Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review
Abstract
1. Introduction
2. Physicochemical Composition and Microbial Diversity of Apple Fruits
2.1. Fresh Apple Composition
2.1.1. Sugars
2.1.2. Organic Acids
2.1.3. Phenolic Compounds
Phenolic Acids
Flavonoids
Flavan-3-ols
Flavonols
Dihydrochalcone
2.1.4. Lipids
2.1.5. Vitamins
2.1.6. Minerals
2.2. Microbial Ecology of Apple Fruit and Cider
3. Cider-Making Process
4. Impact of Apple Juice Composition and Microbial Diversity on Alcoholic Fermentation in the Cider Production Process
4.1. Impact of Apple Juice Composition on Fermentation
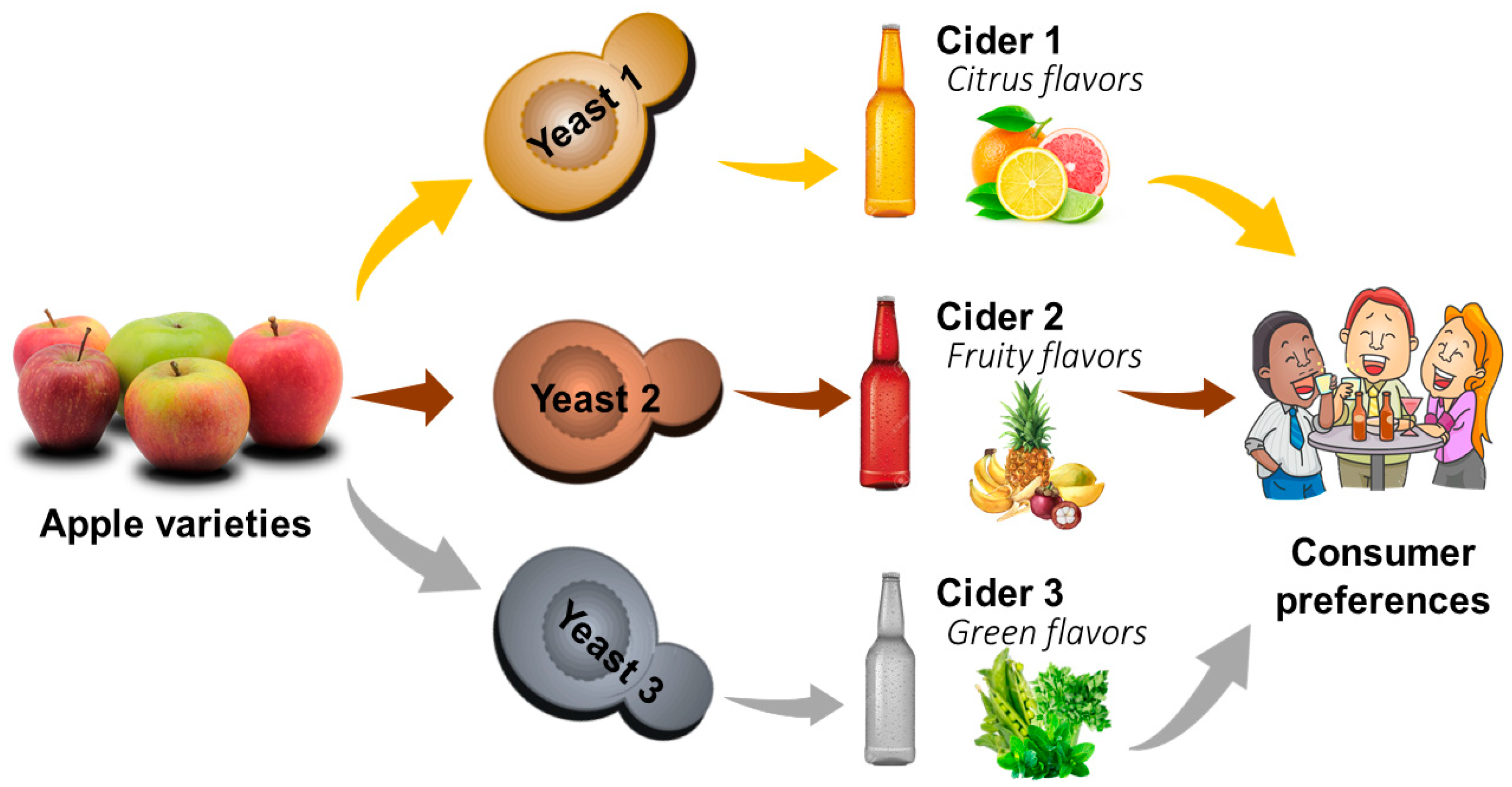
4.2. Impact of Yeast on Fermentation
5. Conclusions
Funding
Conflicts of Interest
References
- Ferree, D.C.; Warrington, I.J. Apples Botany, Production and Uses; CABI Publishing: Oxfordshire, UK, 2015; Volume 1, ISBN 9788578110796. [Google Scholar]
- United States Department of Agriculture. Fresh Apples Fresh Domestic Consumption by Country in MT; U.S. Department of Agriculture: Washington, DC, USA, 2019.
- Centro Nacional De Alimentación. Tablas Peruanas de Composición de Alimentos; Centro Nacional De Alimentación: Madrid, Spain, 2009; ISBN 9789972857737. [Google Scholar]
- Hansen, P. The Effect of cropping on the growth and uptake of nutrients by apple trees at different levels of nitrogen, potassium, magnesium and phosphorus. Acta Agric. Scand. 1973, 23, 87–92. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Zhang, L.-S.; Li, B.-Z.; HAN, M.-Y. Mineral nutrition elements and their roles in growth and development of apple trees in arid areas. J. Northwest For. Univ. 1997, 22, 111–115. [Google Scholar]
- Perring, M.A.; Holland, D.A. The effect of orchard factors on the chemical composition of apples. V. Year-to-year variations in the effects of NPK fertilizers and sward treatment on fruit composition. J. Hortic. Sci. 1985, 60, 37–46. [Google Scholar] [CrossRef]
- Fallahi, E.; Conway, W.S.; Hickey, K.D.; Sams, C.E. The role of calcium and nitrogen in postharvest quality and disease resistance of apples. HortScience 1997, 32, 831–835. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W. Food Chemistry Berlin Allemagne. Springer: Berlin, Germany, 2009; ISBN 9783540699330. [Google Scholar]
- Renard, C.M.G.C.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Valois, S.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization of fermented cider apple cultivars grown in upstate New York. J. Am. Pomol. Soc. 2006, 60, 113–128. [Google Scholar]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Richard-Forget, F.C.; Goupy, P.M. Enzymatic browning reactions in apple and apple products. Crit. Rev. Food Sci. Nutr. 1994, 34, 109–157. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Phenolics of apple, pear, and white grape juices and their changes with processing and storage. A review. J. Agric. Food Chem. 1992, 40, 1478–1487. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- de Rijke, E.; Out, P.; Niessen, W.M.A.; Ariese, F.; Gooijer, C.; Brinkman, U.A.T. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. golden delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.-F. Reversed-Phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a french cider apple variety (Malus domestica Var. Kermerrien). J. Agric. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Jawad, M.; Schoop, R.; Suter, A.; Klein, P.; Eccles, R. Perfil de eficacia y seguridad de Echinacea purpurea en la prevención de episodios de resfriado común: Estudio clínico aleatorizado, doble ciego y controlado con placebo. Rev. Fitoter. 2013, 13, 125–135. [Google Scholar]
- Podsedek, A.; Wilska-Jeszka, J.; Anders, B.; Markowski, J. Compositional characterisation of some apple varieties. Eur. Food Res. Technol. 2000, 210, 268–272. [Google Scholar] [CrossRef]
- Cabranes, C.; Moreno, J.; Mangas, J.J. Dynamics of yeast populations during cider fermentation in the Asturian Region of Spain. Appl. Environ. Microbiol. 1990, 56, 3881–3884. [Google Scholar] [CrossRef]
- Campo, G.; Santos, J.I.; Berregi, I.; Velasco, S.; Ibarburu, I.; Dueñas, M.T.; Irastorza, A.; Brew, J.I. Ciders produced by two types of presses and fermented in stainless steel and wooden vats. J. Inst. Brew. 2003, 109, 342–348. [Google Scholar] [CrossRef]
- Valles, B.S.; Pando Bedriñana, R.; Tascón, N.F.; Simón, A.Q.; Madrera, R.R. Yeast species associated with the spontaneous fermentation of cider. Food Microbiol. 2007, 24, 25–31. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Magan, N.; Viñas, I. Microbial population dynamics on golden delicious apples from bud to harvest and effect of fungicide applications. Ann. Appl. Biol. 1999, 134, 109–116. [Google Scholar] [CrossRef]
- Abadias, M.; Cañamás, T.P.; Asensio, A.; Anguera, M.; Viñas, I. Microbial quality of commercial ‘Golden Delicious’ apples throughout production and shelf-life in Lleida (Catalonia, Spain). Int. J. Food Microbiol. 2006, 108, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cruz, S.; Alvarez-Parrilla, E.; de la Rosa, L.; Martinez-Gonzalez, A.I.; Ornelas-Paz, J.D.J.; Mendoza-Wilson, A.M.; Gonzalez-Aguilar, G.A.; Obregon, C. Effect of different sanitizers on microbial, sensory and nutritional quality of fresh-cut jalapeno peppers. Am. J. Agric. Biol. Sci. 2010, 5, 331–341. [Google Scholar] [CrossRef]
- Tournas, V.H.; Heeres, J.; Burgess, L. Moulds and yeasts in fruit salads and fruit juices. Food Microbiol. 2006, 23, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Graça, A.; Santo, D.; Esteves, E.; Nunes, C.; Abadias, M. Evaluation of microbial quality and yeast diversity in fresh-cut apple. Food Microbiol. 2015, 51, 179–185. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef]
- Beech, F. Cider making and cider research: A review. J. Inst. Brew. 1972, 78, 477–491. [Google Scholar] [CrossRef]
- Coton, E.; Coton, M.; Levert, D.; Casaregola, S.; Sohier, D. Yeast ecology in French cider and black olive natural fermentations. Int. J. Food Microbiol. 2006, 108, 130–135. [Google Scholar] [CrossRef]
- Morrissey, W.F.; Davenport, B.; Querol, A.; Dobson, A.D.W. The role of indigenous yeasts in traditional Irish cider fermentations. J. Appl. Microbiol. 2004, 97, 647–655. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R.; Maifreni, M. Glycerol and other fermentation products of apiculate wine yeasts. J. Appl. Microbiol. 1997, 82, 615–618. [Google Scholar] [CrossRef]
- Terpou, A.; Dimopoulou, M.; Belka, A.; Kallithraka, S.; Nychas, G.E.; Papanikolaou, S. Effect of myclobutanil pesticide on the physiological behavior of two newly isolated Saccharomyces cerevisiae strains during very-high-gravity alcoholic fermentation. Microorganisms 2019, 7, 666. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Zapparoli, G.; Azzolini, M.; Carvalho, C.; Sampaio, J.P. Sporobolomyces agrorum sp. nov. and Sporobolomyces sucorum sp. nov., two novel basidiomycetous yeast species isolated from grape and apple must in Italy. Int. J. Syst. Evol. Microbiol. 2019, 69, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.R.; Santamaría, P.; Epifanio, S.; Garijo, P.; Ópez, R.L. Ecology of spontaneous fermentation in one winery during 5 consecutive years. Lett. Appl. Microbiol. 1999, 29, 411–415. [Google Scholar] [CrossRef]
- Suárez Valles, B.; Pando Bedriñana, R.; González García, A.; Querol Simón, A. A molecular genetic study of natural strains of Saccharomyces isolated from Asturian cider fermentations. J. Appl. Microbiol. 2007, 103, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Suitability of the Lebanese “Ace Spur” apple variety for cider production using Hanseniaspora sp. yeast. Fermentation 2020, 6, 32. [Google Scholar] [CrossRef]
- Carr, J.G.; Davies, P.A. Homofermentative Lactobacilli of ciders including Lactobacillus mali nov. spec. J. Appl. Bacteriol. 1970, 33, 768–774. [Google Scholar] [CrossRef]
- Marshall, C.R.; Walkley, V.T. Some aspects of microbiology applied to commercial apple juice production. I. Distribution of microorganisms on the fruit. J. Food Sci. 1951, 16, 448–456. [Google Scholar] [CrossRef]
- Weiss, N.; Schillinger, U.; Kandler, O. Lactobacillus lactis, Lactobacillus leichmannii and Lactobacillus bulgaricus, subjective synonyms of Lactobacillus delbrueckii, and description of Lactobacillus delbrueckii subsp. lactis comb. nov. and Lactobacillus delbrueckii subsp. bulgaricus comb. nov. Syst. Appl. Microbiol. 1983, 4, 552–557. [Google Scholar] [CrossRef]
- Laplace, J.M.; Jacquet, A.; Travers, I.; Simon, J.P.; Auffray, Y. Incidence of land and physicochemical composition of apples on the qualitative and quantitative development of microbial flora during cider fermentations. J. Inst. Brew. 2001, 107, 227–234. [Google Scholar] [CrossRef]
- Carr, J.G.; Davies, P.A. The ecology and classification of strains of Lactobacillus collinoides nov. spec.: A bacterium commonly found in fermenting apple juice. J. Appl. Bacteriol. 1972, 35, 463–471. [Google Scholar] [CrossRef]
- Garai, G.; Duenas, M.T.; Irastorza, A.; Moreno-Arribas, M.V. Biogenic amine production by lactic acid bacteria isolated from cider. Lett. Appl. Microbiol. 2007, 45, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Sauvageot, N.; Gouffi, K.; Laplace, J.M.; Auffray, Y. Glycerol metabolism in Lactobacillus collinoides: Production of 3-hydroxypropionaldehyde, a precursor of acrolein. Int. J. Food Microbiol. 2000, 55, 167–170. [Google Scholar] [CrossRef]
- Duenas, M.; Irastorza, A.; Fernandez, K.; Bilbao, A. Heterofermentative Lactobacilli causing ropiness in basque country ciders. J. Food Prot. 1995, 58, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Claisse, O.; Lonvaud-funel, A. Détection de bactéries lactiques produisant du 3-hydroxypropionaldéhyde (précurseur d’acroléine) à partir du glycérol par tests moléculaires. Lait 2001, 81, 173–181. [Google Scholar] [CrossRef][Green Version]
- Whiting, G.C.; Carr, J.G. Chlorogenic acid metabolism in cider fermentation. Nature 1957, 180, 1479. [Google Scholar] [CrossRef]
- Dellaglio, F.; Torriani, S.; Felis, G.E. Reclassification of Lactobacillus cellobiosus Rogosa et al. 1953 as a later synonym of Lactobacillus fermentum Beijerinck 1901. Int. J. Syst. Evol. Microbiol. 2004, 54, 809–812. [Google Scholar] [CrossRef]
- Marieta, C.; Ibarburu, I.; Duenas, M.; Irastorza, A. Supramolecular structure and conformation of a (1-->3)(1-->2)-beta-D-glucan from Lactobacillus suebicus CUPV221 as observed by tapping mode atomic force microscopy. J. Agric. Food Chem. 2009, 57, 6183–6188. [Google Scholar] [CrossRef]
- Kleynmans, U.; Heinzl, H.; Hammes, W.P. Lactobacillus suebicus sp. nov., an obligately heterofermentative lactobacillus species isolated from fruit mashes. Syst. Appl. Microbiol. 1989, 11, 267–271. [Google Scholar] [CrossRef]
- Salih, A.G.; Drilleau, J.F.; Cavin, F.F.; Divies, C.; Bourgeois, C.M. A survey of microbiological aspects of cider making. J. Inst. Brew. 1988, 94, 5–8. [Google Scholar] [CrossRef]
- Fleet, G.H. Growth of yeasts during wine fermentations. J. Wine Res. 1990, 1, 211–223. [Google Scholar] [CrossRef]
- Sánchez, A.; Rodríguez, R.; Coton, M.; Coton, E.; Herrero, M.; García, L.A.; Díaz, M. Population dynamics of lactic acid bacteria during spontaneous malolactic fermentation in industrial cider. Food Res. Int. 2010, 43, 2101–2107. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Maroun, R.G.; Louka, N.; Vorobiev, E. Ultrasound-assisted fermentation for cider production from Lebanese apples. Ultrason. Sonochem. 2020, 63, 104952. [Google Scholar] [CrossRef] [PubMed]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Vorobiev, E.; Maroun, R.G.; Louka, N. Control of the sugar/ethanol conversion rate during moderate pulsed electric field-assisted fermentation of a Hanseniaspora sp. strain to produce low-alcohol cider. Innov. Food Sci. Emerg. Technol. 2020, 59, 102258. [Google Scholar] [CrossRef]
- AL Daccache, M.; Salameh, D.; Chamy, L.E.L.; Koubaa, M.; Maroun, R.G.; Vorobiev, E.; Louka, N. Evaluation of the fermentative capacity of an indigenous Hanseniaspora sp. strain isolated from Lebanese apples for cider production. FEMS Microbiol. Lett. 2020, 367, fnaa093. [Google Scholar] [CrossRef]
- Andrew, L.G.H.; Piggott, J.R. Cidermaking. In Fermented Beverage Production; Kluver Academic/Plenum: New York, NY, USA, 2003; ISBN 9780306477065. [Google Scholar]
- Alonso-Salces, R.M.; Guyot, S.; Herrero, C.; Berrueta, L.A.; Drilleau, J.F.; Gallo, B.; Vicente, F. Chemometric characterisation of Basque and French ciders according to their polyphenolic profiles. Anal. Bioanal. Chem. 2004, 379, 464–475. [Google Scholar] [CrossRef]
- Mangas, J.J.; Rodríguez, R.; Suárez, B.; Picinelli, A.; Dapena, E. Study of the phenolic profile of cider apple cultivars at maturity by multivariate techniques. J. Agric. Food Chem. 1999, 47, 4046–4052. [Google Scholar] [CrossRef]
- Nogueira, A.; Guyot, S.; Marnet, N.; Lequéré, J.M.; Drilleau, J.F.; Wosiacki, G. Effect of alcoholic fermentation in the content of phenolic compounds in cider processing. Braz. Arch. Biol. Technol. 2008, 51, 1025–1032. [Google Scholar] [CrossRef]
- Lata, B. Relationship between apple peel and the whole fruit antioxidant content: Year and cultivar variation. J. Agric. Food Chem. 2007, 55, 663–671. [Google Scholar] [CrossRef]
- van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef]
- Symoneaux, R.; Baron, A.; Marnet, N.; Bauduin, R.; Chollet, S. Impact of apple procyanidins on sensory perception in model cider (part 1): Polymerisation degree and concentration. LWT Food Sci. Technol. 2014, 57, 22–27. [Google Scholar] [CrossRef]
- Park, J. Characterizing and Improving the Oral Sensations and Preference of Polyphenol-Rich Aronia Berry Juice; Honors Scholar Theses.348; University of Connecticut: Storrs, CT, USA, 2014. [Google Scholar]
- Arroyo-López, F.N.; Orlić, S.; Querol, A.; Barrio, E. Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. Int. J. Food Microbiol. 2009, 131, 120–127. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, D.; Corbo, M.R.; Del Nobile, M.A.; Sinigaglia, M. Effects of temperature, ammonium and glucose concentrations on yeast growth in a model wine system. Int. J. Food Sci. Technol. 2006, 41, 1152–1157. [Google Scholar] [CrossRef]
- Boudreau, T.F.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free amino nitrogen concentration correlates to total yeast assimilable nitrogen concentration in apple juice. Food Sci. Nutr. 2018, 6, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Rosend, J.; Kuldjarv, R.; Rosenvald, S.; Paalme, T. The effects of apple variety, ripening stage, and yeast strain on the volatile composition of apple cider. Heliyon 2019, 5, e01953. [Google Scholar] [CrossRef]
- Lawless, H.T.; Horne, J.; Giasi, P. Astringency of organic acids is related to pH. Chem. Senses 1996, 21, 397–403. [Google Scholar] [CrossRef]
- Whiting, G.C. Organic acid metabolism of yeasts during fermentation of alcoholic beverages—A review. J. Inst. Brew. 1976, 82, 84–92. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, F.; Ji, B.; Nout, R.M.J.; Fang, Q.; Yang, Z. Determination of organic acids evolution during apple cider fermentation using an improved HPLC analysis method. Eur. Food Res. Technol. 2008, 227, 1183–1190. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Castilleja, D.E.M.; Aldrete Tapia, J.A.; Arvizu Medrano, S.M.; Hernández Iturriaga, M.; Muñoz, L.S.; Martinez Peniche, R.Á. Growth kinetics for the selection of yeast strains for fermented beverages. In Yeast—Industrial Applications Conversion; InTech: London, UK, 2017; pp. 67–87. [Google Scholar]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. Fems Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Barnett, J.A. A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850–1880. Yeast 2000, 16, 755–771. [Google Scholar] [CrossRef]
- Ubeda, J.; Briones, A. Characterization of differences in the formation of volatiles during fermentation within synthetic and grape musts by wild Saccharomyces strains. LWT Food Sci. Technol. 2000, 33, 408–414. [Google Scholar] [CrossRef]
- Dubourdieu, D.; Tominaga, T.; Masneuf, I.; Peyrot des Gachons, C.; Murat, M.L. The role of yeast in grape flavour development during fermentation: The example of Sauvignon blanc. Am. J. Enol. Vitic. 2006, 57, 81–88. [Google Scholar]
- Ugliano, M.; Bartowsky, E.J.; McCarthy, J.; Moio, L.; Henschke, P.A. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. J. Agric. Food Chem. 2006, 54, 6322–6331. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I. The Genetic Analysis and Tailoring of Wine Yeasts. In Functional Genetics of Industrial Yeasts; de Winde, J.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 99–142. [Google Scholar]
- Pretorius, I.S.; van der Westhuizen, T.J.; Augustyn, O.P.H. Yeast biodiversity in vineyards and wineries and its importance to the South African wine industry. South Afr. J. Enol. Vitic. 1999, 20, 61–75. [Google Scholar] [CrossRef]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef]
- Fleet, G.H.; Lafon-Lafourcade, S.; Ribereau-Gayon, P. Evolution of yeasts and lactic acid bacteria during fermentation and storage of Bordeaux wines. Appl. Environ. Microbiol. 1984, 48, 1034–1038. [Google Scholar] [CrossRef]
- Martínez, J.; Millán, C.; Ortega, J.M. Growth of natural flora during fermentation of inoculated musts from “Pedro Ximenez” grapes. South Afr. J. Enol. Vitic. 1989, 10, 31–35. [Google Scholar]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 1–20. [Google Scholar] [CrossRef]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1998, 14, 199–203. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romanib, C.; Lencioni, L.; Mannazzud, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolina, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Suzzi, G. Higher alcohol and acetoin production by Zygosaccharomyces wine yeasts. J. Appl. Bacteriol. 1993, 75, 541–545. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Moreira, N.; Mendes, F.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as pure and mixed cultures in grape must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef]
- Moreira, N.; Mendes, F.; Hogg, T.; Vasconcelos, I. Alcohols, esters and heavy sulphur compounds production by pure and mixed cultures of apiculate wine yeasts. Int. J. Food Microbiol. 2005, 103, 285–294. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef] [PubMed]
- Lemos Junior, W.J.F.; Binati, R.L.; Felis, G.E.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Volatile organic compounds from Starmerella bacillaris to control gray mold on apples and modulate cider aroma profile. Food Microbiol. 2020, 89, 103446. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Kubodera, S.; Yanagida, F. Distribution of phenolic yeasts and production of phenolic off-flavors in wine fermentation. J. Biosci. Bioeng. 2000, 90, 90–97. [Google Scholar] [CrossRef]
- Cûs, F.; Jenko, M. The influence of yeast strains on the composition and sensory quality of Gewürztraminer wine. Food Technol. Biotechnol. 2013, 51, 547–553. [Google Scholar]
- López, M.C.; Mateo, J.J.; Maicas, S. Screening of β-Glucosidase and β-Xylosidase activities in four non-Saccharomyces yeast isolates. J. Food Sci. 2015, 80, C1696–C1704. [Google Scholar] [CrossRef]
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of beta-glucosidase activity in yeasts of enological origin. J. Appl. Bacteriol. 1994, 77, 519–527. [Google Scholar] [CrossRef]
- Spagna, G.; Barbagallo, R.N.; Palmeri, R.; Restuccia, C.; Giudici, P. Properties of endogenous beta—Glucosidase of a Saccharomyces cerevisiae strain isolated from Sicilian musts and wines. Enzym. Microb. Technol. 2002, 31, 1030–1035. [Google Scholar] [CrossRef]
- de Arruda Moura Pietrowski, G.; dos Santos, C.M.E.; Sauer, E.; Wosiacki, G.; Nogueira, A. Influence of fermentation with Hanseniaspora sp. yeast on the volatile profile of fermented apple. J. Agric. Food Chem. 2012, 60, 9815–9821. [Google Scholar] [CrossRef]
- Wosiacki, G.; Nogueira, A.; Silva, N.C.C.; Denardi, F.; Vieira, R.G. Quality profile of samples of 139 apples. Acta Aliment. 2008, 37, 9–22. [Google Scholar] [CrossRef]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Röcker, J.; Strub, S.; Ebert, K.; Grossmann, M. Usage of different aerobic non-Saccharomyces yeasts and experimental conditions as a tool for reducing the potential ethanol content in wines. Eur. Food Res. Technol. 2016, 242, 2051–2070. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachancea thermotolerans and its influence on mannose-containing polysaccharides wine composition. Amb Express 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [PubMed]
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen-Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61. [Google Scholar] [CrossRef]
- Zelle, R.M.; de Hulster, E.; van Winden, W.A.; de Waard, P.; Dijkema, C.; Winkler, A.A.; Geertman, J.-M.A.; van Dijken, J.P.; Pronk, J.T.; van Maris, A.J.A. Malic acid production by Saccharomyces cerevisiae: Engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl. Environ. Microbiol. 2008, 74, 2766–2777. [Google Scholar] [CrossRef]

| Country | Apple Production (kt) | Fresh Domestic Consumption (kt) |
|---|---|---|
| China | 33,000 | 38,050 |
| European Union | 15,442 | 7400.6 |
| United States | 5564 | 2589.4 |
| Turkey | 3306 | 2630.5 |
| Iran | 3085 | 1813.9 |
| Russia | 1656 | 1884.4 |
| Chile | 1393 | 229.6 |
| Ukraine | 1211 | 1066.2 |
| Brazil | 1156 | 1325.9 |
| Compounds | Concentrations |
|---|---|
| Glucose * | 1.8 |
| Fructose * | 5.6 |
| Sucrose * | 2.6 |
| Arabinose ** | 109 |
| Rhamnose ** | 12 |
| Fucose ** | 8.5 |
| Galactose ** | 71.5 |
| Glucose ** | 288 |
| Mannose ** | 21 |
| Xylose ** | 57 |
| Galacturonic Acid ** | 227 |
| Compounds | Apple Flesh | Apple Peel |
|---|---|---|
| Procyanidin B1 | 1.0 ± 0.1 | 1.9 ± 1.6 |
| (+) -catechin | 1.1 ± 0.9 | 5.1 ± 3.0 |
| Procyanidin B2 | 1.6 ± 0.3 | 7.1 ± 1.5 |
| Procyanidin C1 | n.d. | 6.7 ± 1.2 |
| (−) -epicatechin | 1.0 ± 0.7 | 8.8 ± 4.9 |
| Procyanidin A2 | 2.5 ± 1.2 | 7.8 ± 2.9 |
| Total Flavanols | 6.4 ± 2.5 | 36.1 ± 8.8 |
| Gallic acid | 1.6 ± 0.2 | |
| Protocatechuic acid | 0.1 ± 0.0 | 1.3 ± 0.6 |
| Chlorogenic acid | 2.1 ± 0.8 | 5.6 ± 0.7 |
| Caffeic acid | 0.8 ± 0.5 | 0.8 ± 0.5 |
| P-coumaric acid | 0.5 ± 0.1 | 1.8 ± 0.4 |
| Ferulic acid | 0.1 ± 0.1 | 0.9 ± 0.4 |
| Total Phenolic Acids | 5.2 ± 1.3 | 14.3 ± 2.1 |
| Phloridzin | 1.1 ± 0.7 | 4.8 ± 3.5 |
| Hyperoside | n.d. | 84.2 ± 57.1 |
| Isoquercitrin | n.d. | 16.6 ± 8.6 |
| Rutin | n.d. | 5.4 ± 3.3 |
| Reynoutrin | n.d. | 17.0 ± 7.6 |
| Avicularin | n.d. | 21.4 ± 8.4 |
| Quercitrin | n.d. | 25.4 ± 13.1 |
| Quercetin | n.d. | 13.4 ± 5.2 |
| Total flavonols | n.d. | 183.5 ± 99.6 |
| Total polyphenols | 12.6 ± 4.4 | 239.4 ± 118.6 |
| TPC | 179.5 ± 52.3 | 914.7 ± 331.3 |
| Compounds | % of Total Lipids |
|---|---|
| Triacylglycerols | 5 |
| Glycolipids | 17 |
| Phospholipids | 47 |
| Sterols | 15 |
| Sterol esters | 2 |
| Sulfolipids | 1 |
| Others | 13 |
| Minerals | mg/100 g Dry Matter |
|---|---|
| Potassium | 840 |
| Sodium | 7.9 |
| Calcium | 38 |
| Magnesium | 40 |
| Iron | 1.6 |
| Aluminum | 0.43 |
| Phosphorus | 73 |
| Zinc | 0.65 |
| Manganese | 0.3 |
| Copper | 0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review. Molecules 2020, 25, 3698. https://doi.org/10.3390/molecules25163698
Al Daccache M, Koubaa M, Maroun RG, Salameh D, Louka N, Vorobiev E. Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review. Molecules. 2020; 25(16):3698. https://doi.org/10.3390/molecules25163698
Chicago/Turabian StyleAl Daccache, Marina, Mohamed Koubaa, Richard G. Maroun, Dominique Salameh, Nicolas Louka, and Eugène Vorobiev. 2020. "Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review" Molecules 25, no. 16: 3698. https://doi.org/10.3390/molecules25163698
APA StyleAl Daccache, M., Koubaa, M., Maroun, R. G., Salameh, D., Louka, N., & Vorobiev, E. (2020). Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review. Molecules, 25(16), 3698. https://doi.org/10.3390/molecules25163698









