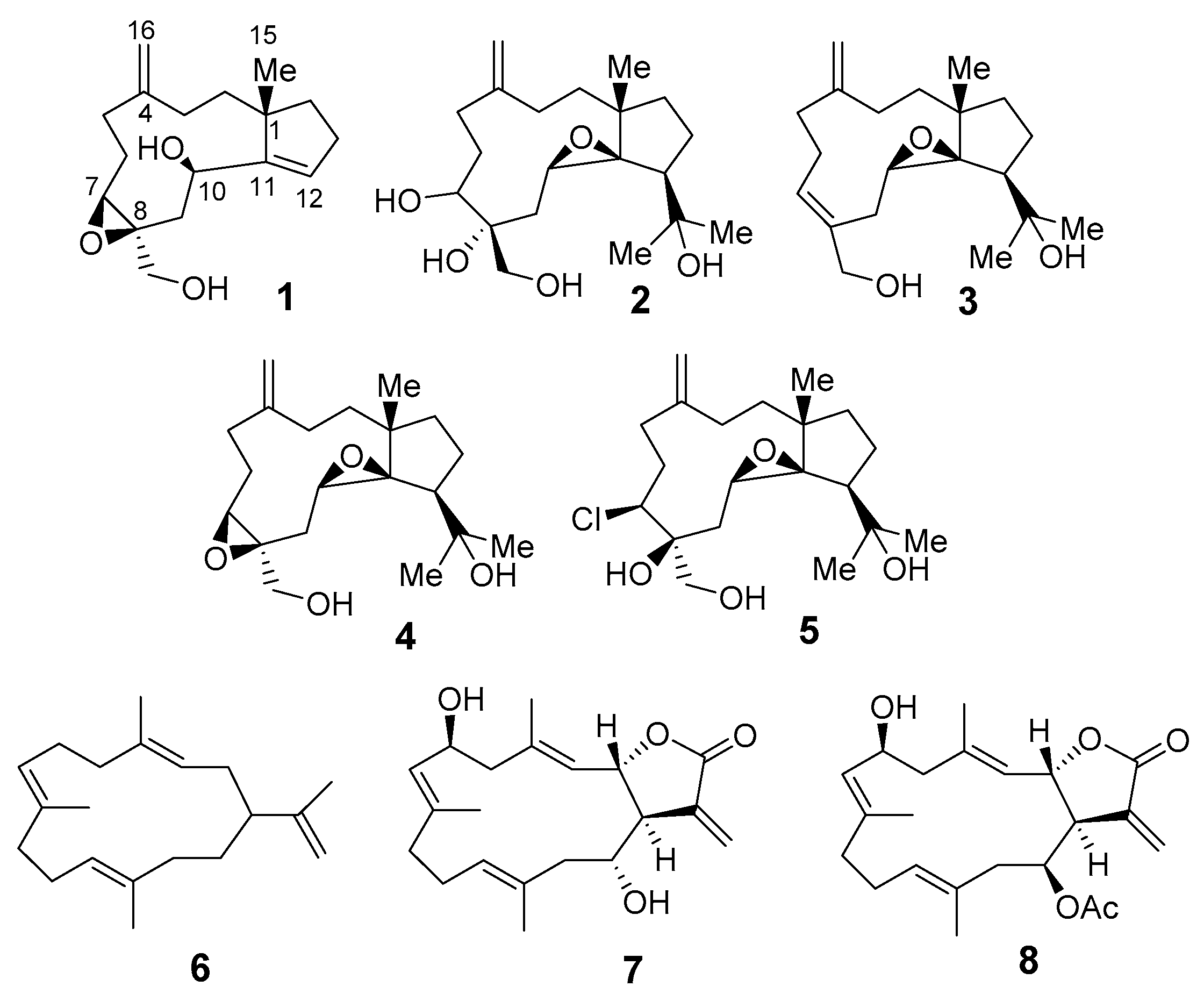
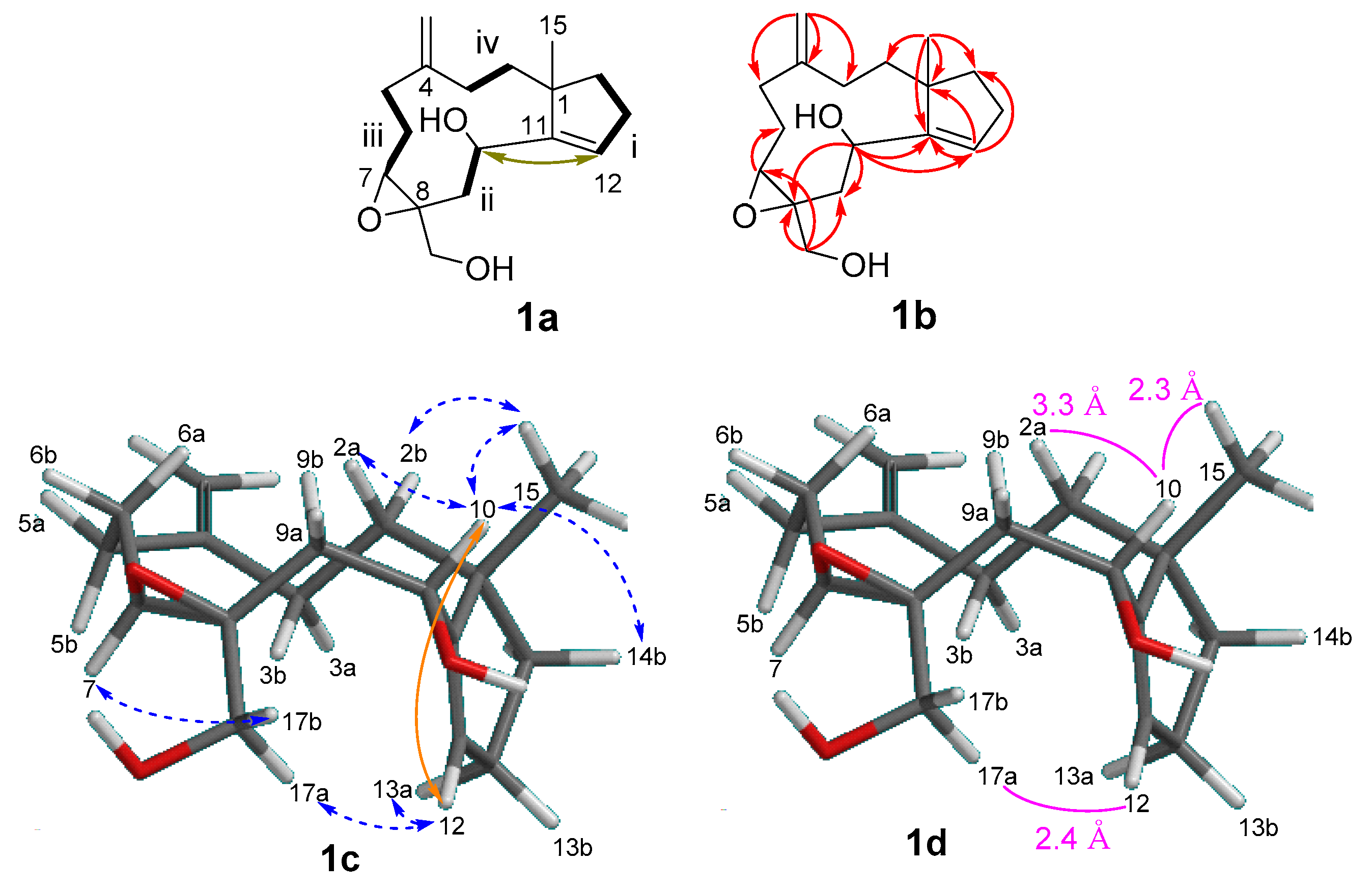
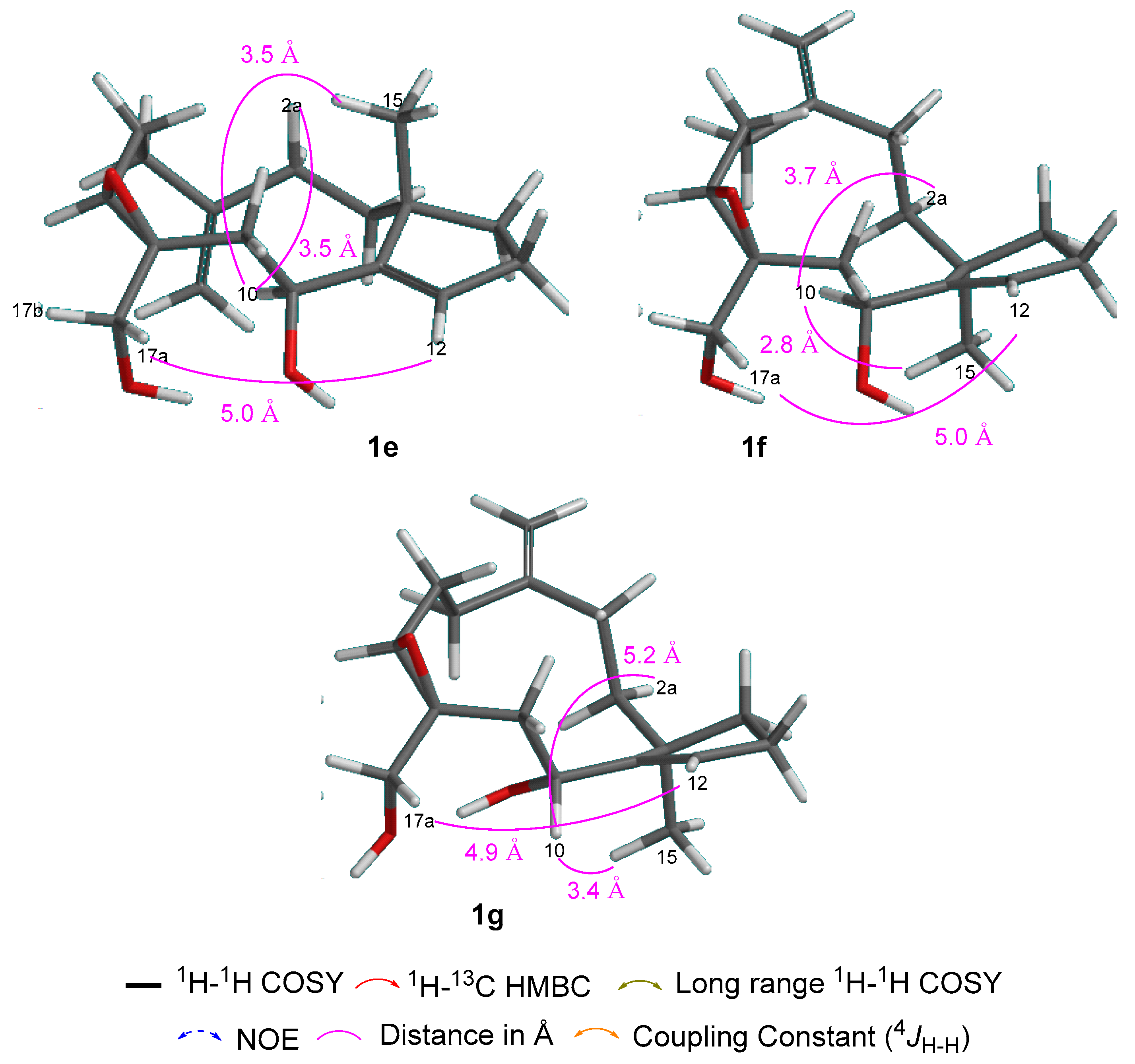
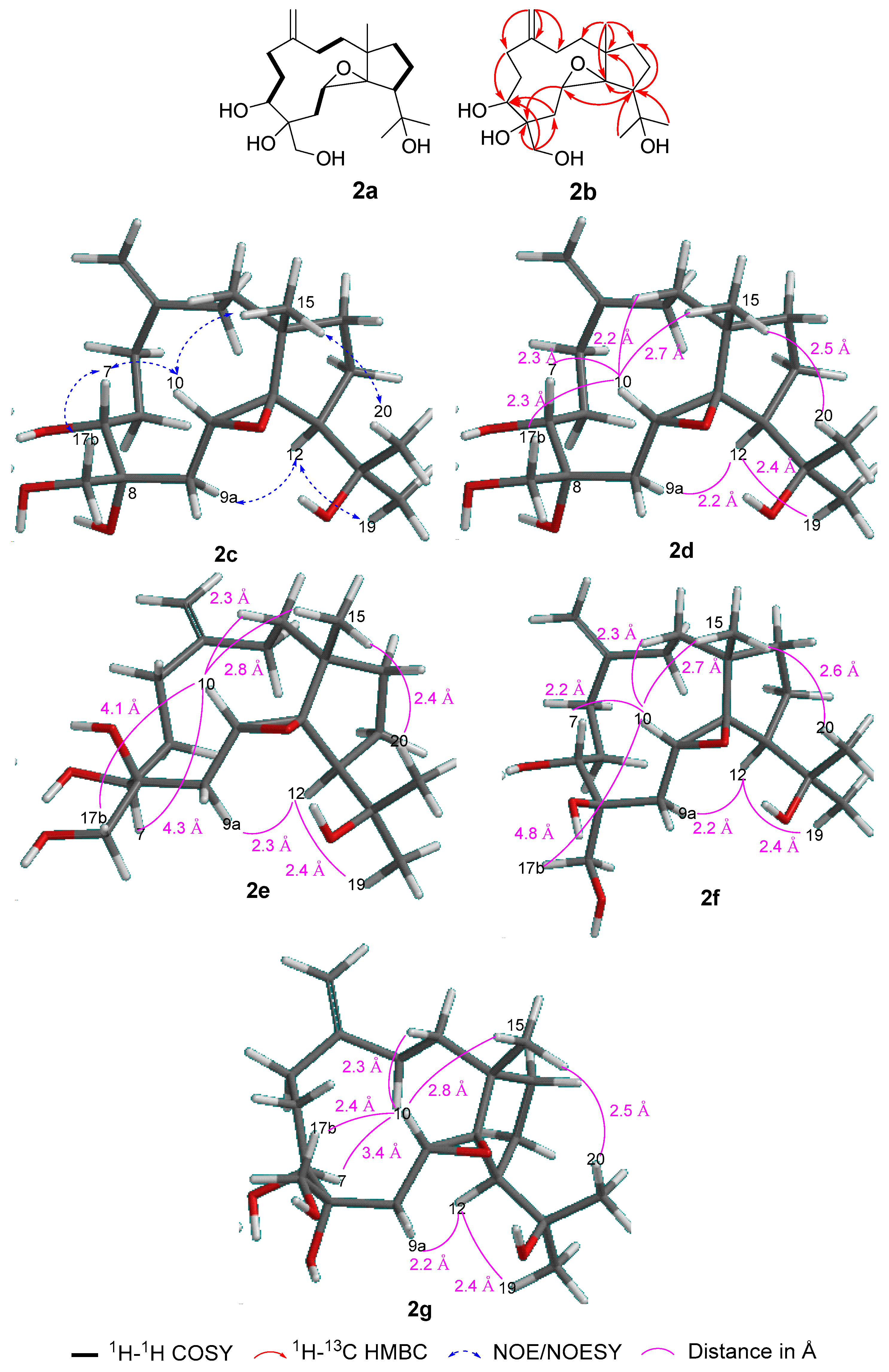
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Animal Material
3.3. Extraction and Isolation
3.3.1. Sangiangol A (1)
3.3.2. Sangiangol B (2)
3.4. Cytotoxicity Assay
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Hanif, N.; Murni, A.; Tanaka, C.; Tanaka, J. Marine natural products from Indonesian waters. Mar. Drugs 2019, 17, 364. [Google Scholar] [CrossRef] [PubMed]
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Hanif, N.; Murni, A.; Yamauchi, M.; Higashi, M.; Tanaka, J. A new trinor-guaine sesquiterpene from and Indonesian soft coral Anthelia sp. Nat. Prod. Commun. 2015, 10, 1907–1910. [Google Scholar] [PubMed]
- Coval, S.J.; Scheuer, P.J.; Matsumoto, G.K.; Clardy, J. Two new xenicin diterpenoid from the octocoral Anthelia edmonsoni. Tetrahedron 1984, 40, 3823–3828. [Google Scholar] [CrossRef]
- Murni, A.; Hanif, N.; Tanaka, J. A new cytotoxic dolabellane from the Indonesian soft coral Anthelia sp. Indones. J. Chem. 2013, 13, 216–220. [Google Scholar] [CrossRef][Green Version]
- Green, D.; Carmely, S.; Benayahu, Y.; Kashman, Y. Antheliolide A & B: Two new C24-acetoacetylated diterpenoids of the soft coral Anthelia glauca. Tetrahedron Lett. 1988, 29, 1605–1608. [Google Scholar]
- Smith, A.B.; Carrol, P.J.; Kashman, Y.; Green, D. Revised structure of antheliolides A and B. Tetrahedron Lett. 1989, 30, 3363–3364. [Google Scholar] [CrossRef]
- Sjsstrand, U.; Bohlin, L.; Fisher, L.; Colin, M.; Djerassi, C. Minor and trace sterol from marine invertebrate 28: Novel polyhydroxylated sterol from soft coral Anthelia glauca. Steroids 1981, 38, 347–354. [Google Scholar] [CrossRef]
- Mason, J.W.; Schmid, C.L.; Bohn, L.M.; Roush, W.R. Stolonidiol: Synthesis, target identification, and mechanism for choline acetyltransferase activation. J. Am. Chem. Soc. 2017, 139, 5865–5869. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Iguchi, K.; Yamada, N.; Yamada, Y.; Inouye, Y. Stolonidiol, a new marine diterpenoid with a strong cytotoxic activity from the Japanese soft coral. Tetrahedron Lett. 1987, 28, 5673–5676. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, Y.; Ko, C.; Kuo, Y.; Chen, C. New dolabellanes from the Taiwanese soft coral Clavularia inflate. J. Chin. Chem. Soc. 2003, 50, 471–476. [Google Scholar] [CrossRef]
- Ireland, C.; Faulkner, D.J.; Finer, J.; Clardy, J. A novel diterpene from Dollabella californica. J. Am. Chem. Soc. 1976, 98, 4664–4665. [Google Scholar] [CrossRef] [PubMed]
- Vanderah, D.J.; Rutledge, N.; Schmitz, F.J.; Ciereszko, L.S. Marine natural products: Cembrene-A and cembrene-C from a soft coral, Nephthea species. J. Org. Chem. 1978, 43, 1614–1616. [Google Scholar] [CrossRef]
- Kobayashi, M.; Son, B.W.; Kyogoku, Y.; Kitagawa, I. Kericembrenolides A, B, C, D, and E, five new cytotoxic cembrenolides from the Okinawan soft coral Clavularia koellikeri. Chem. Pharm. Bull. 1986, 34, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, M.; Matsumoto, Y.; Takahashi, H.; Iguchi, K. New marine cembrane-type diterpenoids from the Okinawan soft coral Clavularia koellikeri. J. Nat. Prod. 2000, 63, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Position | Sangiangol A (1) | Sangiangol B (2) | ||||||
|---|---|---|---|---|---|---|---|---|
| δC * | mult. | δH ** | J in Hz | δC * | mult. | δH ** | J in Hz | |
| 1 | 50.2 | C | 44.7 | C | ||||
| 2a | 38.4 | CH2 | 1.63 | m *** | 42.8 | CH2 | 1.97 | m |
| 2b | 1.50 | ddd (14.4, 11.6, 7.2) | 1.25 | m *** | ||||
| 3a | 29.6 | CH2 | 2.10 | m *** | 25.0 | CH2 | 2.09 | dt (15.4, 10.0) |
| 3b | 1.73 | m *** | 1.64 | dd (13.3, 10.0) | ||||
| 4 | 150.1 | C | 149.3 | C | ||||
| 5a | 30.8 | CH2 | 2.42 | dt (14.6, 5.4) | 34.3 | CH2 | 2.45 | td (13.6, 4.6) |
| 5b | 2.22 | dd (14.6, 7.7) | 2.29 | brdd (13.6, 4.6) | ||||
| 6a | 24.4 | CH2 | 1.73 | m *** | 27.4 | CH2 | 1.79 | m |
| 6b | 1.73 | m *** | 1.50 | m | ||||
| 7 | 61.3 | CH | 3.06 | t (6.5) | 72.9 | CH | 3.56 | d (11.4) |
| 8 | 63.7 | C | 75.3 | C | ||||
| 9a | 36.0 | CH2 | 2.20 | dd (15.7, 3.0) | 33.6 | CH2 | 1.95 | dd (4.5, 2.7) |
| 9b | 2.00 | dd (15.7, 6.3) | 1.92 | dd (5.5, 1.6) | ||||
| 10 | 65.1 | CH | 4.40 | dd (6.3, 1.3) | 54.5 | CH | 3.02 | dd (5.4, 2.7) |
| 11 | 153.0 | C | 76.7 | C | ||||
| 12 | 127.0 | CH | 5.88 | brt (2.5) | 50.2 | CH | 2.18 | d (10.8, 2.0) |
| 13a | 29.7 | CH2 | 2.31 | m *** | 27.8 | CH2 | 1.89 | m |
| 13b | 2.10 | m *** | 1.60 | m | ||||
| 14a | 36.7 | CH2 | 1.84 | ddd (12.7, 9.0, 7.1) | 39.0 | CH2 | 1.79 | m |
| 14b | 1.63 | m *** | 1.74 | m | ||||
| 15 | 27.4 | CH3 | 1.09 | s | 24.0 | CH3 | 0.84 | s |
| 16a | 111.4 | CH2 | 4.76 | s | 112.7 | CH2 | 4.96 | s |
| 16b | 4.70 | s | 4.79 | s | ||||
| 17a | 67.2 | CH2 | 4.07 | d (12.2) | 67.2 | CH2 | 3.89 | d (11.4) |
| 17b | 3.38 | d (12.2) | 3.52 | d (11.4) | ||||
| 18 | 75.2 | C | ||||||
| 19 | 29.6 | CH3 | 1.21 | s | ||||
| 20 | 26.0 | CH3 | 1.26 | s | ||||
| Compound | Concentration (µg/mL) |
|---|---|
| 1 | 5 |
| 2 | 10 |
| 3 | 10 |
| 4 | 1 |
| 5 | 0.5 |
| 6 | 10 |
| 7 | 1 |
| 8 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, N.; Murni, A.; Tanaka, J. Sangiangols A and B, Two New Dolabellanes from an Indonesian Marine Soft Coral, Anthelia sp. Molecules 2020, 25, 3803. https://doi.org/10.3390/molecules25173803
Hanif N, Murni A, Tanaka J. Sangiangols A and B, Two New Dolabellanes from an Indonesian Marine Soft Coral, Anthelia sp. Molecules. 2020; 25(17):3803. https://doi.org/10.3390/molecules25173803
Chicago/Turabian StyleHanif, Novriyandi, Anggia Murni, and Junichi Tanaka. 2020. "Sangiangols A and B, Two New Dolabellanes from an Indonesian Marine Soft Coral, Anthelia sp." Molecules 25, no. 17: 3803. https://doi.org/10.3390/molecules25173803
APA StyleHanif, N., Murni, A., & Tanaka, J. (2020). Sangiangols A and B, Two New Dolabellanes from an Indonesian Marine Soft Coral, Anthelia sp. Molecules, 25(17), 3803. https://doi.org/10.3390/molecules25173803